
·Basic
Research·Current
Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
Effect of angiotensin II type 1
receptor blocker and angiotensin converting enzyme inhibitor on the intraocular
growth factors and their receptors in streptozotocin-induced diabetic rats
Ik Soo Byon1,2, Dong Hyun Lee1, Eun Sook Jun3,
Min Kyu Shin4, Sung Who Park2,3, Ji Eun Lee2,3
1Department of
Ophthalmology, Research Institute for Convergence of Biomedical Science and
Technology, Pusan National University Yangsan Hospital, Yangsan 50612, Korea
2College of Medicine, Pusan
National University, Yangsan 50612, Korea
3Department of
Ophthalmology, Biomedical Research Institute, Pusan National University
Hospital, Busan 49241, Korea
4Balgeunsesang Eye Clinic,
Busan 47286, Korea
Correspondence
to: Ji
Eun Lee. Department of Ophthalmology, Pusan National University Hospital, 179
Guduk-Ro, Seo-gu Busan 49241, Korea. jlee@pusan.ac.kr
Received:
2016-05-31
Accepted: 2017-03-24
Abstract
AIM: To investigate
the effect of angiotensin II type 1 receptor blocker (ARB) and angiotensin
converting enzyme inhibitor (ACEI) on intraocular growth factors and their
receptors in streptozotocin-induced diabetic rats.
METHODS: Forty
Sprague-Dawley rats were divided into 4 groups: control, diabetes mellitus
(DM), candesartan-treated DM, and enalapril-treated DM (each group, n=10).
After the induction of DM by streptozotocin, candesartan [ARB, 5 mg/(kg·d)] and
enalapril [ACEI, 10 mg/(kg·d)] were administered to rats orally for 4wk. Vascular
endothelial growth factor (VEGF) and angiotensin II (Ang II) concentrations in
the vitreous were measured using enzyme-linked immunosorbent assays, and VEGF
receptor 2 and angiotensin II type 1 receptor (AT1R) levels were assessed at
week 4 by Western blotting.
RESULTS: Vitreous Ang II
levels were significantly higher in the DM group and candesartan-treated DM
group than in the control (P=0.04 and 0.005, respectively). Vitreous
AT1R increased significantly in DM compared to the other three groups (P<0.007).
Candesartan-treated DM rats showed higher vitreal AT1R concentration than the
enalapril-treated DM group and control (P<0.001 and P=0.005,
respectively). No difference in vitreous Ang II and AT1R concentration was
found between the enalapril-treated DM group and control. VEGF and its receptor
were below the minimum detection limit in all 4 groups.
CONCLUSION: Increased Ang II
and AT1R in the hyperglycemic state indicate activated the intraocular
renin-angiotensin system, which is inhibited more effectively by systemic ACEI
than systemic ARB.
KEYWORDS: angiotensin converting enzyme inhibitor; angiotensin
II type 1 receptor blocker; diabetic rat; intraocular renin-angiotensin system
DOI:10.18240/ijo.2017.06.10
Citation: Byon IS,
Lee DH, Jun ES, Shin MK, Park SW, Lee JE.Effect of angiotensin II type 1
receptor blocker and angiotensin converting enzyme inhibitor on the intraocular
growth factors and their receptors in streptozotocin-induced diabetic rats.
Int J Ophthalmol 2017;10(6):896-901
Article Outline
INTRODUCTION
The
renin-angiotensin system (RAS) is known to play an important role in
physiologic and pathologic conditions of the systemic vascular system.
Angiotensin II (Ang II) is a major effector molecule that regulates various
growth factors in the RAS[1-4].
Independent of the systemic RAS, local RASs have been observed in several
organs including the eye[5-9].
Intraocular RAS is associated with intraocular pressure, ocular blood
flow, and the production of vascular endothelial growth factor (VEGF)[8-11].
Previous
studies suggest that intraocular RAS has pathogenic roles on vascular
proliferation and macular edema in diabetic retinopathy (DR)[12-13]. Elevation of intraocular Ang II promotes VEGF and
vascular endothelial growth factor receptor 2 (VEGFR-2) by stimulating
angiotensin II type 1 receptor (AT1R) and induces vascular permeability and
retinal neovascularization in the pathologic condition[1,6,13-15]. On the
basis of these findings, RAS inhibition has been highlighted to treat and
prevent DR. To inhibit the RAS pathway, two major therapeutic agents,
angiotensin II type 1 receptor blocker (ARB) and angiotensin converting enzyme
inhibitor (ACEI), have been widely used. Clinical and experimental studies
showed that treatment with ARB and ACEI had a beneficial effect on retinopathy
progression in diabetes mellitus (DM) patients[15-20], and improvements were seen in blood retinal barrier
breakdown, hyperpermeability, and retinal neovascularization in DR rats[21-23]. However, inhibiting the RAS
using systemic ARB and ACEI have not shown consistent outcomes for DR[24-26].
Focusing on
the effects of systemic ARB and ACEI on the intraocular RAS pathway and their
pathologic receptors, we investigated the concentrations of Ang II, VEGF, AT1R,
and VEGFR-2 in the vitreous after oral administration of candesartan (ARB) and
enalapril (ACEI) in diabetic rats.
MATERIALS AND METHODS
Animal Preparation Forty
inbred male Sprague-Dawley (SD) rats, weighing 200-250 g at 6wk of age, were
purchased from Koatech Inc (Pyeongtaek, Korea). The rats were housed in standard
12h dark-light cycles at room temperature of approximately 23℃
and permitted free access to deionized water and standard rat chow for 7d after
their arrival. The rats were divided to 4 groups (10 rats per group): control,
DM, candesartan-treated DM, and enalapril-treated DM.
Care, use, and treatment of all animals in this study
were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic
and Vision Research and the institutional guidelines of Pusan National
University. This study was approved by the Institutional Animal Care and Use
Committee (PNU-IACUC, approval number: PNU-2013-0373) of Pusan National
University.
Induction of Diabetes Diabetes was induced with a single intraperitoneal
injection of streptozotocin (STZ) (Sigma-Aldrich, St. Louis, MO, USA; 60 mg/kg,
in 10 mmol/L citrate buffer, pH 4.5). Rats in the control group received an
equivalent amount of citrate buffer alone. Two days after STZ injection, we
checked the rats’ blood glucose levels using a blood glucose meter (New
caresens N, I-Sens, Seoul, Korea) on the tips of their tails. Diabetes was
defined as a blood glucose level >300 mg/dL 24h after STZ injection. Blood
glucose levels were examined once per week to verify the maintenance of the
diabetic condition throughout the study.
Oral Administration of Angiotensin II type 1 Receptor
Blocker and Angiotensin Converting Enzyme Inhibitor for Diabetic Rats We administered candesartan [Atacand; Astrazeneca AB,
Karlebyhus, Astraallén Södertälje, Sweden, 5 mg/(kg·d)] to the
candesartan-treated DM group and enalapril [Lenipril; JW Pharmaceutical,
Dangjin, South Korea, 10 mg/(kg·d)] to the enalapril-treated DM group orally via
gastric sonde (BioGenomics, Seongnam, South Korea) for 4wk. Control and DM
groups received the same volume of phosphate-buffered saline (Sigma-Aldrich,
St. Louis, MO, USA; 10 mL/kg) orally.
Preparation of Retina and Vitreous At week 4, we sacrificed the rats by CO2
gas inhalation, followed by enucleation. The eyes were stored immediately at -80℃.
After the anterior segment was removed, the vitreous was meticulously separated
and transferred into sterile tubes. A full-thickness specimen including the
retina and sclera was obtained at the same distance inferiorly from the optic
disc. The tissues were prefixed with 2.5% glutaraldehyde (4℃;
phosphate buffer, pH 7.2) and were postfixed with 1% osmium tetroxide in the
same buffer. The materials were dehydrated with a series of graded ethyl
alcohol and were embedded in epoxy resin (Epon 812 mixture). Sections (1 mm)
were stained with 1% toluidine blue for light microscopy. Thin sections were
then examined with a light microscope (BX-50, Olympus, Japan).
Western Blot Analysis The
separated vitreous were homogenized in protein extraction solution (PRO-PREP,
iNtRON, Korea) and placed on ice for 30min. The supernatants were collected after
centrifugation at 12 000 rpm for 10min at 4℃. Protein
concentrations were quantified using a Bicinchoninic acid Protein Assay Kit (BCA,
Pierce, USA) according to the manufacturer’s instructions. Extracted proteins
were resolved on a 8% SDS-polyacrylamide gel and transferred onto a
nitrocellulose membrane (Hybond-ECL, Amersham Pharmacia Biotech, USA) by
electroblotting. Nitrocellulose blots were blocked with 5% non-fat dry milk for
1h in Tris buffered saline with Tween-20 (TBS-T) buffer (20 mmol/L Tris pH 7.4,
137 mmol/L NaCl, and 0.1% Tween-20) at room temperature. Blots were incubated
overnight at 4℃ with the appropriate primary antibody (VEGF Receptor
2, #2479, Cell Signaling, USA-dilution; 1:500) (AT1R, ADI-905-743, Enzo Life
Science, USA-dilution; 1:300) (GAPDH, ab8245, Abcam, UK-dilution; 1:2000) in
TBS-T buffer. Immunoreactive bands were detected using an anti-rabbit
peroxidase-conjugated secondary antibody (Amersham Pharmacia Biotech,
Piscataway, NY, USA) and visualized by enhanced chemiluminescence (ECL
detection kit, Amersham Pharmacia Biotech, Piscataway, NY, USA). Protein
expression was calculated by height, area, and optical density of the bands
using a gel imaging system (UVIpro, UVItec Limited, UK).
Enzyme-linked
Immunosorbent Assay for Vitreous The undiluted vitreous
samples from frozen eyes were obtained and transferred into sterile tubes. The vitreous
samples were immediately stored at -80℃ until use. The frozen
biopsies were defrosted, homogenized in protein extraction solution (PRO-PREP,
iNtRON, Korea), and placed on ice for 30min. The supernatants were collected
after centrifugation at 12 000 rpm for 10min at 4℃. The VEGF and Ang II
protein levels in the vitreous were determined using the rat VEGF Quantikine
ELISA kit (R&D Systems, Minneapolis, MN, USA) and Ang II enzyme immunoassay
kit (Spi Bio Bertin Pharma, Montigny le Bretonneux, France) according to the
manufacturer’s instructions. Optical density was read with a microplate reader
(Emax, Molecular Devices, USA) at 450 nm or 405 nm (for VEGF and Ang II,
respectively). The VEGF and Ang II concentrations (pg/mL) were calculated from
a standard curve. The minimum detectable concentrations using the ELISA kits
were 8.4 pg/mL for VEGF and 1.5 pg/mL for Ang II.
Statistical
Analysis Statistical comparisons
were performed with one way of analysis of variance (ANOVA), and the
Kruskal-Wallis test. Statistical analysis was performed with the PASW
statistics software (IBM SPSS software, New York, USA).
RESULTS
Blood Glucose Level, Body Weight, and Histology Blood glucose and body weight measured of the rats at
week 4 are shown in Table 1. DM, candesartan-treated, and enalapril treated DM
groups maintained high non-fasting blood glucose levels through week 4. Body
weights were reduced in DM, candesartan-treated DM, and enalapril-treated DM
groups compared to baseline (P<0.05), but the control group gained
body weight through week 4.
Table 1 Body weight change 4wk after induction of
diabetes
|
Groups |
Body weight (g) |
|
|
Baseline |
4wk |
|
|
Control |
334.50±13.82 |
378.33±67.50 |
|
DM |
294.33±14.87 |
225.16±40.47 |
|
Candesartan-treated
DM |
310.83±8.54 |
264.00±21.84 |
|
Enalapril-treated
DM |
315.67±16.22 |
243.67±44.22 |
DM: Diabetes mellitus.
Histology showed no apparent abnormal findings in the
retinal layers of all four groups (Figure 1).
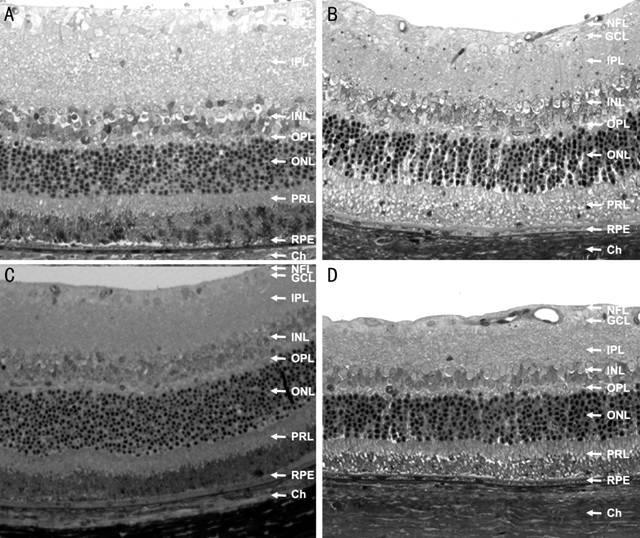
Figure 1 Histologic findings of retinal tissues The type of specimen: full thickness retina &
choroid, original magnification: ×100, and stain: 1% toluidine blue A: Controls; B: DM; C:
Candesartan-treated DM; D: Enalapril treated DM groups. NFL; Nerve fiber layer:
GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer;
OPL: Outer plexiform layer; ONL: Outer nuclear layer; PRL: Photo receptor
layer; RPE: Retinal pigment epithelium; Ch: Choroid.
Levels of Angiotensin II and Vascular Endothelial
Growth Factor in Vitreous Table 2 presents vitreous Ang II concentration in the
control, the DM group, the candesartan-treated DM group, and the
enalapril-treated DM group. Vitreous Ang II levels were significantly higher in
DM and candesartan-treated DM groups than in the control group (P=0.04
and 0.005, respectively), and there was no difference between the
enalapril-treated DM group and control.
Table 2 Vitreous angiotension II levels at week 4
|
Groups |
Angiotensin II level (pg/mL) |
P |
|
Control |
26.89±9.15 |
|
|
DM |
42.45±9.39 |
0.04a |
|
Candesartan-treated
DM |
47.11±14.63 |
0.005b |
|
Enalapril-treated
DM |
36.09±12.35 |
0.15c |
DM: Diabetes mellitus. aMann-Whitney U
test was conducted between control and DM group; bMann-Whitney U
test was conducted between control and candesartan-treated DM group; cMann-Whitney
U test was conducted between control and enalapril-treated DM group.
VEGF concentrations were below the minimum detection
limit in all 4 groups.
Vitreous
Concentrations of Angiotensin II Receptor Type 1 and Vascular Endothelial
Growth Factor Receptor 2 Figure 2 shows AT1R
expression by Western blot assay. The DM group had a significantly higher concentration
of vitreous AT1R than candesartan-treated DM (P<0.001),
enalapril-treated DM (P=0.006), and control groups (P=0.001).
Concentration of AT1R was higher in the enalapril-treated DM group than the
control (P=0.005) and the candesartan-treated DM group (P<0.001).
AT1R expression was comparable between the enalapril-treated DM group and the
control group.
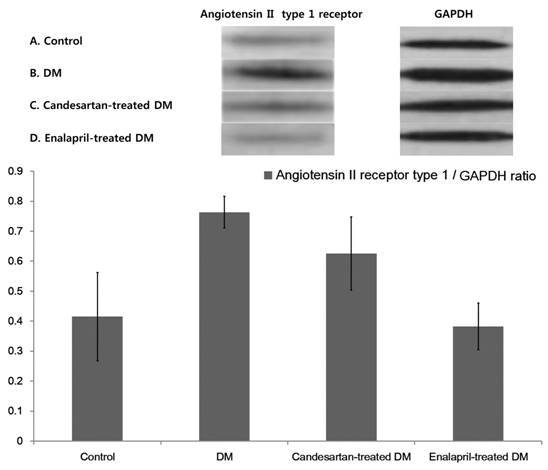
Figure 2 Western blotting for AT1R in vitreous.
The
concentration of VEGFR-2 was below the minimum detection limit in all 4 groups.
DISCUSSION
The present
study showed that intraocular Ang II and AT1R significantly increased in rats
with DM. These results agree with previous studies, showing that RAS in the
plasma as well as eye were correlated positively with severity of DM or
retinopathy[12,14,27].
Systemic enalapril effectively inhibited the expression of Ang II and
AT1R in the vitreous. Although candesartan also reduced the vitreous AT1R
concentration, it was less effective than enalapril, and did not prevent
elevation of Ang II in the vitreous of DM rat.
Among the
various proteins associated with the RAS, such as AT1R, AT2R and Mas receptor,
AT1R is known as a major pathologic receptor in the eye. Ang II secretion can
be promoted by hyperglycemic and hypoxic conditions, and may cause reduction in
retinal capillary blood flow, increases in retinal vascular hyperpermeability,
and decreased neovascularization by activation of AT1R[21,23-24,28]. It has
been suggested that inhibiting the RAS will be beneficial in management of DR.
Both ACEI and
ARB inhibit RAS, but at different steps. ACEI inhibits the conversion of Ang I
into Ang II, and ARB blocks AT1R selectively. Regarding clinical trials for DR,
DM patients on lisinopril, another ACEI, showed a 50% reduction in DR
progression compared to placebo after adjusting glycemic status[16]. Candesartan also reduced progression of DR in early
stages[17-18]. Both enalapril
(ACEI) and losartan (ARB) reduced DR progression in type 1 DM patients[19]. However, the effect of systemic RAS inhibition on
the intraocular RAS is still unclear.
Comparison of
systemic ACEI and ARB administration to inhibit intraocular RAS was performed
by Moravski et al[15]. They demonstrated
that the ACEI lisinopril reduced VEGF and AT1R mRNA expression in retinal
tissues of Ren2 rats, but the ARB losartan did not. However, this outcome might
have resulted from the different characteristics or dosages of drugs, rather
than the common pharmacological action of ACEI or ARB, because ARB telmisartan
inhibited VEGF mRNA expression in bovine retinal pericytes[29].
Our results demonstrated that systemic enalapril reduced the vitreous Ang II
and AT1R in DM rats to the level of control rats, but candesartan did not. In
human studies, we previously reported that proliferative diabetic retinopathy
(PDR) patients receiving various systemic ARBs did not have reduction of
vitreous VEGF concentration[25], but Hogeboom van
Buggenum et al[26] demonstrated that PDR
patients receiving various ACEIs did show a decrease in vitreous VEGF
expression. Accordingly, these findings imply that systemic ACEI may
be more effective for inhibiting the intraocular RAS pathway than systemic ARB,
which selectively blocks receptors.
VEGF and VEGFR
also were reportedly elevated in intraocular tissues in diabetic conditions[14,22-23,25-26,29], which is associated with
activated AT1R by Ang II [1,6,13-15]. But, we did not find VEGF and
VEGFR-2 in either control or DM rats. These conflicting outcomes may result
from different specimens used. Previous in vivo experimental studies
showing high VEGF expression in diabetes measured VEGF and VEGFR concentration
in retinas[21-24], whereas we
assessed the vitreous. An elevation of VEGF and VEGFR in retinal tissues may
not be enough to spill over into the vitreous cavity in our study. In human
studies, increased vitreous VEGF has been reported in PDR patients[13-14,25-26],
an advanced stage of DR. If the retina were in a more hypoxic condition such as
in the oxygen induced retinopathy (OIR) model, an advanced DR model with new
vessel formation, elevated VEGF in the vitreous may be detectable, although in
previous studies VEGF in the vitreous was 10-fold lower than in the retina[30-31].
There are some
limitations to our study. First, it is based on an animal model and the study
was designed to investigate short-term changes. The response to systemic ACEI
and ARB may differ in rats compared to humans. Thus, our results from this
experimental animal model may not reflect the clinical course in humans
directly. Second, this study could lack sufficient power to detect the
beneficial effect of ACEI and ARB, due to the small number of animals used. A
larger number of animals tested could generate a different outcome. Finally,
the intraocular activity of systemic medication may vary according to formulas
or doses, and the direct measurement of intraocular concentrations of drug and
intraocular growth factors will provide further information regarding the
efficacy of systemic ACEI and ARB.
In conclusion,
vitreal Ang II and AT1R can increase in DM. In such a condition, systemic ACEI
is likely to be more effective to inhibit the pathologic activation of
intraocular AT1R than ARB.
ACKOWLEDGEMENTS
Authors’
Contributions: design and conduct of study (Lee JE and Byon IS); data collection (Lee
DH, Shin MK, Jun ES, Park SW, Lee JE, Byon IS); data management, analysis, and
interpretation (Lee DH, Shin MK, Park SW, Lee JE, Byon IS); and manuscript
preparation, review, and approval (Shin MK, Park SW, Lee JE, Byon IS).
Foundation:
Supported
by Biomedical Research Institute Grant (PNU-2013-0373), Pusan National
University Hospital.
Conflicts
of Interest: Byon IS, None; Lee DH, None; Jun ES, None; Shin MK,
None; Park SW, None; Lee JE, None.
REFERENCES
1 Otani A, Takagi H, Suzuma K, Honda Y.
Angiotensin II potentiates vascular endothelial growth factor-induced
angiogenic activity in retinal microcapillary endothelial cells. <ii>Circ
Res</ii> 1998;82(5):619-628. [CrossRef]
2 Santos RA, Ferreira AJ, Verano-Braga
T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new
players of the renin-angiotensin system. <ii>J Endocrinol</ii>
2013;216(2):R1-R17. [CrossRef]
3 Zhong J, Guo D, Chen CB, Wang W,
Schuster M, Loibner H, Penninger JM, Scholey JW, Kassiri Z, Oudit GY.
Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and
fibrosis by angiotensin-converting enzyme 2. <ii>Hypertension </ii>
2011;57(2):314-322. [CrossRef]
4 Delafontaine P, Lou H. Angiotensin II
regulates insulin-like growth factor 1 gene expression in vascular smooth
muscle cells. <ii>J Biol Chem</ii> 1993;268(22):16866-16870. [PubMed]
5 Deschepper CF, Mellon SH, Cumin F,
Baxter JD, Ganong WF. Analysis by immunocytochemistry and in situ hybridization
of renin and its mRNA in kidney, testis, adrenal, and pituitary of the rat.
<ii>Proc Natl Acad Sci U S A</ii> 1986;83(19):7552-7556. [CrossRef]
6 Otani A, Takagi H, Oh H, Suzuma K,
Matsumura M, Ikeda E, Honda Y. Angiotensin II-stimulated vascular endothelial
growth factor expression in bovine retinal pericytes. <ii>Invest
Ophthalmol Vis Sci</ii> 2000;41(5):1192-1199. [PubMed]
7 Wilkinson-Berka JL, Agrotis A,
Deliyanti D. The retinal renin-angiotensin system: roles of angiotensin II and
aldosterone. <ii>Peptides </ii> 2012;36(1):142-150. [CrossRef]
8 Ozawa Y, Kurihara T, Sasaki M, Ban N,
Yuki K, Kubota S, Tsubota K. Neural degeneration in the retina of the
streptozotocin-induced type 1 diabetes model. <ii>Exp Diabetes Res
</ii> 2011;2011:108328. [CrossRef]
9 Giese MJ, Speth RC. The ocular
renin-angiotensin system: a therapeutic target for the treatment of ocular
disease. <ii>Pharmacol Ther</ii> 2014;142(1): 11-32. [CrossRef]
10 Verma A, Shan Z, Lei B, Yuan L, Liu
X, Nakagawa T, Grant MB, Lewin AS, Hauswirth WW, Raizada MK, Li Q. ACE2 and
Ang-(1-7) confer protection against development of diabetic
retinopathy.<ii> Mol Ther</ii> 2012;20(1):28-36. [CrossRef]
11 Marin Garcia PJ, Marin-Castaño ME.
Angiotensin II-related hypertension and eye diseases. <ii>World J
Cardiol</ii> 2014;6(9):968-984. [CrossRef]
12 Danser AHJ, van den Dorpel MA, Deinum
J, Derkx FH, Franken AA, Peperkamp E, de Jong PT, Schalekamp MA. Renin,
prorenin, and immunoactive rennin in vitreous fluid from eyes with and without
diabetic retinopathy. <ii>J Clin Endocrinol Metab </ii> 1989;68(1):160-167.
[CrossRef]
13 Funatsu H, Yamashita H, Nakanishi Y,
Hori S. Angiotensin II and vascular endothelial growth factor in the viteous
fluid of patients with proliferative diabetic retinopathy. <ii>Br J
Ophthalmol</ii> 2002;86(3):311-315. [CrossRef]
14 Aiello LP, Avery RL, Arrigg PG, Keyt
BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV,
Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular
fluid of patients with diabetic retinopathy and other retinal
disorders.<ii> N Eng J Med</ii> 1994;331(22):1480-1482. [CrossRef]
15 Moravski CJ, Kelly DJ, Cooper ME,
Gilbert RE, Bertram JF, Shahinfar S, Skinner SL, Wilkinson-Berka JL. Retinal
neovascularization is prevented by blockade of the renin-angiotensin system.
<ii>Hypertension</ii> 2000;36(6):1099-1104. [CrossRef]
16 Chaturvedi N, Sjolie AK, Stephenson
JM, Abrahamian H, Keipes M, Castellarin A, Rogulja-Pepeonik Z, Fuller JH.
Effect of lisinopril on progression of retinopathy in normotensive people with
type 1 diabetes. The EUCLID Study Group. EURODIAB controlled trial of
lisinopril in insulin-dependent diabetes mellitus. <ii>Lancet</ii>
1998;351(9095):28-31. [CrossRef]
17 Chaturvedi N, Porta M, Klein R,
Orchard T, Fuller J, Parving HH, Bilous R, Sjølie AK; DIRECT Programme Study
Group. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression
(DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomized,
placebo-controlled trials. <ii>Lancet</ii> 2008;372(9647):1394-1402.
[CrossRef]
18 Sjølie AK, Klein R, Porta M, Orchard
T, Fuller J, Parving HH, Bilous R, Chaturvedi N; DIRECT Programme Study Group.
Effect of candesartan on progression and regression of retinopathy in type 2
diabetes (DIRECT-Protect 2): a randomized placebo-controlled trial.
<ii>Lancet</ii> 2008;372(9647):1385-1393. [CrossRef]
19 Mauer M, Zinman B, Gardiner R, Suissa
S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R.
Renal and retinal effects of enalapril and losartan in type 1 diabetes.
<ii>N Engl J Med </ii> 2009;361(1):40-51. [CrossRef]
20 Harindhanavudhi T, Mauer M, Klein R,
Zinman B, Sinaiko A, Caramori ML; Renin Angiotensin System Study (RASS) group.
Benefits of Renin-Angiotensin blockade on retinopathy in type 1 diabetes vary
with glycemic control.<ii> Diabetes Care</ii> 2011;34(8):1838-1842.
[CrossRef]
21 Gilbert RE, Kelly DJ, Cox AJ,
Wilkinson-Berka JL, Rumble JR, Osicka T, Panagiotopoulos S, Lee V, Hendrich EC,
Jerums G, Cooper ME. Angiotensin converting enzyme inhibition reduces retinal
overexpression of vascular endothelial growth factor and hyperpermeability in
experimental diabetes. <ii>Diabetologia</ii> 2000;43(11):1360-1367.
[CrossRef]
22 Kim HW, Kim JL, Lee HK, Hur DY, Yun
IH, Kim SD. Enalapril alters expression of key growth factors in experimental
diabetic retinopathy. <ii>Curr Eye Res </ii> 2009;34(11):976-987. [CrossRef]
23 Kim JH, Kim JH, Yu YS, Cho CS, Kim
KW. Blockade of angiotensin ΙΙ attenuates VEGF-mediated blood-retinal barrier
breakdown in diabetic retinopathy. <ii>J Cereb Blood Flow
Metab</ii> 2009;29(3):621-628. [CrossRef]
24 Nakamura S, Tsuruma K, Shimazawa M,
Hara H. Candesartan, an angiotensin II type 1 receptor antagonist, inhibits
pathological retinal neovascularization by down regulating VEGF receptor-2
expression. <ii>Eur J Pharmacol</ii> 2012;685(1-3):8-14. [CrossRef]
25 Byon IS, Jeon HS, Kim HW, Lee SJ, Lee
JE, Oum BS. The effect of a systemic angiotensin receptor blocker on vascular
endothelial growth factor in the vitreous of patients with proliferative diabetic
retinopathy. <ii>Curr Eye Res</ii> 2013;38(7):774-780. [CrossRef]
26 Hogeboom van Buggenum IM, Polak BC,
Reichert-Thoen JW, de Vries-Knoppert WA, van Hinsbergh VW, Tangelder GJ.
Angiotensin converting enzyme inhibiting therapy is associated with lower
vitreous vascular endothelial growth factor concentrations in patients with
proliferative diabetic retinopathy. <ii>Diabetologia</ii>
2002;45(2):203-209. [CrossRef]
27 Luetscher JA, Kraemer FB, Wilson DM,
Schwartz HC, Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus.
A marker of microvascular complications. <ii>N Engl J Med</ii>
1985;312(22):1412-1417. [CrossRef]
28 Nagai N, Noda K, Urano T, Kubota Y,
Shinoda H, Koto T, Shinoda K, Inoue M, Shiomi T, Ikeda E, Tsubota K, Suda T,
Oike Y, Ishida S. Selective suppression of pathologic, but not physiologic,
retinal neovascularization by blocking the angiotensin II type 1 receptor.
<ii>Invest Ophthalmol Vis Sci</ii> 2005;46(3):1078-1084. [CrossRef]
29 Okada Y, Yamanaka I, Sakamoto T, Hata
Y, Sassa Y, Yoshikawa H, Fujisawa K, Ishibashi T, Inomata H. Increased
expression of angiotensin-converting enzyme in retinas of diabetic rats.
<ii>Jpn J Ophthalmol</ii> 2001; 45(6):585-591. [CrossRef]
30 Naug HL, Browning J, Gole GA, Gobé G.
Vitreal macrophages express vascular endothelial growth factor in
oxygen-induced retinopathy.<ii> Clin Exp Ophthalmol </ii>
2000;28(1):48-52. [CrossRef]
31 Hartnett ME. Pathophysiology and
mechanisms of severe retinopathy of prematurity.
<ii>Ophthalmology</ii> 2015:122(1):200-210. [CrossRef]