
·Basic Research·Current Issue·
·Achieve·
·Search Articles· ·Online Submission· ·About IJO· PMC
Protection of retinal ganglion
cells against optic nerve injury by induction of ischemic preconditioning
Xia Liu1,2, Jiu-Ping Liang1, Ou Sha3,
Song-Juan Wang4, Heng-Guo Li1, Eric Y.P. Cho2
1Medical Imaging Center,
the First Affiliated Clinical Hospital of Jinan University, Guangzhou 510632, Guangdong Province, China
2School of Biomedical Sciences, the
Chinese University of Hong Kong, Shatin, Hong Kong 999077, China
3Department of Preclinical Medicine, School
of Medicine, Shenzhen University, Shenzhen 518060, Guangdong Province, China
4Shiyan People’s Hospital of Baoan
District, Shenzhen 518108, Guangdong Province, China
Correspondence
to: Heng-Guo Li. Medical Imaging Center, the First Affiliated Clinical
Hospital of Jinan University, Guangzhou 510632, Guangdong Province,
China. lhgjnu@263.net; Eric Y.P. Cho. School of
Biomedical Sciences, the Chinese University of Hong Kong, Shatin, 325A, Lo
Kwee-Seong Integrated Biomedical Sciences Building, Area 39, Hong Kong 999077,
China. eric-cho@cuhk.edu.hk
Received:
2016-10-25
Accepted: 2017-04-14
Abstract
AIM: To explore if
ischemic preconditioning (IPC) can enhance the survival of retinal ganglion
cells (RGCs) after optic nerve axotomy.
METHODS: Twenty-four
hours prior to retinal ischemia 60min or axotomy, IPC was applied for ten
minutes in groups of (n=72) animals. The survival of RGCs, the cellular
expression of heat shock protein 27 (HSP27) and heat shock protein 70 (HSP70)
and the numbers of retinal microglia in the different groups were quantified at
7 and 14d post-injury. The cellular expression of HSP27 and HSP70 and changes
in the numbers of retinal microglia were quantified to detect the possible
mechanism of the protection of the IPC.
RESULTS: Ten minutes of
IPC promoted RGC survival in both the optic nerve injury (IPC-ONT) and the
retinal ischemia 60min (IPC-IR60) groups, examined at 7d and 14d post-injury.
Microglial proliferation showed little correlation with the extent of benefit
effects of IPC on the rescue of RGCs. The number of HSP27-positive RGCs was
significantly higher in the IPC-ONT group than in the sham IPC-ONT group,
although the percentage of HSP27-positive RGCs did not significantly differ
between groups. For the IPC-IR60 group, neither the number nor the percentage
of the HSP27-positive RGCs differed significantly between the IPC and the
sham-operated groups. The number of HSP70-positive RGCs was significantly
higher for both the IPC-ONT and the IPC-IR60 experimental groups, but the
percentages did not differ.
CONCLUSION: The induction of
IPC enhances the survival of RGCs against both axotomy and retinal ischemia.
KEYWORDS: ischemic preconditioning; retinal ganglion cells;
axotomy; retinal ischemia/reperfusion; heat shock protein 27 and 70
DOI:10.18240/ijo.2017.06.05
Citation: Liu X,
Liang JP, Sha O, Wang SJ, Li HG, Cho EY. Protection of retinal ganglion cells
against optic nerve injury by induction of ischemic preconditioning. Int J
Ophthalmol 2017;10(6):854-861
Article Outline
INTRODUCTION
Ischemic preconditioning (IPC), also known as ischemic
tolerance or ischemic resistance, refers to the phenomenon that brief nonlethal
periods of ischemia can protect local or remote organs from subsequent
prolonged periods of critical ischemia. IPC was first described with a canine
myocardium model in 1986, which showed that four cycles of short periods of
ischemia and reperfusion 40min prior to coronary artery occlusion, reduced by
75%, the size of the ultimate myocardial infarct compared to cases without IPC[1]. Subsequent studies disclosed that IPC provides both
histological and psychological protection through eliciting endogenous
protective mechanisms, providing a promising strategy for protecting tissues
and organ systems with high sensitivity to ischemia, such as the myocardium[2], muscle flaps, the stomach, kidneys, lungs and liver,
and the central nervous system[3]. In 1998, one
study showed that IPC in the rat retina alleviated functional impairment and
cell death and also provided complete protection against retinal
ischemia/reperfusion injury[4].
We have earlier shown that application of remote
ischemic post-conditioning can promote the survival of retinal ganglion cells
(RGCs) after optic nerve axotomy[5]. Induction of
ischemic tolerance has also been reported to be a promising strategy to protect
RGCs against diabetic retinopathy[6], increasing
survival and function of retinal neurons in a model of glaucomatous retinopathy[7]. Given this background we tested the hypothesis that
induction of ischemic tolerance would rescue ganglion cells from degeneration after
subsequent optic nerve axotomy and compare the protective effects of IPC after
retinal ischemia/reperfusion injury. Retinal microglia and the expression of
heat shock protein 27 (HSP27) and heat shock protein 70 (HSP70) were
quantitated to investigate the possible mechanism of IPC in surviving RGCs.
MATERIALS AND METHODS
The experiments were performed in adult 8 to
12-week-old Syrian golden hamsters (Mesocricetus auratus). Surgical
manipulations were performed after induction of general anesthesia by intraperitoneal
(i.p.) injection of ketamine/xylazine (200 mg/20 mg per kg body weight). All
hamsters were randomly assigned to one of two different groups: optic nerve
injury (optic nerve transection, ONT) and retinal ischemia/reperfusion (IP)
groups. In the ONT group, the RGCs of one eye were damaged by a complete
transection of the optic nerve proximal to the orbit, whereas in the IP group,
retinal ischemia was induced by ligature of the ophthalmic vessels (LOV)
lasting 60min after the induction of a 10min IPC performed 24h previously. The
survival of RGCs was quantified at 7 and 14d post-injury, as was the cellular
expression of HSP27 and HSP70 and changes in the numbers of retinal microglia
in the different groups. The experimental protocols have been approved by the
Animal Ethics Committee of the Chinese University of Hong Kong.
Ligature of the Ophthalmic Vessels to Induce Ischemic
Preconditioning Twenty-four hours prior to retinal ischemia or
axotomy, IPC was applied for 10min in groups of animals (n=72).
Following the method described by Lafuente et al[8]
with slight modifications, LOV was induced to produce transient retinal
ischemia, followed by retinal reperfusion upon releasing the suture. In this
procedure, the optic nerve was first exposed, and the superior dural sheath was
opened longitudinally. A 10/0 nylon suture was inserted between the dural
sheath and the optic nerve, the two ends of the suture were tied with a loose
knot around the dura, and the vessels were also ligated within the dura. During
the surgery, great care was taken to avoid damage to the optic nerve. Ten
minutes later, the suture was released and removed with great care in order to
ensure resumption of retinal perfusion. For each group, a corresponding sham
IPC procedure group was prepared in similar manner, i.e. with exposure
and loose suturing of the ophthalmic vessels, but without ligation of the
suture.
Transection of the Optic Nerve to Induce Ganglion Cell
Axonal Injury Twenty-four hours after the 10min of IPC, the optic
nerve of the right eye was cut both in the IPC-ONT and sham IPC-ONT groups. In
this procedure, the animals were re-anesthetized as above. The optic nerve of
the right eye was exposed in the orbit and transected with microsurgical scissors
2 mm behind the eyeball, taking care not to injure the ophthalmic artery
running along the inferior aspect of the dura.
Ligature of the Ophthalmic Vessels to Induce Transient
Retinal Ischemia/Reperfusion The LOV method to induce retinal ischemia/reperfusion
was performed as described above. The interruption of the retinal blood flow
was assessed by ophthalmoscopy of the eye fundus through an operating
microscope (Figure 1). Animals not showing a complete interruption of the
retinal blood flow underwent a second LOV operation until complete interruption
was observed. The duration of the ischemic period lasted 60min, whereupon the
suture was released for subsequent reperfusion. The sham IPC-IR60 group was
processed in the same manner, but without LOV. Before release of the suture,
observation of the eye fundus through the operating microscope corroborated and
confirmed the interruption throughout the targeted time. After the suture was
released and the skin was closed, resumption of the retinal blood flow was
investigated every five minutes, and the total time to complete reperfusion
time was recorded. Animals not exhibiting a complete recovery of retinal blood
flow within the first 10min after release were excluded from the study.
Transient cloudiness of the lens was occasionally observed during the LOV
process, which might have hampered the observation of reperfusion time.
However, the cloudiness reversed spontaneously within a few minutes after the
onset of reperfusion. The observation of the fundus was easy and non-invasive,
providing a reliable and feasible measure to confirm the cessation and recovery
of blood flow for each experimental animal.
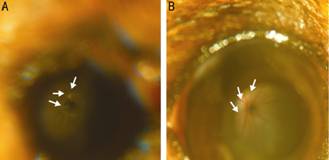
Figure 1
The conditions of the blood flow in the Syrian golden hamster retina Images (A) and (B) are both from the
operated eye; the funduscopic examination pictures were taken during the
process of LOV (A) and after reperfusion (B). A: The entire fundus looked pale
with clear white vessels branching from the optic disc initially, and the
arrows point to the whitening branches; B: The blood flow of the retina was
resumed after reperfusion, here the arrows point to the red branches.
Magnification: 4×.
Quantification of Ganglion Cell Survival Survival of ganglion cell at 7 or 14d post-optic nerve
injury or post-retinal ischemia/reperfusion was assessed by immunostaining with
anti-βIII-tubulin (clone TuJ1, CovanceInc., USA). The immunohistochemical
staining and quantification of the surviving RGCs followed the methods
described in previous paper[5,9].
In the ONT groups, retrograde labeling was also applied to confirm the results at
14d post-optic nerve injury. In this procedure, several crystals of the
fluorescent dye 4-[4-(didecylamino)styryl]-N-methylpyridinium iodide
(4-Di-10ASP; Molecular Probes), were placed on the cut surface of the proximal
stump upon surgical re-exposure of the
right optic nerve with truncation to 0.5 mm from the orbit. The wound
was closed and the animals recovered for a period of 2d after dye application,
namely at 14d post-optic nerve injury. At this time, the animals were killed
and the orbit resected. The entire retina fixed as a whole mount in glycerol
and observed under epifluorescence to quantify the number of surviving
retrogradely labelled RGCs, as in TuJ1 staining.
Changes in Microglia Number in the Optic Nerve Injury
Groups Results of previous studies suggest that the number
and activity of microglia increase after neuronal injury, and influence
neuronal survival. To test the relationship between microglial activation and
beneficial effects of IPC on RGC survival, we quantified retinal microglial
numbers in the IPC-ONT and sham IPC-ONT groups at 7d post-optic nerve section.
Whole mount retinas were incubated in a medium containing anti-βIII-tubulin and
anti-Iba-1 (1:1000 rabbit polyclonal, Wako). Anti-Iba-1 is a pan-microglia
marker, which is visualized with anti-rabbit-biotin plus streptavidin-Cy2. The
number of stained microglia per retina was quantified with Neurolucida as
described previously[5].
Expression of Heat Shock Protein 27 and Heat Shock
Protein 70 After Optic Nerve Transection or Ischemia/Reperfusion 60min The expression of HSP27 by RGCs was examined in the
IPC-ONT group, the IPC-IR60 group, and the corresponding sham groups. At 7d
post-injury, the whole mount retina was processed for double immunofluorescence
with antibodies against TuJ1 and HSP27 (rabbit polyclonal, StressGen; 1:1000)
according to procedures described previously[5].
The numbers of TuJ1- and HSP27-stained ganglion cells were quantified and the
percentage of HSP27-expressing ganglion cells in the surviving population (as
identified by double labeling with TuJ1) were calculated[4].
For the expression of HSP70 by RGCs in the IPC-ONT or
IPC-IR60 groups, paraffin-embedded sagittal sections across the optic disc were
selected and processed. At 7d post-injury, the retinal sections were processed
for double immunofluorescence with antibodies against TuJ1 and HSP70 (rabbit
polyclonal, Chemicals USA, Inc.; 1:100). TuJ1 staining was visualized by anti-mouse-Cy3,
whereas HSP70 staining was visualized by anti-rabbit-biotin, followed by
application of streptavidin-Cy2 (Jackson; 1:500).
The mean densities of TuJ1- and HSP70-positive RGCs in
the retinal sections were obtained from the average of 8 counted grids; 6
sections were counted for every sample, and mean was obtained. The number of
HSP70-positive RGCs in the sections was counted in four grids (200×200 μm2)
for each the superior and inferior portions of the retina. Furthermore, the
percentage of HSP70-positive RGCs was calculated for every section. In
addition, the retinal thickness at the center and the periphery was measured, i.e.
in the first and the last grids in the superior and inferior portions. To
reduce sampling errors, six sections were measured, and the mean thickness of
the center and periphery of the retina were obtained for every sample.
Statistical Analysis For the quantification of the survival of
TuJ1-positive RGCs, and also for quantification of HSP27- or HSP70-positive
ganglion cells, each experimental group consisted of four or five animals. All
quantitative data were presented as the means±SEM. For statistical comparison
of the outcomes between two groups, the two-tailed Student's t-test was
used with the level of statistical significance set at P<0.05.
RESULTS
Ischemic Preconditioning Promoted Survival of Retinal
Ganglion Cells After Optic Nerve Transection Ten minutes of IPC at 24h prior to axotomy had a
protective effect on the survival of RGCs at 7 and 14d post-axotomy (Figure 2A,
2C). In the IPC-ONT group (40 229±1205), the number of TuJ1-positive RGCs was
significantly higher than that in the sham IPC-ONT group (35 298±847; t-test,
P=0.02; Figure 2A vs 2B) at 7d post-axotomy. The number of
surviving RGCs was approximately 5000 to 7000 RGCs higher in the IPC-ONT
treatment group at 7d post-axotomy than in the sham IPC-ONT group. In the case
of the retinas examined at 14d post-axotomy, the number of TuJ1-positive RGCs
was 22% higher in the IPC-ONT group (25 358±1326) compared with the sham group
(20 788±1435; t-test, P=0.001; Figure 2C vs 2D). RGCs
survival as accessed by retrograde labelling with dye at 14d post-optic nerve
injury also confirmed the beneficial effects of the IPC pre-treatment. The
number of surviving ganglion cells was 14.7% higher in the treatment group
compared to the sham group (10 749±287 vs 9165± 463; P=0.02)
(Figure 2E vs 2F).
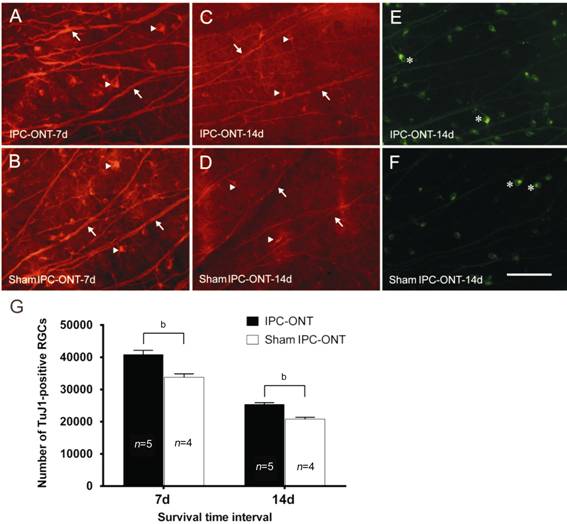
Figure 2 TuJ1-positive RGCs IPC-ONT
group (A) and sham IPC-ONT group (B) at 7d post-optic nerve section; IPC or
sham IPC was induced for 10min and 24h later, ONT was conducted; there were
more surviving RGCs in IPC-ONT group than in the sham conditioning group.
IPC-ONT group (C) and shamIPC-ONT group (D) at 14d post-optic nerve section;
IPC or sham IPC was applied for 10min and 24h later, ONT was conducted; there
were significantly more surviving RGCs in IPC-ONT group than in sham
conditioning group. IPC-ONT group (E) and sham IPC-ONT group (F) at 14d
post-optic nerve cut labeled by 4-Di-10ASP in a retina; there were more
surviving ganglion cells (some marked by asterisk) in the retina of animals
pretreated with IPC. The number of TuJ1-positive RGCs (G) in the different
groups at 7 and 14d post-optic nerve cut; significantly more RGCs were observed
in the IPC treatment group than in the sham group at both 7 and 14d
post-axotomy (t-test, bP<0.01). The arrows indicate
axons extending from the RGCs; the arrowheads indicate the bodies of RGCs
labeled by TuJ1; the asterisks indicate the surviving ganglion cells labeled by
4-Di-10ASP in a retina. Error bar=SEM. Scale bar=100 µm (A-F).
Increased Ganglion Cell Survival did not Correlate
with Changes in Microglia Number Microglia are located in different laminae of the
healthy retina, mainly in: 1) the ganglion cell layer (GCL); 2) inner plexiform
layer (IPL); 3) outer plexiform
layer (OPL). In our analysis we focused on microglia counts in the GCL, due to
the intimate association of microglia in those regions with RGCs and their
axons (Figure 3A). Morphologically, normal retinal microglia has small cell
bodies and short fine branches, which are extended without overlapping (Figure
3A). The total number of microglia in the GCL of normal retina amounted to
4394±200 (n=4) per retina (data not shown). According to our results,
the number of microglia increased to about 100 000 at 7d post-optic nerve sectioning
(Figure 3B). Microglia counts also increased to a similar extent in both the
IPL and OPL but to similar extents in the IPC and sham-pretreated groups (data
not shown). Thus, the extent of microglial proliferation was unaltered by the
induction of IPC, and seeming without relation to RGC survival after axotomy.
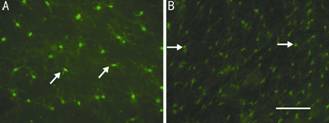
Figure 3
The activation of retinal microglia at 7d after optic nerve cut compared to the
normal group A: GCL microglia from the
normal group; B: GCL microglia from the retina at 7d post-optic nerve cut.
Arrows indicate microglia in GCL. Scale bar=100 µm.
Different Expression of HSP27 Induced by IPC Plus ONT
or IR60 Under normal conditions, retinal blood vessels and astrocytes
are labeled by HSP27, but no HSP27-positive RGCs are seen in the whole-mount
retina. Seven days after injury, in both the axotomy and 60min of retinal
ischemia groups, the expression of HSP27 in the RGCs was significantly
increased. We could distinguish RGCs labeled with HSP27 from astrocytes because
HSP27-positive RGCs are morphologically distinct, having a larger soma size
with several processes (Figure 4B and 4E). Furthermore, in combination with the
TuJ1 staining, some of HSP27-positive cells were confirmed as being RGCs.
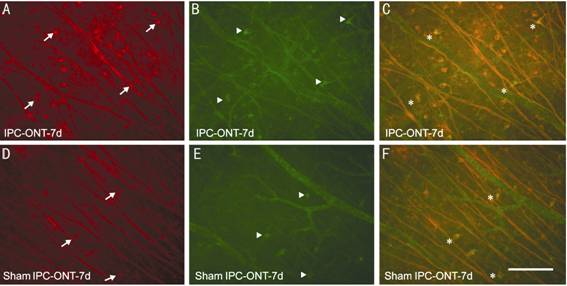
Figure 4
TuJ1-positive surviving RGCs (A, D), HSP27-positive RGCs in the same field of
view (B, E) and merged images (C, F)
Arrows
indicate RGCs labeled by TUJ-1 (red), arrowheads indicate RGCs labeled by HSP27
(green), asterisks indicate RGCs double-labeled by TuJ1 and HSP27 (yellow),
respectively. Note that more HSP27-positive RGCs are observed in the IPC group
(B) compared to the sham IPC group (E). A, B and C show surviving TuJ1-positive
and HSP27-positive RGCs at 7d after optic nerve axotomy in the IPC group and D,
E and F show corresponding results them in the sham IPC group. Scale bar=100
µm.
Significantly more HSP27-positive RGCs were observed
in the IPC-ONT group (1018±34) than in the sham IPC-ONT group (865±35) (Figure
5B). Although significantly more surviving TuJ1-positive RGCs were also
consistently observed in the IPC-ONT group, the percentage of HSP27-positive
RGCs (double-stained with TuJ1) did not differ between the two groups (2.5% in
the IPC-ONT group and 2.4% in the sham IPC-ONT group) (Figure 5C).
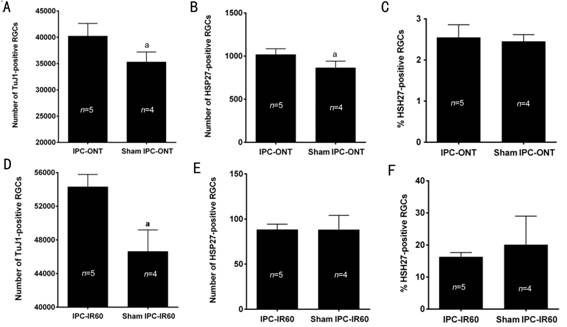
Figure 5
The numbers of TuJ1-positive RGCs (A, D) and HSP27-positive RGCs (B, E) in the
IPC-ONT, IPC-IR60 and their corresponding sham groups at 7d post-ischemia Significantly more TuJ1-positive RGCs
were found in the IPC-ONT group than in the sham IPC-ONT group at 7d
post-ischemia (A), and a significant difference in number of HSP27-positive
RGCs was observed between IPC-ONT and sham IPC-ONT groups (B), while no
difference in percentage of HSP27-positive RGCs between IPC-ONT and sham
IPC-ONT groups (C). Meanwhile, significantly more surviving RGCs were found in
the IPC-IR60 group than in the sham IPC-IR60 group at 7d post-ischemia (D), but
no difference in number and percentage of HSP27-positive RGCs was observed
between IPC-IR60 and sham IPC-IR60 groups (E, F). t-test, aP<0.05;
Error bar=SEM.
Although the number of TuJ1-positive RGCs was
significantly higher in the IPC-IR60 group (54 304±1474) than in the sham
IPC-IR60 group (46 633±2565) at 7d post-ischemia (P=0.03) (Figure 5D),
the numbers of HSP27-positive RGCs were not different between the two groups
(88±6 in the IPC-IR60 group and 88±16 in the sham IPC-IR60 group (Figure 5E).
In addition, much fewer HSP27-positive RGCs were found in the IPC-IR60 group
than in the IPC-ONT group, although the numbers of TuJ1-positive RGCs were
slightly higher in the IPC-IR60 group than in the IPC-ONT group; fewer than 100
HSP27-positive RGCs were found in the IPC-IR60 and sham IPC-IR60 groups,
whereas more than 800 HSP27-positive RGCs were found in the ONT groups.
Similar Expression of HSP70 Induced by IPC Plus ONT or
IR60 HSP70-positive cells were RGCs and astrocytes in the
GCL; cells that were double-stained with both TuJ1 and HSP70 were recognized as
RGCs and counted (Figure 6).
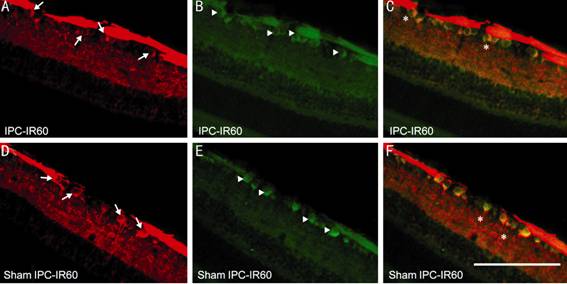
Figure 6 Micrographs
showing surviving TuJ1-positive RGCs (A, D), HSP70-positive cells (B, E) and
the merged images (C, F) in the central retinal section 7d after 60min of
retinal ischemia A, B and C are from the
IPC-IR60 group, which received 10min of IPC 1d before 60min of retinal ischemia
and survived 7d. D, E and F are from the sham IPC-IR60 group, which received
10min of sham IPC 1d before 60min of retinal ischemia and survived 7d. Arrows
indicate RGCs positive for TuJ1 (red); arrowheads indicate RGCs positive for
HSP70; asterisks indicate RGCs double-labeled by antibodies for both TuJ1 and
HSP70. Scale bar=100 µm.
For the axotomy groups, the mean density of the
HSP70-positive RGCs in the IPC-ONT group was significantly higher than in the
sham IPC-ONT group at 7d post-axotomy. However, the percentage of
HSP70-positive RGCs did not differ significantly with IPC treatment when
compared with the sham or control groups 89.1% and 83.8% of TuJ1-positive RGC
population were labeled with HSP70 in the IPC and the sham IPC treatment
groups, respectively (Figure 6).
For the IPC-IR60 group, a mean of 7.15 (±0.50)
HSP70-positive RGCs were found on the retinal sagittal sections, versus only
4.92 (±0.46) HSP70-positive RGCs in the sham IPC-IR60 group at 7d post-ischemia
(Figure 7A). Consistent with the results of the TuJ1-positive RGCs,
significantly more TuJ1-positive RGCs were found in the IPC-IR60 group than in
the shamIPC-IR60 group. Similar to findings in the axotomy group, the
percentage of HSP70-positive RGCs did not differ between the two groups; 85.2%
and 86.2% of TuJ1-positive RGCs were labeled with HSP70 in the IPC-IR60 or the
sham IPC-IR60 groups, respectively
(Figure 7B).
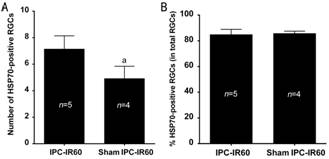
Figure 7 The number and percentage of HSP70-positive
RGCs in the IPC-IR60 and the sham IPC-IR60 groups 7d post-ischemia respectively
Significantly more HSP70-positive RGCs
were found in the IPC-IR60 group than in the sham IPC-IR60 group at 7d
post-ischemia (A), while percentage of HSP70-positive RGCs did not show
significant difference between two groups (B). t-test, aP<0.05;
Error bar=SEM.
DISCUSSION
We chose to use LOV in order to induce an IPC
stimulus, and subsequently evoked lethal ischemia/reperfusion injury of retinal
neurons in our experiment because this model scenario is most similar to
central retinal artery occlusion, a clinical disease leading to ischemia. The
advantage of our selected method is that reversible ligature can induce pure
retinal ischemia without an obvious mechanical effect on the retina. In
contrast, high intraocular pressure (HIOP) is sometimes used to induce IPC, but
at the risk of generating pressure-related mechanical injury to the retina[10]. Furthermore, the duration of ischemia can be readily
adjusted with timely removal of the suture. When performed with precision and
skill, the LOV surgical operation should not lead to optic nerve damage. LOV
has not previously been used as an IPC stimulus, although it has commonly been
used as a model of retinal ischemia per second.
Results of our study indicate that the loss of RGCs
induced by optic nerve section was efficiently rescued with the prior
application of IPC. As far as we know, this is the first report that has investigated
the effect of IPC on RGCs after axotomy, although IPC-induced protection
against subsequent ischemic injury has been previously reported[1-4]. A multitude of strategies have proven
to promote the survival and regeneration of RGCs after axotomy, such as
application of peripheral nerve, intravitreal injection of various neurotrophic
factors, and stem cell replacement therapy. Among the possible therapeutic
mechanisms of IPC, it has been supposed that induction of IPC could activate
endogenous protective mechanisms, thus potentially rescuing RGCs from a number
of types of injuries and it is assumed that IPC may also be effective on other
types of injuries. Thus, ischemic tolerance induced by six weekly rounds of
retinal ischemic stimulus prior to the onset of experimental diabetes has been
examined to protect the retina from diabetic retinopathy by preserving retinal
function (as measured by electroretinogram) and also the local integrity of the
blood-retinal barrier and by decreasing the circulating levels of vascular
endothelial growth factor[10]. The same research
group found that a brief ischemic pulse applied for 6 successive weeks prior to
experimental glaucoma protected the rat retina from glaucomatous damage through
both functional and morphological preservation[7].
These findings indicated that the induction of ischemic tolerance is a
promising therapy for treating different types of retinal injuries.
Microglia have been considered as a “sensor” for
pathological events in the central nervous system, given their rapid
proliferation in response to minor injury of diverse etiologies[3]. As such, the extent of microglial proliferation might
reflect the efficacy of protective interventions. In the present study we saw
similar retinal microgliosis not only in the GCL but also in the IPL and OPL.
However, the loss of RGCs and the increase of microglia in the GCL region
exhibited a significant positive correlation at 7d after the different injuries
(data not shown). There was no significant difference between the IPC treatment
and sham-operated groups with respect of activated microglia in response to the
IPC and axotomy. There has been no previous quantitative study of microglia
proliferation after IPC and subsequent severe insult, nor has the relationship
between microglia counts in the GCL or elsewhere in the retina and the number
of surviving RGCs after axotomy been reported previously. Our results suggest
that the effect of IPC played a minor role in the activation of microglia, or,
in other words, the protective effects of IPC on RGCs survival are not
importantly mediated by microglia.
A great number
of cellular and molecular mechanisms have been associated with the benefits of
preconditioning, and many key molecules may be involved as candidate protective
mechanisms and pathways in IPC. The inhibition of cyclooxygenase (COX) enzymes[11], HSP27 up-regulation[12-13], hypoxia-inclucible factor-1 transcription factor[14], б-opioid receptor activation[15],
and inducible nitric oxide synthase[16] have all
been implicated in IPC. Ganglion cells in uninjured retinas do not normally
express HSP27, but after axotomy, a small population of surviving ganglion
cells start to express this marker, with a significant positive correlation
existing between HSP27 expression and axonal regeneration[17].
In our study, the number of HSP27-positive RGCs showed dramatically different
responses to different injuries, namely optic nerve cut and retinal ischemia
60min. In particular, there were nearly 10 times more HSP27-positive RGCs in
optic nerve cut groups (865±35 in the sham IPC-ONT group) than in the retinal
ischemia groups (88±16 in the sham IPC-IR60 group), although the number of
surviving RGCs was slightly greater in the retinal ischemia 60min group. More
importantly, given the correlation of HSP27 expression with the ability of
neurons to regenerate their axons[18], its
expression in ganglion cells after ischemic or optic nerve injury may be
relevant to their different regenerative propensities. This observation
suggests a greater capacity for axonal regeneration after axotomy, when more
HSP27 positive RGCs are present. On the other hand, HSPs may be a marker for
damage rather than protective mechanism[19],
since axotomy is a more severe insult to the retina and leads to greater RGC
loss than did 60min of retinal ischemia, such that more HSP27-positive RGCs
were found in both the IPC- and sham IPC-ONT groups.
We observed that the number of HSP70-positive cells
was markedly higher in the IPC-IR60 group, although the percentage of the
HSP70-positive RGCs did not show a significant difference between the IPC-IR60
and sham IPC-IR60. This result implies that, the expression of HSP70 may play
only a small role in IPC-induced neuroprotection of RGCs.
This study has several limitations that should be
addressed in future work. The activation of HSP27 and HSP70 were investigated
only in the groups that received axotomy or retinal ischemia for 60min, but
effects of longer duration of retinal ischemia were not explored. Thus, the
expression of HSP27 and HSP70 after retinal ischemia lasting 120min or longer
is unknown. Furthermore, the neuronal expression of HSP27 exhibited dramatic
differences between the groups with optic nerve injury and retinal ischemia, a
phenomenon that merits further examination.
In conclusion, the neuroprotection provided by IPC can
protect RGCs against not only subsequent retinal ischemia, but also optic nerve
axotomy. Endogenous protective mechanisms activated by IPC maintained the
thickness of the retina and the GCL almost within the normal range. Although
direct or local IPC can protect vulnerable tissues against ischemia/reperfusion
injury, its application necessarily entails direct stress to the target organ
and mechanical trauma to major vascular structures, which has hitherto limited
its clinical application. Recently, the concept of remote ischemic preconditioning
(RIPC) has emerged, in which brief ischemia of one tissue confers protection to
important distant organs without direct stress to those organs, presumably
through the release of blood-born factors. Encouragingly, brief IPC of a hind
limb provided remote protection to the heart in children who underwent
cardiopulmonary bypass surgery for congenital heart disease[18].
Furthermore, the induction of RIPC protected the retina against
ischemia/reperfusion injury in rats[20-21].
We suppose that the induction of RIPC should provide a promising strategy to
protect organs against subsequent injury.
ACKNOWLEDGEMENTS
Conflicts
of Interest: Liu X, None; Liang JP, None; Sha O, None; Wang
SJ, None; Li HG, None; Cho EY, None.
REFERENCES
1 Hausenloy DJ, Yellon DM.
Preconditioning and postconditioning: underlying mechanisms and clinical
application. <ii>Atherosclerosis</ii> 2009;204(2):334-341. [PubMed]
2 Wever KE, Hooijmans CR,
Riksen NP, Sterenborg TB, Sena ES, Ritskes-Hoitinga M, Warlé MC. Determinants
of the efficacy of cardiac ischemic preconditioning: a systematic review and
Meta-analysis of animal studies. <ii>PLoS One
</ii>2015;10(11):e0142021. [PMC free article] [PubMed]
3 Kanoria S, Jalan R,
Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote
ischemic preconditioning: a novel method for reducing ischemia reperfusion
injury. <ii>Transplantation </ii>2007;84(4):445-458. [PubMed]
4 Roth S, Li B, Rosenbaum
PS, Gupta H, Goldstein IM, Maxwell KM, Gidday JM. Preconditioning provides
complete protection against retinal ischemic injury in rats. <ii>Invest
Ophthalmol Vis Sci</ii> 1998;39(5):777-785. [PubMed]
5 Liu X, Sha O, Cho EY.
Remote ischemic postconditioning promotes the survival of retinal ganglion
cells after optic nerve injury<ii>. J Mol Neurosci</ii>
2013;51(3):639-646. [PubMed]
6 Rosenstein RE, Fernandez
DC. Induction of ischemic tolerance as a promising treatment against diabetic
retinopathy. <ii>Neural Regen Res </ii>2014;9(17):1581-1584. [PMC free article] [PubMed]
7 Belforte N, Sande PH, de
Zavalía N, Fernandez DC, Silberman DM, Chianelli MS, Rosenstein RE. Ischemic
tolerance protects the rat retina from glaucomatous damage. <ii>PLoS One
</ii>2011;6(8):e23763. [PMC free article] [PubMed]
8 Lafuente MP,
Villegas-Pérez MP, Sellés-Navarro I, Mayor-Torroglosa S, Miralles de Imperial
J, Vidal-Sanz M. Retinal ganglion cell death after acute retinal ischemia is an
ongoing process whose severity and duration depends on the duration of the
insult. <ii>Neuroscience </ii>2002;109(1):157-168. [CrossRef]
9 Wong WK, Cheung AW, Yu SW,
Sha O, Cho EY. Hepatocyte growth factor promotes long-term survival and axonal
regeneration of retinal ganglion cells after optic nerve injury: comparison
with CNTF and BDNF. <ii>CNS Neurosci Ther </ii>2014;20(10):916-929.
[PubMed]
10 Osborne NN, Casson RJ,
Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage
and potential therapeutic strategies. <ii>Prog Retin Eye Res
</ii>2004;23(1):91-147. [PubMed]
11 Fleischman A, Oron Y,
Geyer O. COX-2 inhibition improves retinal function in rats' ischemic eyes.
<ii>J Ocul Pharmacol Ther </ii>2014;30(8):634-641. [PubMed]
12 Li Y, Roth S, Laser M, Ma
JX, Crosson CE. Retinal preconditioning and the induction of heat-shock protein
27. <ii>Invest Ophthalmol Vis Sci </ii>2003;44(3):1299-1304. [CrossRef]
13 Whitlock NA, Lindsey K,
Agarwal N, Crosson CE, Ma JX. Heat shock protein 27 delays Ca2+-induced cell
death in a caspase-dependent and -independent manner in rat retinal ganglion
cells. <ii>Invest Ophthalmol Vis Sci</ii> 2005;46(3):1085-1091. [PubMed]
14 Whitlock NA, Agarwal N,
Ma JX, Crosson CE. Hsp27 upregulation by HIF-1 signaling offers protection
against retinal ischemia in rats. <ii>Invest Ophthalmol Vis
Sci</ii> 2005;46(3):1092-1098. [PubMed]
15 Husain S, Potter DE,
Crosson CE. Opioid receptor-activation: retina protected from ischemic injury.
<ii>Invest Ophthalmol Vis Sci</ii> 2009;50(8): 3853-3859. [PubMed]
16 Sakamoto K, Yonoki Y,
Kubota Y, Kuwagata M, Saito M, Nakahara T, Ishii K. Inducible nitric oxide
synthase inhibitors abolished histological protection by late ischemic
preconditioning in rat retina. <ii>Exp Eye Res
</ii>2006;82(3):512-518. [PubMed]
17 Hebb MO, Myers TL, Clarke
DB. Enhanced expression of heat shock protein 27 is correlated with axonal regeneration
in mature retinal ganglion cells. <ii>Brain Res</ii>
2006;1073-1074:146-150. [PubMed]
19 Windisch BK, LeVatte TL,
Archibald ML, Chauhan BC. Induction of heat shock proteins 27 and 72 in retinal
ganglion cells after acute pressure-induced ischaemia. <ii>Clin Exp
Ophthalmol</ii> 2009;37(3):299-307. [CrossRef]
20 Brandli A, Johnstone DM,
Remote ischemic preconditioning protects retinal photoreceptors: evidence from
a rat model of light-induced photoreceptor degeneration. <ii>Invest
Ophthalmol Vis Sci </ii>2016;57(13): 5302-5313. [PubMed]
21 Brandli A. Remote limb
ischemic preconditioning: a neuroprotective technique in rodents. <ii>J
Vis Exp </ii>2015;(100):e52213. [CrossRef]