
·Basic
Research·Current
Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
Effect of pyridone agent on
blood-retinal barrier in diabetic mice
Si-Qi Xiong, Hai-Bo Jiang, Hui-Zhuo Xu, Xiao-Bo Xia
Department of
Ophthalmology, Xiangya Hospital, Central South University, Changsha 410008, Hunan
Province, China
Correspondence
to:
Xiao-Bo Xia. Department of Ophthalmology, Xiangya Hospital, Central South
University, Changsha 410008, Hunan Province, China. xbxia21@163.com
Received:
2017-02-18
Accepted: 2017-04-10
Abstract
AIM: To evaluate the
therapeutic effect of fluorofenidone on disrupted blood-retinal barrier in the
diabetic mice and uncover its underlying mechanism.
Methods: db/db mice were
randomly chosen for treatment with daily doses of fluorofenidone or placebo at
5-week-old, treatment continued until mice reach 24-week-old. Then, expression
of transcriptiona factor insulin gene enhancer binding protein-1 (Islet-1) and
vascular endothelial growth factor (VEGF) in murine retinas were evaluated.
Retinal vascular permeability was assessed by examining the level of albumin in
db/db murine retinas. Furthermore, the retinal vessel tight junction was
estimated by checking the level of occludin in the murine retinal tissues.
Results: After occurrence
of diabetic retinopthy in db/db mice, expressions of
transcritpional factor Islet-1 was found to be upregulated in db/db
murine retinas compared with non-diabetic controls. Similar to
expression pattern of Islet-1, VEGF were also demonstrated to be increased in
retinas of db/db mice, which was accompanied by increased retinal
vascular leakage and decreased tight junction protein level. Systemetic
administration of fluorofenidone repaired broken retinal vascular tight
junction by restoring occludin expression in db/db retinal tissue. Consequently, retinal vascular
premeability were indicated to be reduced by examining the transudative albumin
level in diabetic retinal tissues. Both Islet-1 and VEGF expression were
inhibited in the retinas of db/db mice after treatment with
fluorofenidone.
Conclusion: Fluorofenidone
significantly protectes retinal tight junction and reduces retinal vascular
leakage. The phenomenon can be partially attributed to reducing overexpression
of Islet-1 and VEGF in diabetic retinal tissues.
Keywords: pyridone agent; diabetic retinopathy; blood- retinal barrier
DOI:10.18240/ijo.2017.06.09
Citation: Xiong SQ,
Jiang HB, Xu HZ, Xia XB. Effect of pyridone agent on blood-retinal barrier in
diabetic mice. Int J Ophthalmol
2017; 10(6):890-895
Article outline
Introduction
Diabetic
retinopathy is one of the diseases that cause vision loss in the world.
Approximately 75% of all diabetic patients show clinical signs of
retinopathy within 15y after onset of diabetes[1].
Diabetic macular edema arising from vascular leakage due to inner blood-retinal
barrier (iBRB) damage is the major cause of loss of vision in patients with
diabetic retinopathy[2]. Several cytokines have
been demonstrated to particpate in the pathogenesis of iBRB breakdown in
diabetic patients. Growing evidence indicates that vascular endothelial growth
factor (VEGF) is related to iBRB damage in diabetic retinopathy. It has been
demonstrated VEGF levels dramaticallty upregulated in patients with diabetic
macular edema and associated with vascular leakage, making it a highly
important therapeutic target[3-5].
More recently, efforts with anti-VEGF therapy have produced promising results
in patients with diabetic macular edema[6]. It has
been confirmed that hypoxia inducible factor-1 (HIF-1) could regulate VEGF
expression at transcriptional level. Treatment
targeting HIF-1α could reduce the leakage of
retinal blood vessels by inhibiting the expression of VEGF[7]. In addition to HIF-1α, a
growing number of transcription factors, such as PPARg-coactivator-1a[8], have been shown to be
involved in the regulation of VEGF expression. Figure out the regulation pathway of VEGF expression, and
found more transcription factor which involved in regulating the expression of
VEGF, would help us have better understanding the pathogenesis of diabetic
retinopathy and providing better way to treat diabetic retinopathy.
Islet-1 is a
LIM domain transcription factor[9]. The functions
of Islet-1 involve in cell fate specification and embryonic development[10]. Recently, exogenous Islet-1 has been proven to
possess ability to enhance proliferative, migratory and tube formation
properties of the vascular endothelial cells, which is attributed to increased
secretion of VEGF[11]. Moreover, accumulated date
indicate that Islet-1 gene mutation is correlated with type 2 diabetes. It
could manipulate body weight and glucose homeostasis via the activation
of proglucagon gene expression. Islet-1, as a
transcription factor, has a role in promoting angiogenesis and associated with
diabetes. However, until now, there is
no research on whether endogenous Islet-1 is involved in the occurrence of
diabetic retinopathy. Therefore, the current study is to investigate
whether the transcription factor Islet is related to the disruption of
iBRB in diabetic mice.
It has been
shown that pirfenidone, as a pyridone agent, could ameliorate fibrosis in
different tissues[12-13]. Oral
administration of pirfenidone was approved to be safe for suppression of
fibrosis in clinical trials. Fluorofenidone (AKF-PD) is an improved analog of
pirfenidone. The difference in structures between fluorofenidone and pirfenidone
is that the hydro- at the metaposition of the benzene ring in pirfenidone is
replaced by fluoro- in fluorofenidone. Alteraton of this chemical structure
could result in promotion of absorption and transmission ability and increasing
physiological activity. Since now, it has been demonstrated that fluorofenidone could attenuate diabetic nephropathy and
kidney fibrosis in different anmial models[14-15]. It is widely believed that diabetic retinopathy and
nephropathy are two major microvascular complications of diabetes mellitus,
which lead to blindness and end-stage renal disease. Diabetic retinopathy
always find to be accompanied by diabetic nephropathy in clinic. It has also
been cofirmed that diabetic nephropathy was related to the severity of diabetic
macular edema[16]. These findings drive us to
imagine whether fluorofenidone has a therapeutic
effect on diabetic retinopathy.
In order to find the answers to these questions. db/db mice were used to figure out changes of Islet-1 gene expression in murine retinal tissues during the process of diabetic retinopathy, and to find correlation between Islet-1 and diabetic retinopathy. The second purpose of our study was to evaluate the therapeutic effects of fluorofenidone on the disrupted blood-retinal barrier and to explore its underlying mechanism in diabetic mice.
MATERIALS AND METHODS
Animals C57BL/KsJ db/db male and age-matched
db/m mice were obtained from Silaike (Shanghai, China), which were bred and
maintained in a pathogen-free environment with a 12-hour light/dark cycle. The
experimental animals were composed of following groups: normal control group
(db/m mice, n=16), negative control group (1% CMC-Na was used to treat
db/db mice, n=16) and treatment group (db/db mice treated by oral gavage
at dose of 500 mg/(kg·d) of fluorofenidone, n=16).
Treatment started at the age of 5wk with an end point at 24-week old. Body
weight of mice was measured.
Analysis of
Blood Glucose and Serum Lipids Following a 12-hour
overnight fast, blood from the tail vein was collected. Blood glucose meter was
used to measure blood glucose levels in mice (LifeScan, Milpitas, CA, USA). Meanwhile,
automatic analyzer model 7170 (Hitachi Co., Ltd., Japan) was adopted to exmine
serum levels of triglyceride and cholesterol.
Determination
of Disruption of Blood-retinal Barrier
In
order to analyze the extent of damage of the blood-retinal barrier in db/db
mice, the level of albumin leaking from retinal vessels was evaluated. Once
deeply anesthetized, the chest of the mice was opened followed by insertin of a
catheter into the left ventricle with a small incision on the right atrium.
Phosphate buffer saline was infused. Consequently, mice were sacrificed and
retina was isolated, extravascular level of albumin in murine retina was
assessed by using the Western blot technique.
Western
Blot Total protein of murine retinas were
collected and resolved on sodium dodecyl sulfate (SDS)-polyacrylamide gel, then
it was transferred onto a nitrocellulose membrane and incubated with
anti-Islet-1 (Abcam, UK), anti-VEGF (Abcam, UK), anti-Albumin(Abcam, UK),
anti-occludin (Abcam, UK) and anti-β-actin antibodies (Sigma, USA). Membranes
were incubated with peroxidase-conjugated secondary antibodies and developed
using the ECL system.
Statistical
Analysis All the data were expressed as mean± SEM
and processed by SPSS20.0 statistical package. One-way analysis of variance
followed by the LSD test were utilized to assess significant differences. P<0.05
would be considered to be statistically significant.
Results
Clinical
Characteristics of the Mice After Fluorofenidone Treatment Serum lipid together with blood
glucose and body weight were monitored in db/db mice before and after
fluorofenidone treatment. Body weights (db/db vs db/m: 50.97±2.95 vs
33.02±0.44 g, P<0.05) and blood glucose concentrations of db/db mice
(db/db vs db/m: 41.11±3.61 vs 9.51±0.72 mmol/L, P<0.05)
were dramatically increased compared with db/m mice. Similarly, we also found
serum concentrations of cholesterol (db/db vs littermates: 3.08±0.62 vs
2.04±0.21 mmol/L, P<0.05) and triglycerides (db/db vs
littermates: 1.04±0.15 vs 0.41±0.05 mmol/L, P<0.05) were upregulated
in db/db mice when compared with db/m mice. Conversely, the difference between
fluorofenidone-treated db/db and placebo-treated db/db mice was insignificant.
Treatment with fluorofenidone did not affect serum levels of triglycerides
(fluorofenidone vs db/db: 1.00±0.11 vs 1.04±0.15 mmol/L, P>0.05),
cholesterol (fluorofenidone vs db/db: 43.06±0.13 vs 3.08±0.62
mmol/L, P>0.05), glucose (fluorofenidone vs db/db: 41.17±2.78 vs
41.11±3.61 mmol/L, P>0.05) compared with placebo-treated mice.
Fluorofenidone did not change body weight of the mice (fluorofenidone vs
db/db: 47.75±4.83 vs 50.97±2.95 g, P>0.05) (Figure 1). In
summary, with progression of diabetes, db/db mice manifested as increased body
weight and elevated level of blood glucose and serum lipid.
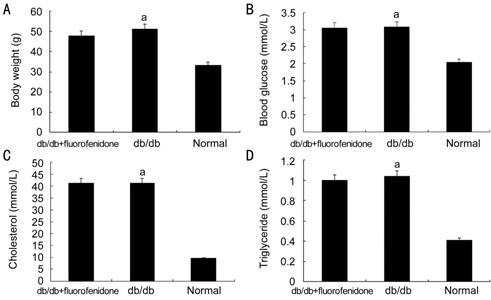
Figure 1
Body weight and serum concentration of glaucose or lipid in db/db mice after
fluorofenidone treatment A: Body weight of
db/db mice and normal control mice were measured; B: Blood glucose of mice were
examined; C, D: Serum concentrations of cholesterol and triglyceride were
evaluated. The difference between placebo-treated db/db mice and normal control
mice (nondiabetic mice) was statistically significant (aP<0.05).
Downregulation
of Retinal Islet-1 Expression in the Retinas of db/db Mice by
Fluorofenidone To figure out the
relationship between Islet-1 and diabetic retinopathy, Islet-1 level in the
retinas of db/db mice was evaluated at age 24wk, Diabetic retinopathy has been occurring in the retinal
tissues at this time. We found that expression of Islet-1 in db/db murine
retina was significantly increased compared with normal control (P<0.05,
Figure 2). This indicates that the occurrence of diabetic retinopathy
can induce the expression of Islet-1, we infer that there is a positive
correlation between the expression of Islet-1 and diabetic retinopathy and Islet-1
may play a potential role in this process. We also found, compared with
placebo-treated db/db mice, systematic administration of fluorofenidone
suppressed the expresssion of Islet-1 in the db/db retinas (P<0.05)
and almost completely reversed this pathological upregulation which was induced
by diabetes to normal level (Figure 2).

Figure 2
Effect of fluorofenidone on Islet-1 expression in murine retinas The level of trascriptional factor
Islet-1 in the retinas of mice was assessed by Western blot. Islet-1 expression
in the db/db murine retinas were dramatically increased compared with normal
control mice. Systematic adminstration of fluorofenidone almost completely
attenuated upregulation of Islet-1 expression in the retinas of db/db mice. The
difference between placebo-treated db/db mice and db/db mice treated with
fluorofenidone was statistically significant (aP<0.05).
The difference between placebo-treated db/db mice and normal control mice was
statistically significant (cP<0.05).
Effect of
Fluorofenidone on Vascular Endothelial Growth Factor Expression in the Retinas
of db/db Mice Overexpression of VEGF has
been demontrated in diabetic retinopathy, which result in disruption of
blood-retinal barrier and vascular leakage. Be consistent with it, we detected
an increased level of VEGF in db/db retinas with high glucose compared with
normal control mice (P<0.05, Figure 3). Then, effects of
fluorofenidone on retinal VEGF expression in db/db mice was evaluated.
Compared with placebo-treated db/db mice, VEGF expression was dramatically
decreased in the retinas of db/db mice which was systematically
adniminstrated with fluorofenidone (P<0.05, Figure 3). It has been proven that Islet-1 could promote the
angiogenesis by increasing the expression of VEGF. This indicates that
Islet directly or indirectly participate in the regulation of VEGF expression. We obseved inbibition of the expression of Islet-1 in
retinal tissues by fluorofenidone, which may
consequently downregulate expression of VEGF on transcriptional level in the
retina of diabetic mice.
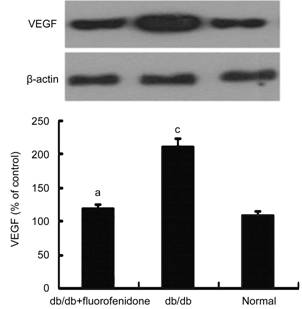
Figure 3 Effect of fluorofenidone on VEGF expression in murine retinas VEGF expression was statistically upregulated in retinas of db/db mice, which was suppressed by fluorofenidone treatment. The difference between placebo-treated db/db mice and db/db mice treated with fluorofenidone was statistically significant (aP<0.05). The difference between placebo-treated db/db mice and normal control mice was statistically significant (cP<0.05).
Effect of Fluorofenidone on Blood-retina Barrier in db/db Mice In order to examine the therapeutic efficacy of fluorofenidone on the blood-retinal barrier, we detected the level of albumin in murine retinal tissues. As shown in Figure 4, the level of albumin was significantly increased in the retinas of db/db mice compared with that of normal control (P<0.05). Systematical administration of fluorofenidone significantly decreased extravascular leakage of albumin in db/db mice (Figure 4, P<0.05). To figure out whether this phenomenon was related to alteration of the tight junction protein expression, we assessed the level of occludin in the retina of db/db mice. It demonstrated that the expression of occludin downregulated in db/db mice compared with non-diabetic littermates (Figure 5, P<0.05). Fluorofenidone treatment almost completely restored retinal occludin expression in db/db mice (Figure 5, P<0.05).
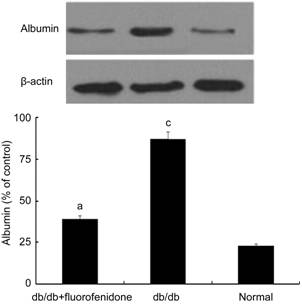
Figure 4
Albumin expression in murine retinas
Albumin
content was higher in the retinas of db/db mice when compared with non-diabetic
littermates. Systematical administration of fluorofenidone significantly
decreased extravascular leakage of albumin in db/db mice. The difference
between placebo-treated db/db mice and db/db mice treated with fluorofenidone
was statistically significant (aP<0.05). The difference
between placebo-treated db/db mice and normal control mice was statistically
significant (cP<0.05).
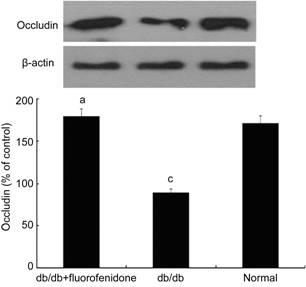
Figure 5
Occludin expression in murine retinas
Retinal
occludin level significantly decreased in db/db mice compared with that in non-diabetic
littermates. Fluorofenidone treatment almost completely restored retinal
occludin expression. The difference between placebo-treated db/db mice and
db/db mice treated with fluorofenidone was statistically significant (aP<0.05).
The difference between placebo-treated db/db mice and normal control mice was
statistically significant (cP<0.05).
Discussion
Significant
upregulation of Islet-1 expression were found in the retinas of db/db mice.
Islet-1 transcriptional activity play important roles in tissue specification
and correlate with the activity of the insulin and glucagon genes[17-20]. It has been shown that Islet-1
could promote postnatal angiogenesis and vasculogenesis, which is attributed to
increased secretion of VEGF[11]. In current
study, along with elevated expression of Islet-1, the level of VEGF also
increased significantly in db/db mice. Previous reports have demonstrated that
VEGF could improve vascular permeability in diabetic patients and correlated
with the diabetic macular edema[21]. In this
study, expression of Islet-1 was significantly increased in parallel with
elevated VEGF levels, vascular leakage and tight junction damage in the retinas
of db/db mice. These findings suggest that Islet-1 may participate in
diabetes-induced VEGF expression and iBRB breakdown.
Our findings
showed that fluorofenidone significantly reversed retinal vascular leakage. To
further unveil whether fluorofenidone has direct effects on the blood retinal
barrier, we evlauated the effect of fluorofenidone on retinal tight junction.
Our findings indicated that fluorofenidone attenuated the downregulation of
tight junction protein-occludin in the retinas of db/db mice. Several studies
have demonstrated that VEGF-mediated disruption of
endothelial transmembrane tight-junction proteins is contributed to the
breakdown of iBRB in diabetic retinopathy[22]. In
accord with previous study, downregulation of tight junction protein is
associated with decrease of VEGF levels in the retinal tissues after
administration of fluorofenidone. We show here that protein expression of
Islet-1 were suppressed by treatment with fluorofenidone. Downregulation expression
of Islet-1 could concomitant attenuation of VEGF levels in retinals of db/db
mice. These findings indicate an important role of Islet-1 in retinal vascular
leakage through regulation of VEGF expression. Moreover, some other unknown
factors implicated in iBRB breakdown may also be regulated by Islet-1.
Inhibition of Islet-1 by systematically administration of fluorofenidone may
suppress retinal vascular leakage through decreasing Islet-1 regulated other
downstream genes besides VEGF. Further study is necessary to elucidated it.
In summary,
our study suggested that Islet-1 expression is upregulated in association with
VEGF expression in the retinas of db/db mice, which is attibuted to retinal
vascular leakage and tight junction diruption. Fluorofenidone could reverse
retinal tight junction and reduce retinal vascular leakage in db/db mice. The
therapeutic efficacy of fluorofenidone on blood-retinal barrier is at least in
part mediated by the inhibition of VEGF expression via attenuation of
Islet-1 levels in diabetic retinal tissues.
Acknowledgements
We thank Dr.
Li-Jian Tao and Dr. Xuan Xiong for providing db/db mice with fluorofenidone
treatment.
Foundations: Supported by National
Natural Science Foundation of China (No.81000388); Health and Family Planning
Commission of Hunan Province (No.132015-016); Natural Science Foundation of
Hunan Province (No.12JJ3120).
Conflicts
of Interest: Xiong SQ, None; Jiang HB, None; Xu HZ, None; Xia
XB, None.
REFERENCES
1 Klein R, Klein BE, Moss SE.
Epidemiology of proliferative diabetic retinopathy. <ii>Diabetes Care
</ii>1992;15(6):1875-1189. [CrossRef]
2 Lian JX, Gangwani RA, McGhee SM, Chan
CK, Lam CL; Primary Health Care Group, Wong DS. Systematic screening for
diabetic retinopathy (DR) in Hong Kong: prevalence of DR and visual impairment
among diabetic population. <ii>Br J Ophthalmol</ii>
2016;100(2):151-155. [CrossRef]
3 Thomas AA, Feng B, Chakrabarti S.
ANRIL: a regulator of VEGF in diabetic retinopathy.<ii> Invest Ophthalmol
Vis Sci</ii> 2017;58(1):470-480. [CrossRef]
4 Funatsu H, Yamashita H, Noma H, Mimura
T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor
and interleukin-6 in the aqueous humor of diabetics with macular edema.
<ii>Am J Ophthalmol</ii> 2002;133(1):70-77. [CrossRef]
5 Xia XB, Xiong SQ, Song WT, Luo J, Wang
YK, Zhou RR. Inhibition of retinal neovascularization by siRNA targeting VEGF
(165). <ii>Mol Vis</ii> 2008;14:1965-1973. [PMC free article] [PubMed]
6 Ho AC, Scott IU, Kim SJ, Brown GC,
Brown MM, Ip MS, Recchia FM. Anti-vascular endothelial growth factor
pharmacotherapy for diabetic macular edema: a report by the American Academy of
Ophthalmology. <ii>Ophthalmology</ii> 2012;119(10):2179-2188. [CrossRef]
7 Xia XB, Xiong SQ, Xu HZ, Jiang J, Li
Y. Suppression of retinal neovascularization by shRNA targeting
HIF-1alpha.<ii> Curr Eye Res </ii>2008;33(10):892-902. [CrossRef]
8 Saint-Geniez M, Jiang A, Abend S, Liu
L, Sweigard H, Connor KM, Arany Z. PGC-1α regulates normal and pathological
angiogenesis in the retina.<ii> Am J Pathol </ii>2013;182(1):255-265.
[CrossRef]
9 Hunter CS, Rhodes SJ. LIM-homeodomain
genes in mammalian development and human disease, <ii>Mol Biol
Rep</ii> 2005;32(2):67-77. [CrossRef]
10 Wilfinger A, Arkhipova V, Meyer D.
Cell type and tissue specific function of islet genes in zebrafish pancreas
development. <ii>Dev Biol</ii> 2013;378(1):25-37. [CrossRef]
11 Xiong SQ, Jiang HB, Li YX, Li HB, Xu
HZ, Wu ZK, Zheng W, Xia XB. Role of endogenous insulin gene enhancer protein
ISL-1 in angiogenesis. <ii>Mol Vis</ii> 2016;22:1375-1386. [PMC free article] [PubMed]
12 Di Sario A, Bendia E, Macarri G,
Candelaresi C, Taffetani S, Marzioni M, Omenetti A, De Minicis S, Trozzi L,
Benedetti A. The anti-fibrotic effect of pirfenidone in rat liver fibrosis is
mediated by downregulation of procollagen alpha1(1), TIMP-1 and
MMP-2.<ii> Dig Liver Dis</ii> 2004;36(11): 744-751. [CrossRef]
13 Behr J, Bendstrup E, Crestani B, Günther
A, Olschewski H, Skold CM, Wells A, Wuyts W, Koschel D, Kreuter M, Wallaert B,
Lin CY, Beck J, Albera C. Safety and tolerability of acetylcysteine and
pirfenidone combination therapy in idiopathic pulmonary fibrosis: a randomised,
double-blind, placebo-controlled, phase 2 trial. <ii>Lancet Respir
Med</ii> 2016;4(6):445-453. [CrossRef]
14 Li BX, Tang YT, Wang W, Xie YY, Wang
NS, Yuan QJ, Zhang FF, Peng ZZ, Hu GY, Tao LJ. Fluorofenidone attenuates renal
interstitial fibrosis in the rat model of obstructive nephropathy.
<ii>Mol Cell Biochem</ii> 2011;354(1-2):263-273. [CrossRef]
<no>15 Qin J, Xie YY, Huang L, Yuan QJ, Mei WJ,
Yuan XN, Hu GY, Cheng GJ, Tao LJ, Peng ZZ. Fluorofenidone inhibits nicotinamide
adeninedinucleotide phosphate oxidase via PI3K/Akt pathway in the pathogenesis
of renal interstitial fibrosis.<ii> Nephrology (Carlton)</ii> 2013;
18(10):690-699.</no>
16 Romero-Aroca P, Mendez-Marin I,
Baget-Bernaldiz M, Fernéndez-Ballart J, Santos-Blanco E. Review of the
relationship between renal and retinal microangiopathy in diabetes mellitus
patients. <ii>Curr Diabetes Rev </ii>2010;6(2):88-101. [CrossRef]
17 Cai CL, Liang X, Shi Y, Chu PH, Pfaff
SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that
proliferates prior to differentiation and contributes a majority of cells to
the heart. <ii>Dev Cell</ii> 2003;5(6):877-889. [CrossRef]
<no>18 Kim KT, Kim N, Kim HK, Lee H, Gruner HN,
Gergics P, Park C, Mastick GS, Park HC, Song MR. ISL1-based LIM complexes
control Slit2 transcription in developing cranial motor neurons. <ii>Sci
Rep </ii>2016;7;6:36491.</no>
19 Du A, Hunter CS, Murray J, Noble D,
Cai CL, Evans SM, Stein R, May CL. Islet-1 is required for the maturation,
proliferation, and survival of the endocrine pancreas.<ii> Diabetes
</ii>2009; 58(9):2059-2069. [CrossRef]
20 Peng SY, Wang WP, Meng J, Li T, Zhang
H, Li YM, Chen P, Ma KT, Zhou CY. ISL1 physically interacts with BETA2 to
promote insulin gene transcriptional synergy in non-beta cells. <ii>Biochim
Biophys Acta</ii> 2005;1731(3):154-159. [CrossRef]
21 Funatsu H, Yamashita H, Sakata K,
Noma H, Mimura T, Suzuki M, Eguchi S, Hori S. Vitreous levels of vascular
endothelial growth factor and intercellular adhesion molecule 1 are related to
diabetic macular edema. <ii>Ophthalmology</ii> 2005;112(5):806-816.
[CrossRef]
22 Harhaj NS, Felinski EA, Wolpert EB,
Sundstrom JM, Gardner TW, Antonetti DA.VEGF activation of protein kinase C
stimulates occludin phosphorylation and contributes to endothelial
permeability.<ii> Invest Ophthalmol Vis Sci
</ii>2006;47(11):5106-5115. [CrossRef]