
·Basic Research·Current
Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
Effect of sorafenib in a murine
high risk penetrating keratoplasty model
Yang Kyung Cho1, Eun Young Shin2, Hironori Uehara3,
Balamurali K Ambati3
1Department of Ophthalmology,
St.Vincent’s Hospital, College of Medicine, the Catholic University of Korea,
Suwon, Gyeonggi-Do 16247, Korea
2Research Institute of
Medical Science, St.Vincent's Hospital, College of Medicine, the Catholic
University of Korea, Suwon, Gyeonggi-Do 16247, Korea
3Department of
Ophthalmology, University of Utah, School of Medicine, Salt Lake City, Utah
84132, USA
Correspondence
to: Yang
Kyung Cho. Department of Ophthalmology, St.Vincent’s Hospital, College of
Medicine, the Catholic University of Korea, 93 Ji-Dong, Paldal-Gu, Suwon,
Gyeonggi-Do 16247, Korea. yangkyeung@hanmail.net
Received:
2016-11-12
Accepted: 2017-03-25
Abstract
AIM: To evaluate the
effect of sorafenib in murine high risk keratoplasty model.
METHODS: Graft survival,
corneal neovascularization, and corneal lymphangiogenesis were compared among
the sorafenib, dexamethasone, dimethyl sulfoxide (DMSO), and phosphate buffered
saline (PBS) groups following subconjunctival injection in mice that underwent
high risk penetrating keratoplasty (HRPK). Real-time polymerase chain reaction
was performed to quantify the expression of inflammatory cytokines and vascular
endothelial growth factor (VEGF)-A, VEGF-C, vascular endothelial growth factor
receptor (VEGFR)-2, VEGFR-3.
RESULTS: The two-month
graft survival rate for HRPK was 42.86% in sorafenib group, 37.50% in
dexamethasone group, 0 in DMSO group, and 0 in PBS group. Sorafenib
significantly increased graft survival compared to the DMSO and PBS group (P<0.05).
The sorafenib didn’t show significant effect in decreasing neovascularization
compared with dexamethsone, DMSO, and PBS group. The sorafenib showed less
total lymphangiogenesis than the dexamethasone, DMSO, and PBS group (P=0.011,
P<0.001, P<0.001, respectively). The sorafenib group showed
reduced expression of VEGF-C, tumor necrosis factor (TNF)-alpha, interleukin
(IL)-6, VEGFR-2 and VEGFR-3 compared with DMSO group and
PBS group (all P<0.05). The sorafenib group didn’t show difference in
the expression of VEGF-A compared with DMSO, neither with PBS. The sorafenib
group showed reduced expression of VEGFR-3 compared with dexamethasone (P=0.051).
CONCLUSION: The
subconjunctivally administered sorafenib shows significant anti-lymphangiogenic
effect, resulting in increased transplant survival in a murine high risk
keratoplasty model. We suggest that a close linkage between decreased
VEGF-C/VEGFR-2 and -3 signaling and increased corneal graft survival by
sorafenib seems to exist.
KEYWORDS: sorafenib;
neovascularization; graft survival; lymphangiogenesis; dexamethasone
DOI:10.18240/ijo.2017.06.02
Citation: Cho YK, Shin EY, Uehara H, Ambati BK. Effect of
sorafenib in a murine high risk penetrating keratoplasty model. Int J
Ophthalmol 2017;10(6):834-839
Article
Outline
INTRODUCTION
In penetrating
keratoplasty (PK) in high risk patients, even with current immunosuppression,
rejection rates can be over 70%, whereas PK in normal risk patients maintains
the survival rates as high as 90% at the first year[1-4]. Compared to normal risk penetrating keratoplasty (NRPK), eyes with high risk
penetrating keratoplasty (HRPK) exhibited much higher
levels of (lymph) angiogenesis[2-4] and inflammatory chemokines in early
postoperative period[3,5]. Risk
for graft rejection can be corneal (lymph) angiogenesis, regrafts, high
intraocular pressure, trauma, and perioperative inflammation. Corneal (lymph)
angiogenesis is a well known risk for graft rejection and failure[3,5]. Therefore, minimizing corneal
(lymph) angiogenesis has the potential to decrease immunologic graft rejection
and graft failure rates[6-7].
Several anti-(lymph) angiognenic treatment was tried to enhance graft survival[7-8]. However, because lymphatics might
play important roles to heal conjunctivitis or conjunctivalchemosis or probable
corneal edema, anti-lymphangiogenesis (LY) treatment can affect the wound
healing of these structures[9-10].
For example, rapamycin which can inhibit LY through inhibition of vascular endothelial growth factor (VEGF)-C can inhibit wound
healing, even though its therapeutic effect for treatment of malignancy[10].
Especially in
corneal transplantation, LY, not hemangiogenesis has been reported to be a
primary mediator of rejection[7-8]. So, treatment targeting LY have
been studied and developed to treat different tumors and ocular diseases.
However, the other report showed that anti-angiogenic treatment such as strong
VEGF-A trap was more successful in improving long-term graft survival as
compared with anti-lymphangiogenic treatment such as anti VEGF-C and soluble vascular endothelial growth factor receptor (VEGFR) 3[11]. The strong angiogenic
VEGF-A increase hemangiogenesis through VEGFR-2 binding. The strong
lymphangiogenic VEGF-C and -D are the main prolymphangiogenic factors that act
through the activation of VEGFR-3[8,12]. When VEGF binds to VEGFR
(receptor), then activation of the rat sarcom (RAS)/rapidly accelerated fibrosacrcoma (RAF)/extracellular signal regulated kinase (ERK)/mitogen activated protein
kinase (MAPK) starts
signal transduction which leads to endothelial proliferation[8,12].
Sorafenib is a
potent inhibitor of RAS/RAF kinase and tyrosine kinases such as VEGFR-2, PDGFR
β, and VEGFR-3[12-14]. This
multikinase inhibitors interfere with the activation of VEGFRs by preventing
phosphorylation. Sorafenib is already in use as an anticancer drug that aims at
tumor proliferation and neovascularization (NV)[12-14]. Recent reports have suggested new therapeutic role
of sorafenib in ocular disease; age-related macular degeneration (AMD) and
retinopathy of prematurity (ROP)[15-16].
The effect of oral administration of sorafenib on choroidal and corneal NV was
previously reported[17]. From the several
previous reports of the effect of sorafenib in various type of tumor and
neovascular disease in retina, we expected that sorafenib would work on cornea.
We tried to evaluate the effect of sorafenib in neovascular disease of cornea,
the HRPK, which has high graft failure rate due to severe hem/LY. Here, we
studied the effect of subconjunctivally injected sorafenib on the graft
survival, LY and hemangiogenesis in a mouse model of high risk corneal PK.
SUBJETCS AND METHODS
The
experiments were performed with the regulations of Association for Research in
Vision and Ophthalmology and approval by the Institutional Animal Care and Use
Committee of the Catholic University of Korea, St. Vincent’s Hospital.
High Risk
Corneal Transplantation Recipient mice [6 to 8
weeks old, female, Bagg Albino (BALB)/c] and donor mice (C57BL/6) (the Koatech, Pyeongtak, Korea)
were anesthetized by Zoletil®50 (30 mg/kg, Virbac Korea Co. Ltd.)
and xylazine (10 mg/kg) was done. Prior to PK, two corneal sutures (10-0 nylon,
CS140-6, Ethicon, Inc.) were placed between the corneal center and the limbus
to induce vascularization. The corneal sutures were removed and corneal
transplantation was done according to the methods used in the normal risk PK[18].
Subconjunctival
Injection of Anti-angiogenic Agents
Sorafenib
(Santa Cruz Biotechnolology, Inc., Santa Cruz, CA, USA) was dialted with
vehicle dimethyl sulfoxide (DMSO). In four group, the respective treatment;
sorafenib (15 μL, 465 μg/mL, 12 eyes), dexamethasone (15 μL, 500 μg/mL, 12 eyes),
DMSO (15 μL, 12 eyes) and phosphate buffered saline (PBS) (15 μL, 12 eyes) were
injected into the subconjunctival space from the day of transplantation and
weekly to postoperative 8wk. Among these groups, sorafenib group was the case
group. And the dexamethasone, which is already known as antiangiogenic agent
was the positive control group. The sorafenib was diluted with vehicle DMSO, so
the DMSO was the negative control. PBS group was another negative control
group.
Clinical
Evaluation of Rejection Microscopic examination
was done weekly through post-op week 8 and corneal microscopic pictures were
taken. Evaluation of graft clarity according to the grading system was done as
previously described. The opacity grading (0 to 5) was as a previous report[19]: grades 3 and above were considered a graft
rejection.
Analysis of
Angiogenesis and Lymphangiogenesis To know the extent of
corneal NV and LY in the recipient cornea before grafting, two corneal sutures
were placed on 6 corneas between the corneal center and the limbus. After two
weeks, we harvested 6 corneas to evaluate the extent of corneal NV before
grafting. After immune staing with CD31 and LYVE-1, we evaluated the extent of
NV and LY under fluorescent microscope. After PK and the planned injections for
observation periods (8wk after PK), we harvested eyes and the corneas were
trimmed. Vascular and lymphatic endothelial cells were immunestained on corneal
flat mounts as our previous report[20]. After
immune staining and flat mounting of the cornea, images of the corneal
vasculature were captured by a fluorescent microscope (OLYMPUS BX51, Tokyo,
Japan). NV and LY were quantified as a previous report[20].
Total NV (%)=neovascularized area/total cornea area×100%; total LY (%)=LY
area/total cornea area×100%.
Comparison
of Graft Survival, Angiogenesis and Lymphangiogenesis in High Risk Penetrating
Keratoplasty The four groups
(sorafenib, dexamethasone, DMSO and PBS) were compared in HRPK. Graft survival,
NV and LY were compared.
Quantitative
Real-time Polymerase Chain Reaction Analysis of Gene
Expression in the Mouse Cornea After harvesting, the
corneas were trimmed and the expression of VEGF-A, VEGF-C, VEGFR-2, VEGFR-3,
tumor necrosis factor (TNF)-alpha and interleukin (IL)-6 was analyzed using
real-time polymerase chain reaction (RT-PCR) as previous report[20]. We used published primer sequences for mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH)[20], VEGF-A[21], VEGF-C[21] , VEGFR2[21], VEGFR3[21], TNF-alpha[22], and IL-6[23]. Each
gene expression level was analyzed by the Ct method, using GAPDH expression as
an internal control. The relative expression level of each sample is expressed
as a fold change compared to the normal control (PBS).
Statistical
Analysis SPSS 11.5 (Chicago, IL,
USA) was used. Graft survival was analyzed using Kaplan-Meier survival curves
(log rank test). NV and LY was compared with groups using an unpaired
two-tailed t-test. RT-PCR results were compared
using ANOVA (analysis of variance) with post hoc test
and the unpaired t-test. A P<0.05 was considered statistically
significant.
RESULTS
Graft
Survival Figure 1 shows the
comparison of graft survival among the four groups in HRPK. There was no
difference in graft survival between the sorafenib and the dexamethasone groups
(P>0.05). Sorafenib significantly increased graft survival compared
to the DMSO and PBS (P=0.023, P=0.022, respectively).
Dexamethasone showed increased graft survival compared to the DMSO and PBS (P=0.082,
P=0.115, respectively), but they don’t reach statistical significance.
The graft survival was not different between the DMSO and PBS (P>0.05).
At the postoperative eight-week, the graft survival rate for each group was
42.86% in sorafenib, 37.50% in dexamethasone, 0 in DMSO, and 0 in PBS. The
subconjunctivally administered sorafenib showed increased transplant survival
in a murine high risk keratoplasty model.
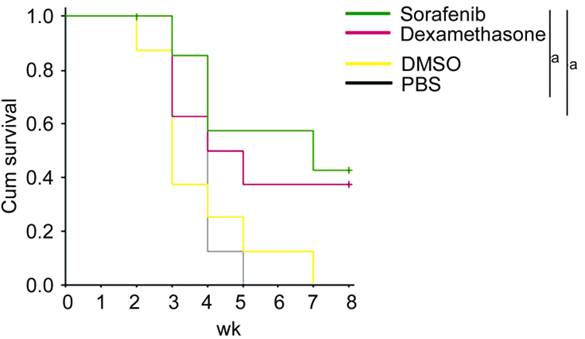
Figure 1
Comparison of graft survival in four groups: sorafenib, dexamethasone, DMSO,
and PBS aP<0.05.
Neovascularization Two weeks after corneal suture,
before grafting, the the hemangiogenesis area in the recipient was 7.38% (mean)
of total corneal area. Eight weeks after PK, the sorafenib group (13.33%±4.03%)
didn’t decreased NV significantly than DMSO group (18.64%±1.74%) (P=0.232).
Dexamethasone group (13.91%±1.68%) showed less total neovascularized area than
DMSO group (18.64%±1.74%), but they don’t reach statistical significance (P=0.087)
(Figures 2 and 3). Sorafenib and dexamethasone were
no different with regard to their effects on NV. Similarly, there was no
difference between DMSO and PBS (18.17%±1.31%) with regard to NV. Sorafenib
showed negligible anti-angiogenesis effect compared with dexamethasone, DMSO
and PBS LY. Two weeks after corneal suture,
before grafting, the LY area in the recipient was 5.16% (mean) of total corneal
area. Eight weeks after PK, The sorafenib group (5.79%±0.81%) showed
less total lymphangiogenenic area than dexamethasone (12.30%±1.88%), DMSO
(18.26%±1.39%), and PBS (18.55%±1.23%) group (P=0.011, P<0.001,
P<0.001, respectively) (Figures 2, 3). The dexamethasone group showed less LY compared to the DMSO
and PBS group (P=0.035, 0.043, respectively). There was no difference of
LY between DMSO and PBS. Sorafenib has significant anti-LY effect on cornea
compared with dexamethasone, DMSO and PBS.
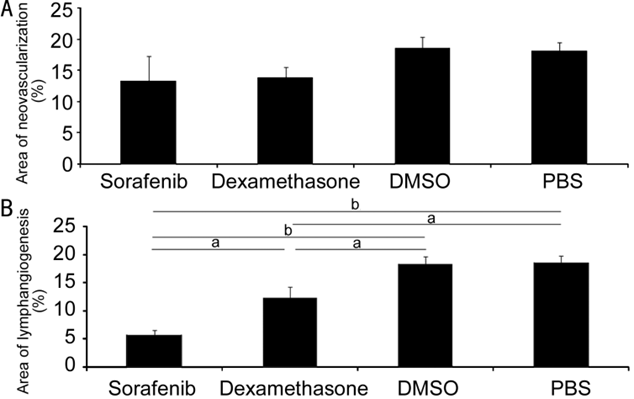
Figure 2
Comparison of NV and LY in four groups: sorafenib, dexamethasone, DMSO, and PBS
A: Comparison of NV; B: Comparison of LY.
aP<0.05, bP<0.01.
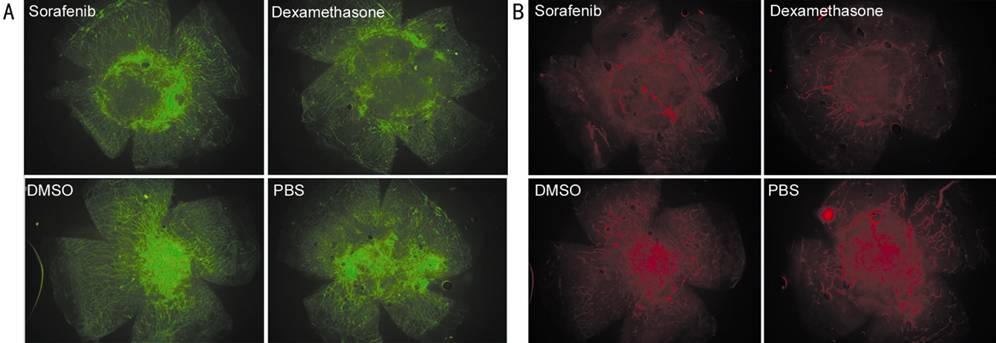
Figure 3
Representative pictures of NV and LY in four groups: sorafenib, dexamethasone,
DMSO, and PBS A: CD31 staining, staining
of blood vessel; B: LYVE-1 staining, staining of lymphatic vessel.
Real-time
Polymerase Chain Reaction The mRNA expression of
VEGF-A, VEGF-C, TNF-alpha, IL-6, VEGFR-2 and VEGFR-3 in each group are shown in
Figure 4. The mRNA expression ratios of VEGF-A, VEGF-C, TNF-alpha, IL-6,
VEGFR-2 and VEGFR-3 are expressed normalized to GAPDH (PBS group=1.0). There
was no significant difference of expression ratio of VEGF-A, VEGF-C, TNF-alpha,
IL-6, VEGFR-2 and VEGFR-3 between DMSO and PBS group.
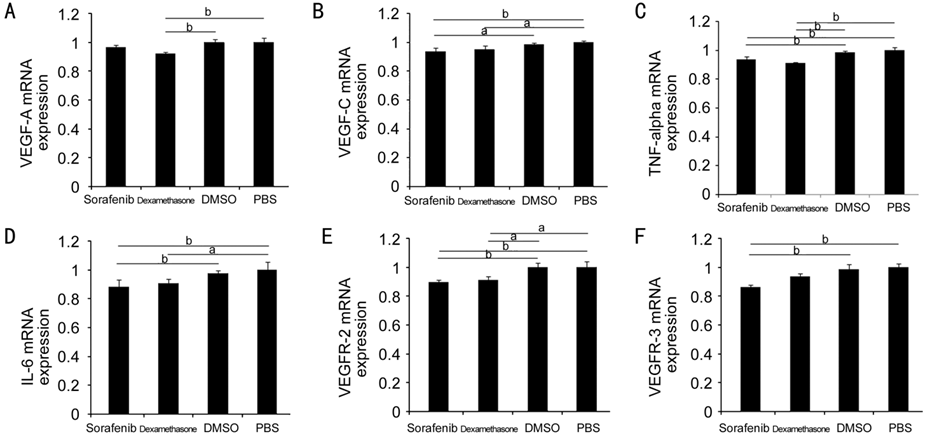
Figure 4
Comparison of mRNA expression in four groups: sorafenib, dexamethasone, DMSO,
and PBS A: VEGF-A; B: VEGF-C; C:
TNF-alpha; D: IL-6; E: VEGFR-2; F: VEGFR-3. aP<0.05, bP<0.01.
The sorafenib
showed reduced VEGF-C, TNF-alpha, IL-6, VEGFR-2 and
VEGFR-3 compared with DMSO group (P=0.03, 0.005, 0.006, 0.003, 0.003,
respectively). The sorafenib showed reduced VEGF-C, TNF-alpha,
IL-6, VEGFR-2 and VEGFR-3 compared with PBS (P=0.004, P=0.001, P=0.002,
P=0.005, P<0.001, respectively). The sorafenib didn’t show
difference in the expression of VEGF-A compared with DMSO, neither with PBS.
The sorafenib group showed reduced expression of VEGFR-3 compared with
dexamethasone (P=0.051) which is already well known anti-(lymph)
angiogenic and anti-inflammatory agent. The dexamethasone group showed reduced
VEGF-A, TNF-alpha, VEGFR-2 compared with DMSO group (P=0.004,
P<0.001, P=0.012, respectively). The dexamethasone group
showed reduced VEGF-A, VEGF-C, TNF-alpha, IL-6, and VEGFR-2
compared with PBS group (P=0.007, P=0.040, P<0.001, P=0.016,
P=0.017, respectively). The dexamethasone group didn’t show difference
in the expression of VEGFR-3 compared with DMSO, neither with PBS.
DISCUSSION
Among human
transplantation surgeries, corneal transplantation is one of the most commonly
performed. The overall 10-year survival rates of corneal grafts reach between
75% and 80%[1-3,5].
However, in the “high-risk” conditions, the survival rate drops to 30%-50% at 3
to 5-year follow-up[2,11,24]. Compared to normal risk keratoplasty, eyes with HRPK
exhibited significantly higher levels of NV, LY[25] and
inflammation in the early postoperative period[3,5,25-26]. The
chemokines expressed in high risk eyes correlated with increased number of
inflammatory cells in high risk recipients[26].
In this study,
IL-6 and TNF-alpha, the inflammatory cytokines were significantly decreased by
sorafenib as effectively as dexamethasone, even in HRPK which is different from
normal risk PK in respect to their postoperative NV and inflammation. The most
common possible reason for graft failure is immunologic rejection. The
conditions that may place the cornea at a higher risk of rejection are corneal
NV, LY, position of the graft close to limbus, and herpes simplex keratitis[5,27]. It has been reported that the
preexisting blood and lymphatic vessels in cornea is a strong risk factor for
immune rejection[28-29].
Ocular immune
privilege can be acquired through avascularity, alymphatics, low major
histocompatibility complex and native immunosuppression[3,5]. Although the normal cornea does not have blood and
lymphatic vessels, NV and LY can be induced after traumatic, chemical,
inflammatory or infectious damage. LY especially constitutes the afferent arm
of the corneal transplantation immunity, and recently, it has been demonstrated
that LY is a primary mediator of corneal transplant rejection[8,30]. So, decreasing lymphangiognesis can enhance graft
survival. The VEGF is the a complex network controlling blood and lymphatic
vessels[31-33]. Several
previous studies have shown that VEGFR-3 mediates LY in the cornea and other
tissues[34-35]. Most recently,
it has been indicated that VEGFR-2 also plays a role in corneal LY, but with
unknown mechanism[30,34-35].
Our study
evaluated the anti-angiognenic and anti-lymphangiogenic effect of sorafenib, as
a therapeutic option against graft rejection in high risk keratoplasty.
Sorafenib, a multikinase inhibitor, has shown promising results for the
treatment of advanced hepatocellular carcinoma in clinical trials. The
mechanisms of sorafenib’s antitumor activities have been well presented.
Evidence has shown that sorafenib inhibits the rapidly accelerated RAF/ MAPK/ERK kinase (MEK)/ERK signal pathways and receptor tyrosine kinases, including
VEGFR-2, VEGFR-3, Flt-3, c-KIT, and platelet-derived growth factor receptor
(PDGFR). the blocking of VEGFR and PDGFR may account for the antiangiogenesis
effect of sorafenib. Sorafenib contains hydrophilic amide groups, and
lipophilic pyridine, and has good biological activity[12-13,36]. Sorafenib is characterized by
good absorbability because of its small molecular weight and the strong tissue.
Its long half-life could reduce intraocular injection times. Additionally, sorafenib
is a synthetic urea derivative and the immunogenicity is low[12-13,36].
Recent reports
have also suggested the role of sorafenib in the treatment of AMD and ROP[15-17]. Our study focused on the
effect of sorafenib on graft survival after corneal transplantation. In
comparison of RNA expression, the results of our study show clearly that
sorafenib significantly reduced the VEGFR-2 and VEGFR-3 in murine corneas. This
is of importance, because VEGFR-2 and 3 in particular plays an important role
in the development of lymphatic vessels, and a close linkage between
VEGF-C/VEGFR-2 and -3 signaling and corneal graft rejection seems to exist. The
sorafenib group showed reduced VEGFR-3 compared with dexamethasone (P=0.051).
We suggest that this result can explain the reduced LY in sorafenib group
compared with dexamethasone. Sorafenib did not affect VEGF-A compared with DMSO
and PBS (P=0.232, 0.087, respectively), resulting in negligible effect
on corneal angiogenesis. In our experimental setup, dexamethasone did not
affect VEGFR-3 (Figure 4F). Dexamethasone mainly affected VEGF-A and VEGFR-2.
Dual blockade
of VEGFR-2 and another key lymphatic receptor, such as VEGFR-3 by sorafenib,
will maximize the anti-lymphangiogenic effect in high risk corneas. In comparison
of NV and LY, both sorafenib and dexamethasone showed more pronounced effect in
decreasing LY rather than NV in our HRPK model. Moreover, sorafenib decreased
LY than dexamethasone. This can explain the results of the enhanced graft
survival compared to DMSO only in sorafenib group, not in dexamethasone group.
Because LY is a key mediator of corneal transplant rejection, in the high-risk
eyes, the rejection rate can be as high as 90%[7-9,30,37].
Unfortunately, many patients who need corneal transplantation fall into this
high risk category, and there is little effective treatment for them even with
steroid treatment.
Our study
indicates that sorafenib may be able to replace the effect of steroid in the
high-risk grafting beds, to improve the survival rate of high-risk transplants.
The anti-lymphangiogenic effect of sorafenib was significantly higher than that
of dexamethasone in HRPK set up in our study (P=0.011), which leads to
increased graft survival. Also, our study might support the importance of LY on
graft survival rather than hemangiogenesis, which warrants further
investigation.
In conclusion,
we investigated the significant anti-lymphangiogenic effect of
subconjunctivally administered sorafenib (off-label use), a
multi-target-receptor tyrosine kinase inhibitor, on increasing transplant
survival in a murine high risk keratoplasty model. These results mandate
further clinical investigation of sorafenib for corneal graft.
ACKNOWLEDGEMENTS
Cho YK
designed the study, performed the animal work and the experiment, wrote the
manuscript. Shin EY assisted the animal work and the experiment. Uehara
Hironori designed and revised the study. Ambati BK designed and revised the
study.
Conflicts
of Interest: Cho YK, None; Shin EY, None; Uehara H, None; Ambati
BK, None.
REFERENCES
1 Santos LN, de Moura LR, Fernandes BF, Cheema DP,
Burnier MN Jr. Histopathological study of delayed regraft after corneal graft
failure. <ii>Cornea </ii>2011;30(2):167-170. [PubMed]
2 Dastjerdi MH, Saban DR, Okanobo A, Nallasamy N,
Sadrai Z, Chauhan SK, Hajrasouliha AR, Dana R. Effects of topical and
subconjunctival bevacizumab in high-risk corneal transplant survival.
<ii>Invest Ophthalmol Vis Sci </ii>2010;51(5):2411-2417. [PMC free
article] [PubMed]
3 Niederkorn JY. High risk corneal allografts and why
they lose their immune privilege. <ii>Curr Opin Allergy Clin Immunol
</ii>2010;10(5):493-497. [PMC free
article] [PubMed]
4 Lam H, Dana MR. Corneal graft rejection.
<ii>Int Ophthalmol Clin </ii>2009; 49(1):31-41. [PubMed]
5 Niederkorn JY, Larkin DF. Immune privilege of
corneal allografts. <ii>Ocul Immunol Inflamn
</ii>2010;18(3):162-171. [PMC free
article] [PubMed]
6 Hos D, Saban DR, Bock F, Regenfuss B, Onderka J,
Masli S, Cursiefen C. Suppression of inflammatory corneal lymphangiogenesis by
application of topical corticosteroids. <ii>Arch Ophthalmol
</ii>2011;129(4):445-452. [PubMed]
7 Hos D, Schlereth SL, Bock F, Heindl LM, Cursiefen C.
Antilymphangiogenic therapy to promote transplant survival and to reduce cancer
metastasis: what can we learn from the eye? <ii>Semin Cell Dev Bio
</ii>2015;38:117-130. [PubMed]
8 Albuquerque RJ, Hayashi T, Cho WG, <ii>et
al</ii>. Alternatively spliced vascular endothelial growth factor
receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth.
<ii>Nat Med </ii>2009;15(9):1023-1030. [PMC free
article] [PubMed]
9 Nakao S, Hafezi-Moghadam A, Ishibashi T. Lymphatics
and lymphangiogenesis in the eye. <ii>J Ophthalmol
</ii>2012;783163.[CrossRef]
10 Huber S, Bruns CJ, Schmid G, Hermann PC, Conrad C,
Niess H, Huss R, Graeb C, Jauch KW, Heeschen C, Guba M. Inhibition of the
mammalian target of rapamycin impedes lymphangiogenesis. <ii>Kidney Int
</ii>2007;71(8):771-777. [PubMed]
11 Dohlman TH, Omoto M, Hua J, Stevenson W, Lee SM,
Chauhan SK, Dana R. VEGF-trap aflibercept significantly improves long-term
graft survival in high-risk corneal transplantation. <ii>Transplantation
</ii>2015;99(4): 678-686. [PubMed]
12 Saranadasa M, Wang ES. Vascular endothelial growth
factor inhibition: conflicting roles in tumor growth. <ii>Cytokine
</ii>2011;53(2):115-129. [PubMed]
13 Wilhelm SM, Adnane L, Newell P, Villanueva A,
Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor
that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling.
<ii>Mol Cancer Ther </ii>2008;7(10):3129-3140. [PubMed]
14 Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib
(BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK
pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. <ii>Meth
Enzymol </ii>2006;407:597-612.[CrossRef]
15 Kernt M, Thiele S, Neubauer AS, Koenig S, Hirneiss
C, Haritoglou C, Ulbig MW, Kampik A. Inhibitoryactivity of ranibizumab,
sorafenib, and pazopanib on light-induced overexpression of platelet-derived
growth factor and vascular endothelial growth factor A and the vascular
endothelial growth factor A receptors 1 and 2 and neurophilin 1 and 2. <ii>Retina
</ii>2012;32(8):1652-1663. [PubMed]
16 Tian LL, Ren B, Gao XW, Luo Y, Cai Y, Zhou K, Du
AJ, Zhao Y. Inhibition of retinopathy of prematurity in rat by intravitreal
injection of sorafenib. <ii>Int J Ophthalmol
</ii>2014;7(2):198-204. [PMC free
article] [PubMed]
17 Park YH, Roh SY, Lee YC. Effect of sorafenib on
experimental choroidal neovascularization in the rat. <ii>Clin Exp
Ophthalmol </ii>2010;38(7): 718-726. [PubMed]
18 Hayashi T, Yamagami S, Tanaka K, Yokoo S, Usui T,
Amano S, Mizuki N. Mouse model of allogeneic corneal endothelial cell
transplantation. <ii>Cornea </ii>2008;27(6):699-705. [PubMed]
19 Sonoda Y, Streilein JW. Orthotopic corneal
transplantation in mice--evidence that the immunogenetic rules of rejection do
not apply. <ii>Transplantation </ii>1992;54(4):694-704. [PubMed]
20 Uehara H, Cho YK, Simonis J, Cahoon J, Archer B,
Luo L, Das SK, Singh N, Ambati BK. Dual suppression of hemangiogenesis and
lymphangiogenesis by splice-shifting morpholinos targeting vascular endothelial
growth factor receptor 2 (KDR). <ii>FASEB J </ii>2013;27(1):76-85. [PMC free
article] [PubMed]
21 Bald T, Quast T, Landsberg J, <ii>et
al</ii>. Ultaviolet-radiation-induced inflammation promotes angiotropism
and metastasis in melanoma. <ii>Nature </ii>2014;507(7490):109-113.
[PubMed]
22 Amaral EP, Kipnis TL, de Carvalho EC, da Silva WD,
Leao SC, Lasundkaia EB. Difference in virulence of Mycobacterium avium isolates
sharing indistinguishable DNA fingerprint determined in murine model of lung
infection. <ii>PLoS One </ii>2011;6:e21673. [PMC free
article] [PubMed]
23 Klein Wolterink RG, Kleinjan A, van Nimwegen M,
Bergen I, de Bruijn M, Levani Y. Hendriks RW. Pulmonary innate lymphoid cells
are major producers of IL-5 and IL-13 in murine models of allergic asthma.
<ii>Eur J Immunol </ii>2012;42(5):1106-1116. [PubMed]
24 Fu H, Larkin DFP, George AJT. Immune modulation in
corneal transplantation. <ii>Transplant Rev (Orlando)
</ii>2008;22(2):105-115. [PubMed]
25 Kim HK, Choi JA, Uehara H, Zhang X, Ambati BK, Cho
YK. Presurgical corticosteroid treatment improves corneal transplant survival
in mice. <ii>Cornea </ii>2013;32(12):1591-1598. [PMC free
article] [PubMed]
26 Yamagami S, Hamrah P, Zhang Q, Liu Y, Huq S, Dana
MR. Early ocular chemokine gene expression and leukocyte infiltration after
high-risk corneal transplantation. <ii>Mol Vis
</ii>2005;11:632-640. [PubMed]
27 Bachmann BO, Bock F, Wiegand SJ, Maruyama K, Dana
MR, Kruse FE, Luetjen-Drecoll E, Cursiefen C. Promotion of graft survival by
vascular endothelial growth factor aneutralization after high-risk corneal
transplantation. <ii>Arch Ophthalmol </ii>2008;126(1):71-77. [PubMed]
28 Bachmann BO, Luetjen-Drecoll E, Bock F, Wiegand SJ,
Hos D, Dana R, Kruse FE, Cursiefen C. Transient postoperative vascular
endothelial growth factor (VEGF)-neutralisation improves graft survival in
corneas with partly regressed inflammatory neovascularisation. <ii>Br J
Ophthalmol </ii>2009;93(8):1075-1080. [PubMed]
29 Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K,
Jackson D, Kruse FE, Wiegand SJ, Dana MR, Streilein JW. Inhibition of hemangiogenesis
and lymphangiogenesis after normal-risk corneal transplantation by neutralizing
VEGF promotes graft survival. <ii>Invest Ophthalmol Vis Sci
</ii>2004;45(8):2666-2673. [PubMed]
30 Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO,
Zahn G, Wiegand S, Chen L, Cursiefen C . Cutting edge: lymphatic vessels, not
blood vessels, primarily mediate immune rejections after transplantation. <ii>J
Immunol </ii>2010;184(2):535-539. [PMC free
article] [PubMed]
31 Ferrara N. Vascular endothelial growth factor:
basic science and clinical progress. <ii>Endocr Rev </ii>2004;25(4):581-611.
[PubMed]
32 Ambati BK, Nozaki M, Singh N, <ii>et
al</ii>. Corneal avascularity is due to soluble VEGF receptor-1.
<ii>Nature </ii>2006;443(7114):993-997. [PMC free
article] [PubMed]
33 Shibuya M. Differential roles of vascular
endothelial growth factor receptor-1 and receptor-2 in angiogenesis. <ii>J
Biochem Mol Biol </ii>2006; 39(5):469-478.[CrossRef]
34 Zhang H, Grimaldo S, Yuen D, Chen L. Combined
blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant
survival. <ii>Invest Ophthalmol Vis Sci </ii>2011;52(9):6529-6535.
[PMC free
article] [PubMed]
35 Yuen D, Pytowski B, Chen L. Combined blockade of
VEGFR-2 and VEGFR-3 inhibits inflammatory lymphangiogenesis in early and middle
stages. <ii>Invest Ophthalmol Vis Sci </ii>2011;52(5):2593-2597. [PMC free
article] [PubMed]
36 Reiberger T, Angermayr B, Schwabl P, Rohr-Udilova
N, Mitterhauser M, Gangl A, Peck-Radosavljevic M. Sorafenib attenuates the
portal hypertensive syndrome in partial portal vein ligated rats. <ii>J
Hepatol </ii>2009;51(5):865-873. [PubMed]
37 Stacker SA, Williams SP, Karnezis T, Shayan R, Fox
SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer.
<ii>Nat Rev </ii>2014;14(3):159-172. [PubMed]