
·Clinical
Research·Current
Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
Effect of human autologous serum and fetal bovine
serum on human corneal epithelial cell viability, migration and proliferation in
vitro
Ming-Feng Wu1,
Tanja Stachon1, Berthold Seitz1, Achim Langenbucher2,
Nóra Szentmáry1,3
1Department of Ophthalmology, Saarland
University Medical Center, Homburg/Saar 66424, Germany
2Experimental Ophthalmology, Saarland
University, Homburg/Saar 66424, Germany
3Department of Ophthalmology,
Semmelweis University, Budapest 1085,
Hungary
Correspondence to: Ming-Feng Wu. Department
of Ophthalmology, Saarland Medical University, Kirrberger Street, Homburg/Saar
66424, Germany. enmengfong@hotmail.com
Received: 2016-05-13
Accepted: 2017-02-06
Abstract
AIM: To analyze the
concentration-dependent effects of autologous serum (AS) and fetal bovine serum
(FBS) on human corneal epithelial cell (HCEC) viability, migration and
proliferation.
METHODS: AS was prepared
from 13 patients with non-healing epithelial defects Dulbecco's modified eagle
medium/Ham’s F12 (DMEM/F12) with 5% FBS, 0.5% dimethyl sulphoxide (DMSO), 10
ng/mL human epidermal growth factor, 1% insulin-transferrin-selenium, then were
incubated in serum media: DMEM/F12 supplemented by 5%, 10%, 15% or 30% AS or
FBS. HCEC viability was analyzed using cell proliferation kit XTT, migration
using a wound healing assay, proliferation by the cell proliferation
enzyme-linked immunosorbent assay (ELISA) BrdU kit. Statistical analysis was
performed using the generalized linear model, the values at 30% AS or 30% FBS
were used as the baselines.
RESULTS: HCEC viability was the highest
at 30% AS or 15% FBS and the lowest at 10% AS or 30% FBS application. HCEC
migration was the quickest through 30% AS or 30% FBS and the slowest through 5%
AS or 5% FBS concentrations. Proliferation was the most increased through 15%
AS or 5% FBS and the least increased through 30% AS or 30% FBS concentrations.
HCEC viability at 10% and 15% AS was significantly worse (P=0.001, P=0.023)
compared to baseline and significantly better at 15% FBS (P=0.003)
concentrations. HCEC migration was significantly worse (P≤0.007) and
HCEC proliferation significantly better (P<0.001) in all
concentration groups compared to baseline.
CONCLUSION: For the best viability of HCEC
30% AS or 15% FBS, for HCEC migration 30% AS or 30% FBS, for proliferation 15%
AS or 5% FBS should be used. Therefore, we suggest the use of 30% AS in
clinical practice.
KEYWORDS: autologous serum; eye drops; serum concentration;
migration; proliferation; viability; human corneal epithelial cells
DOI:10.18240/ijo.2017.06.12
Citation: Wu MF, Stachon T, Seitz B, Langenbucher A, Szentmáry
N. Effect of human autologous serum and fetal bovine serum on human corneal
epithelial cell viability, migration and proliferation in vitro. Int J
Ophthalmol 2017;10(6):908-913
Article
Outline
INTRODUCTION
Tear film is a fluid layer essential for ocular
surface lubrication, nutrition and immunology[1].
Abnormal tear film can result in keratoconjunctivitis sicca (KCS), which is still most commonly
treated by lubricating artificial tears[2].
However, the components of tear film, including electrolytes, proteins, lipids,
mucins, are hardly compensated by the only use of lubricants[1].
Human peripheral blood serum is a natural substitute
of tears. Serum has similar pH and osmotic pressure to tears. Furthermore, it
contains many identical components to tears, such as epidermal growth factor
(EGF), nerve growth factor, insulin-like growth factor, platelet-derived growth
factor, transforming growth factor (TGF)-β, lysozyme, IgA, albumin, vitamin A,
substance P, etc[3]. In 1984, the
beneficial effect of autologous serum (AS) eye drops, as “artificial tear” for
KCS patients, was firstly reported by Fox et al[4].
Thereafter, serum eye drops, mostly autologous and sometimes allogenic, were
used in various ocular surface diseases such as KCS, Sjögren’s syndrome,
persistent corneal epithelial defects, chemical eye burn and neurotrophic
keratitis[5-10].
Like other materials, AS is only optimal and safe for
the human corneal epithelial cells (HCECs) in a certain concentration range. In
2001, using an in vitro cell culture model, Geerling et al[11] found that 50% and 100% AS were toxic to HCECs; they
either decreased cellular ATP level or increased cell membrane permeability.
The 100% AS was less toxic compared to 50% as. A few years later, Liu et al[12] found that 100% human serum was supporting more HCEC
migration than 25% human serum (diluted with isotonic saline). Later it was
also described that the relative cell growth of HCECs was best supported with
human serum diluted to 12%[13]. Beside these in
vitro studies, Akyol-Salman[14] has shown
that 100% AS accelerates rabbit corneal wound healing more than 20% AS.
Nowadays, 20% AS concentration seems to be the most commonly used concentration
of AS in clinical and experimental studies[9,15-20]. However, there is still no
consensus on AS preparation and application; AS concentration can vary from 20%
to 100% among different institutions[3,21].
Recently, AS has also been recommended as an
alternative of fetal bovine serum (FBS) use in cell cultures, in order to
devoid animal-derived products during culturing and expansion of human corneal
limbal epithelial cells, in vitro, with the aim of transplantation[22]. In some previous studies, corneal epithelial cells
cultivated in AS and FBS supplemented media have shown similar morphology and
expression pattern of intercellular junction proteins, basement membrane
proteins and tissue-specific keratins. Likewise, BrdU enzyme-linked
immunosorbent assay (ELISA) cell proliferation assay and colony-forming
efficiency analysis did not demonstrate significant differences between corneal
epithelial cells of both cultures[23-24].
Considering the complicated effects of different concentrations of AS on HCECs
and the inconsistency of AS application, the dose-dependent effects of AS and
the growth factors which may play key role in effects of AS, should be studied
in detail.
The aim of the current study was to analyze the
effects of AS and FBS on HCEC healing function in vitro, and to
determine the impact of growth factors in AS on HCEC healing function.
MATERIALS AND METHODS
The study was performed in accordance with the tenets
of the Declaration of Helsinki. No ethical committee approval was needed for
this study.
Materials Cell proliferation kit XTT
(AppliChem, Darmstadt, Germany), cell proliferation ELISA BrdU (colorimetric)
kit (Roche, Mannheim, Germany), sulfuric acid (Titrisol, Darmstadt, Germany),
phosphate-buffered saline (PBS) (Sigma-Aldrich, Steinheim, Germany), Dulbecco's
modified eagle medium/Ham’s F12 (DMEM/F12) (Life technologies, Paisley, UK),
FBS (Life technologies, Paisley, UK), penicillin-streptomycin (P/S)
(Sigma-Aldrich, USA), dimethylsulfoxide (DMSO) (Central Chemical Storage of
Saarland University, Saarbruecken, Germany), human epidermal growth factor
(hEGF) (Biochrom GmbH, Berlin, Germany), insulin-transferrin-selenium (Life
technologies, Paisley, UK), trypsin-EDTA solution (Sigma-Aldrich, USA). Human
fibroblast growth factor (FGF) basic DuoSet, human hepatocyte growth factor
(HGF) DuoSet, human TGF-β1 DuoSet and human keratinocyte growth factor
(KGF)/FGF-7 DuoSet (R&D systems, Minneapolis, USA).
Preparation of Autologous Serum AS was obtained from 13 patients with
non-healing epithelial defects [38% females, age 69±16 (41 to 92)y] with the
diagnosis non-healing corneal ulcer (6 patients) or corneal erosion (4
patients), Salzmann’s nodular degeneration (1 patient), Sjögren’s syndrome (1
patient), systemic lupus erythematosus (1 patient). Five of the patients had
previous penetrating keratoplasty. All patients had non-healing epithelial
defects and AS was used for their clinical treatment (details not described in
this study). The rests of AS, not used in clinical treatment of our patients
was available for our experiments. No other specific inclusion criteria were
applied. Serological tests for hepatitis B, hepatitis C, HIV, cytomegalovirus
and syphilis were all negative. To prepare the AS, peripheral blood was
obtained by vein puncture, was stored for 1 to 3h at room temperature, then
centrifuged at 3000 rpm (855 g) for 15min. Thereafter, under laminar flow,
serum was pipetted into a sterile container and 1.5 to 2 mL aliquots of serum
were filtered and injected into 5 mL sterile dropper bottles via a
disposable filter connected to a syringe. The serum was stored at -20℃ for
maximal 3mo.
Determination of Human Corneal Epithelial
Cell Viability SV40-Adeno vector
transformed HCECs (cell no. RCB2280), which obtained from RIKEN BioResource
Center, Ibaraki, Japan, were cultured in DMEM/F12 culture medium containing 5%
FBS, 5 μg/mL Insulin, 10 ng/mL hEGF and 0.5% DMSO. HCEC viability was
determined as previous description using cell proliferation kit XTT[25]. Briefly, HCECs in 96-well plates was incubated in
the 5%, 10%, 15% or 30% AS or FBS containing medium (serum media) for 24h,
XTT-containing solution was then added to react with HCECs at 37℃ for 2h.
Finally, the absorbance of the reaction mixture in each well, which
representing the HCEC viability, were measured by a 96-well microplate reader.
Wound Healing Assay of Human Corneal
Epithelial Cells HCECs grew in DMEM/F12
culture medium with 5% FBS, 5 μg/mL Insulin, 10 ng/mL hEGF and 0.5% DMSO until
confluence in 6-well plate. Then the culture medium was replaced by serum media
after rinsing the well twice by PBS, followed by incubation at 37℃ for 20min.
The HCEC monolayers were scratched by 200 μL yellow pipette tips, then 3 photos
of each scratch were taken at the beginning and after 9h incubation (Figure 1).
The pixel areas of the scratch on the photos were measured by the software GNU
Image Manipulation Program. Then, the average widths of the scratches were
calculated and converted into micrometers.
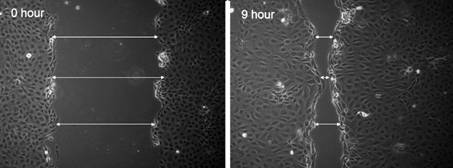
Figure 1 Photos of the scratch were
taken at the beginning and after 9h incubation.
Determination of Human Corneal
Epithelial Cell Proliferation HCEC proliferation was
determined by cell proliferation ELISA BrdU (colorimetric) kit as previous
description[25]. Briefly, HCEC monolayer was
incubated in the serum media for 24h. BrdU labeling solution was added and
incubated at 37℃ for 3h (BrdU incorporation). After removing the serum media,
the cells underwent a series of reactions for photometric detection. The
96-well plates were analyzed by the microplate reader.
Measurement of Growth Factors in
Autologous Serum KGF, FGFb, HGF and TGF-β1
concentrations in AS were measured by taking a 100 μL aliquot of the AS, as
previous description[25].
Statistical Analysis Statistical analysis was done using
the SPSS Statistics 22.0. Mann-Whitney U test was used to compare
viability, migration and proliferation of AS and FBS groups with the same
concentration. We used a generalized linear model to determine the effect of
different concentrations of AS and the impact of growth factor concentrations
in AS on HCEC viability, migration and proliferation. We calculated the
concentration of the growth factors from the concentration measurement results
of 100% AS. P<0.05 was considered statistically significant.
RESULTS
Effect of Different Concentrations of
Autologous Serum and Fetal Bovine Serum on Human Corneal Epithelial Cell
Viability, Migration and Proliferation
Viability,
migration and proliferation of HCECs using different concentrations of AS and
FBS are displayed at Figures 2-4. Effect of different concentrations of AS or
FBS on HCEC viability, migration and proliferation using a generalized linear
model are shown in Table 1. HCEC viability was the highest when 30% AS or 15%
FBS was applied, and lowest when 10% AS or 30% FBS applied. HCEC migration rate
was the highest when 30% AS or 30% FBS applied, and lowest when 5% AS or 5% FBS
applied. Proliferation was the highest when 15% AS or 5% FBS applied, and
lowest when 30% AS or 30% FBS applied. HCEC viability at 10% and 15% AS was
significantly worse (P=0.001, 0.023) compared to baseline and
significantly better at 15% FBS (P=0.003) concentrations. HCEC migration
was significantly worse (P≤0.007) and HCEC proliferation significantly
better (P<0.001) in all concentration groups compared to baseline.
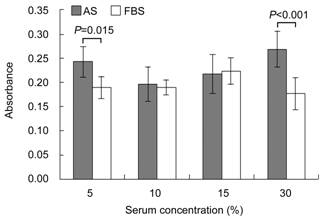
Figure 2 HCEC viability using
different concentrations of AS and FBS Mann-Whitney U test was used
to compare AS and FBS groups with the same concentration. Viability was
significantly higher using 5% AS than 5% FBS (P=0.015) or using 30% AS
than 30% FBS (P<0.001).
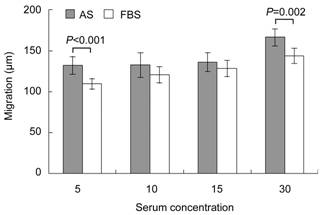
Figure 3 HCEC migration using
different concentrations of AS and FBS
Mann-Whitney
U test was used to compare AS and FBS groups with the same
concentration. Migration was significantly higher using 5% AS than 5% FBS (P<0.001)
or using 30% AS than 30% FBS (P=0.002).
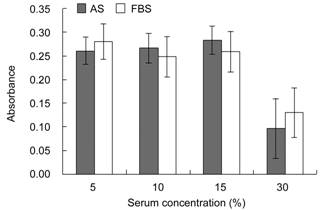
Figure 4 HCEC proliferation using
different concentrations of AS and FBS
Mann-Whitney
U test was used to compare AS and FBS groups with the same
concentration. Proliferation did not differ significantly between AS and FBS
groups with the same concentration (P>0.096).
Table 1 Effect of different concentrations
of AS or FBS on HCEC viability, migration and proliferation using a generalized
linear model
|
Serum |
Concentration (%) |
Viability |
Migration |
Proliferation |
|||
|
RC |
P |
RC |
P |
RC |
P |
||
|
AS |
5 |
-0.026 |
0.244 |
-34.319 |
<0.001 |
0.164 |
<0.001 |
|
10 |
-0.072 |
0.001 |
-33.595 |
<0.001 |
0.170 |
<0.001 |
|
|
15 |
-0.052 |
0.023 |
-30.583 |
<0.001 |
0.187 |
<0.001 |
|
|
30 |
0 |
- |
0 |
- |
0 |
- |
|
|
FBS |
5 |
0.013 |
0.428 |
-34.154 |
<0.001 |
0.150 |
<0.001 |
|
10 |
0.013 |
0.423 |
-23.084 |
<0.001 |
0.118 |
<0.001 |
|
|
15 |
0.047 |
0.003 |
-15.365 |
0.007 |
0.129 |
<0.001 |
|
|
30 |
0 |
- |
0 |
- |
0 |
- |
|
RC: Regression coefficient. P<0.05 was
considered statistically significant, compared to baseline. The values at 30%
AS and 30% FBS were used as baselines.
Effect of Autologous Serum and Fetal
Bovine Serum with the Same Concentration on Human Corneal Epithelial Cell
Viability, Migration and Proliferation
Results
of HCEC viability, migration and proliferation responding to AS and FBS are
shown at Figures 2-4. Viability and migration was significantly higher using 5%
AS than 5% FBS (P=0.015, P<0.001). Viability and migration
were also significantly higher using 30% AS than 30% FBS (P<0.001, P=0.002).
However, viability and migration did not differ significantly using 10% AS vs
10% FBS or 15% AS vs 15% FBS (P>0.077). Proliferation did not
differ significantly between AS and FBS groups with the same concentration (P>0.096).
Effect of Growth Factors in
Autologous Serum on Human Corneal Epithelial Cells Concentrations of KGF, FGFb, HGF and
TGF-β1 in AS of 13 patients are shown in Table 2. The effect of FGFb, HGF and
TGF-β1 concentrations in AS on HCEC viability, migration and proliferation
using a generalized linear model is displayed in Table 3. Effect of KGF on HCEC
viability, migration and proliferation was not considered in Table 3, since KGF
was only measurable in one AS sample. Concentration of the measured growth
factors did not affect HCEC viability (P>0.590). However, FGFb and
HGF concentrations had a positive effect (P<0.001 for both) on HCEC
migration and FGFb and TGF-β1 concentrations a negative effect (P=0.006,
0.008) on HCEC proliferation.
Table 2 Growth factor concentrations
in AS of 13 patients
|
Patient No. |
Growth factors (pg/mL) |
|||
|
KGF |
FGFb |
HGF |
TGF-β1 |
|
|
1 |
0 |
202 |
139 |
2928 |
|
2 |
0 |
204 |
1115 |
2928 |
|
3 |
0 |
130 |
1884 |
2313 |
|
4 |
0 |
122 |
2888 |
3426 |
|
5 |
0 |
56 |
1272 |
3955 |
|
6 |
0 |
0 |
2784 |
16767 |
|
7 |
0 |
300 |
628 |
28906 |
|
8 |
106 |
136 |
5964 |
28218 |
|
9 |
0 |
58 |
1426 |
28873 |
|
10 |
0 |
0 |
1182 |
26046 |
|
11 |
0 |
130 |
308 |
25075 |
|
12 |
0 |
96 |
661 |
25781 |
|
13 |
0 |
131 |
1715 |
35045 |
|
Median |
0 |
130 |
1272 |
25075 |
KGF: Keratinocyte growth factor; FGFb: Basic
fibroblast growth factor; HGF: Hepatocyte growth factor; TGF-β1: Transforming
growth factor-β1.
Table 3 Effect of FGFb, HGF and
TGF-β1 concentrations in AS on HCEC
|
Growth factor |
Viability |
Migration |
Proliferation |
|||
|
RC |
P |
RC |
P |
RC |
P |
|
|
FGFb |
3.3×10-4 |
0.590 |
0.623 |
<0.001 |
-0.002 |
0.006 |
|
HGF |
1.74×10-6 |
0.945 |
0.024 |
<0.001 |
-8.02×10-5 |
0.086 |
|
TGF-β1 |
2.30×10-6 |
0.666 |
3.14×10-4 |
0.808 |
-1.50×10-5 |
0.008 |
Effect of KGF was not considered since it was only
measurable in one AS sample; RC: Regression coefficient. P<0.05 was
considered statistically significant, compared to baseline.
DISCUSSION
From the clinical point of view, migration and
proliferation are the most important functions of HCECs during corneal
epithelial regeneration and wound healing, in order to reach closure of a
corneal epithelial defect/erosion. The 20% AS concentration seems to be the
most commonly used concentration of AS in clinical and experimental studies,
but the most important component of AS with beneficial effect on corneal
epithelial regeneration could not be defined yet[9,15-20]. Appropriate function of the
corneal limbal stem cells (proliferation, differentiation and centripetally
migration) is also indispensable in renewal of the corneal epithelial layer[26].
AS has been used as a substitute for FBS in cultures
of various cells, and supported better cell confluence, enhanced
differentiation of bone marrow mesenchymal cells and increased cell
proliferation rate, more compared to FBS[27-33]. However, as mentioned above, only a few studies
tried to compare differences between AS and FBS in HCEC culture, and until now,
no significant differences between both has been determined. In addition, the
use of animal-derived material is not allowed for humans, or human studies, in
Germany.
In our present study, HCEC viability and migration was
better using AS than FBS. However, concerning proliferation, no difference
could be shown between both groups. HCEC viability and migration were the
highest at 30% AS, but this group increased HCEC proliferation the least. 15%
AS concentration led to lower HCEC viability and migration than 30% AS, but 15%
AS resulted in the best proliferation of the HCECs.
Based on our results, we suggest the clinical use of
30% AS, since the most important in vivo corneal epithelial functions
are migration and viability. In our opinion, this concentration could be
reached through dilution of AS in the remaining tear film of the patients. To
the best of our knowledge, up to now there is no study analyzing the impact of
tear volume on efficacy of AS therapy. Intrestingly, AS improves ocular surface
disease index (OSDI), but does not have an impact on tear osmolarity[34]. Further in vivo studies should analyse the
impact of tears on AS efficacy on corneal wound healing.
The high variability of growth factor concentrations
in AS of different patients, should also be taken into account. It is already
known that FGFb is a beneficial factor in corneal epithelial cell growth or
corneal wound healing[35-36],
and it is also required for corneal epithelial cell proliferation and
differentiation during embryonic development[37].
HGF and FGFb are both major factors initiating proliferation and migration in
the cornea, while TGF-β in the tear film suppresses the proliferation at the
migrating cell front[38].
In the present study we analyzed the effects of FGFb,
HGF and TGF-β1 concentrations on HCEC viability, migration and proliferation.
FGFb and HGF concentrations had a positive effect on HCEC migration, but FGFb
and TGF-β1 concentrations had negative effect on HCEC proliferation. The
interactions of growth factors in human serum and their impact on human
epithelial cell viability, migration and proliferation should be further
analyzed.
In conclusion, HCEC viability is most supported by 30%
AS or 15% FBS, migration by 30% AS or 30% FBS, and proliferation by 15% AS or
5% FBS. In addition, AS better supports HCECs viability and migration than FBS.
Therefore, 30% may be an appropriate AS concentration in clinical practice.
Based on our experiments, we also suggest the use of AS instead of FBS for in
vitro HCEC cultures, especially for ex vivo expansion of limbal stem
cells.
ACKNOWLEDGEMENTS
Conflicts of Interest: Wu MF, None; Stachon T, None;
Seitz B, None; Langenbucher A, None; Szentmáry N, None.
REFERENCES
1 Tiffany JM. The normal tear film. Dev Ophthalmol 2008;41:1-20. [CrossRef]
2 Alves M, Fonseca EC, Alves MF, Malki
LT, Arruda GV, Reinach PS, Rocha EM. Dry eye disease treatment: a systematic
review of published trials and a critical appraisal of therapeutic strategies. Ocul Surf 2013;11(3):181-192. [CrossRef]
3 Rauz S, Saw VP. Serum eye drops,
amniotic membrane and limbal epithelial stem cells-tools in the treatment of
ocular surface disease. Cell Tissue Bank
2010;11(1):13-27. [CrossRef]
4 Fox RI, Chan R, Michelson JB, Belmont
JB, Michelson PE. Beneficial effect of artificial tears made with autologous
serum in patients with keratoconjunctivitis sicca. Arthritis Rheum 1984;27(4):459-461. [CrossRef]
5 Tsubota K, Goto E, Shimmura S,
Shimazaki J. Treatment of persistent corneal epithelial defect by autologous
serum application. Ophthalmology 1999;106(10):1984-1989.
[CrossRef]
6 Noble BA. Comparison of autologous
serum eye drops with conventional therapy in a randomised controlled crossover
trial for ocular surface disease. Br J
Ophthalmol 2004;88(5):647-652. [CrossRef]
7 Jeng BH, Dupps WJ Jr. Autologous serum
50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea 2009;28(10): 1104-1108. [CrossRef]
8 Chiang CC, Chen WL, Lin JM, Tsai YY.
Allogeneic serum eye drops for the treatment of persistent corneal epithelial
defect. Eye (Lond) 2009;23(2):290-293.
[CrossRef]
9 Harritshoj LH, Nielsen C, Ullum H,
Hansen MB, Julian HO. Ready-made allogeneic ABO-specific serum eye drops:
production from regular male blood donors, clinical routine, safety and
efficacy. Acta Ophthalmol 2014;92(8):783-786.
[CrossRef]
10 Semeraro F, Forbice E, Braga O, Bova
A, Di Salvatore A, Azzolini C. Evaluation of the efficacy of 50% autologous
serum eye drops in different ocular surface pathologies. Biomed Res Int 2014;
2014: 826970. [CrossRef]
11 Geerling G, Daniels JT, Dart JK, Cree
IA, Khaw PT. Toxicity of natural tear substitutes in a fully defined culture
model of human corneal epithelial cells. Invest
Ophthalmol Vis Sci 2001;42(5):948-956. [CrossRef]
12 Liu L, Hartwig D, Harloff S,
Herminghaus P, Wedel T, Geerling G. An optimised protocol for the production of
autologous serum eyedrops. Graefes Arch Clin Exp Ophthalmol 2005;243:706-714.
[CrossRef]
13 Liu L, Hartwig D, Harloff S,
Herminghaus P, Wedel T, Kasper K. Corneal epitheliotrophic capacity of three
different blood-derived preparations.
Invest Ophthalmol Vis Sci 2006;47(6):2438-2444.
[CrossRef]
14 Akyol-Salman I. Effects of autologous
serum eye drops on corneal wound healing after superficial keratectomy in
rabbits. Cornea 2006;
25(10):1178-1181. [CrossRef]
15 Lavaju P, Sharma M, Sharma A, Chettri
S. Use of amniotic membrane and autologous serum eye drops in Mooren's ulcer. Nepal J Ophthalmol 2013;5(1):120-123. [CrossRef]
16 Mukhopadhyay S, Sen S, Datta H.
Comparative role of 20% cord blood serum and 20% autologous serum in dry eye
associated with Hansen's disease: a tear proteomic study. Br J Ophthalmol 2015;99(1): 108-112. [CrossRef]
17 Celebi AR, Ulusoy C, Mirza GE. The
efficacy of autologous serum eye drops for severe dry eye syndrome: a
randomized double-blind crossover study. Graefes
Arch Clin Exp Ophthalmol 2014;252(4):619-626. [CrossRef]
18 Urzua CA, Vasquez DH, Huidobro A,
Hernandez H, Alfaro J. Randomized double-blind clinical trial of autologous
serum versus artificial tears in dry eye syndrome. Curr Eye Res 2012;37(8):684-688. [CrossRef]
19 Matsuo H, Tomidokoro A, Tomita G,
Araie M. Topical application of autologous serum for the treatment of
late-onset aqueous oozing or point-leak through filtering bleb. Eye (Lond) 2005;19(1):23-28. [CrossRef]
20 Goto E, Shimmura S, Shimazaki J,
Tsubota K. Treatment of superior limbic keratoconjunctivitis by application of
autologous serum. Cornea 2001;20(8):807-810.
[CrossRef]
21 Geerling G, Maclennan S, Hartwig D.
Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol 2004;88(11):1467-1474. [CrossRef]
22 Mariappan I, Maddileti S, Savy S,
Tiwari S, Gaddipati S, Fatima A, Sangwan VS, Balasubramanian D, Vemuganti GK.
In vitro culture and expansion of human limbal epithelial cells. Nat Protoc 2010;5(8):1470-1479. [CrossRef]
23 Nakamura T, Inatomi T, Sotozono C,
Ang LP, Koizumi N, Yokoi N, Kinoshita S. Transplantation of autologous
serum-derived cultivated corneal epithelial equivalents for the treatment of
severe ocular surface disease. Ophthalmology
2006;113(10):1765-1772. [CrossRef]
24 Nakamura T, Ang LP, Rigby H, Sekiyama
E, Inatomi T, Sotozono C, Fullwood NJ, Kinoshita S. The use of autologous serum
in the development of corneal and oral epithelial equivalents in patients with
Stevens-Johnson syndrome. Invest
Ophthalmol Vis Sci 2006;47(3):909-916. [CrossRef]
25 Wu MF, Stachon T, Langenbucher A,
Seitz B, Szentmáry N. Effect of amniotic membrane suspension (ams) and amniotic
membrane homogenate (AMH) on human corneal epithelial cell viability, migration
and proliferation in vitro. Curr Eye Res 2017;42(3):351-357.
[CrossRef]
26 Yoon J, Ismail S, Sherwin T. Limbal
stem cells: central concepts of corneal epithelial homeostasis. World J Stem Cells 2014;6(4):391-403. [CrossRef]
27 Goodarzi P, Arjmand B, Emami-Razavi
SH, Soleimani M, Khodadadi A, Mohamadi-Jahani F, Aghayan HR. Human autologous
serum as a substitute for fetal bovine serum in human Schwann cell culture. Acta Med Iran 2014;52(4):241-245. [PubMed]
28 Rouhi L, Kajbafzadeh AM, Modaresi M,
Shariati M, Hamrahi D. Autologous serum enhances cardiomyocyte differentiation
of rat bone marrow mesenchymal stem cells in the presence of transforming
growth factor-β1 (TGF-β1). In Vitro Cell
Dev Biol Anim 2013;49(4):287-294. [CrossRef]
29 Bahn JJ, Chung JY, Im W, Kim M, Kim
SH. Suitability of autologous serum for expanding rabbit adipose-derived stem
cell populations. J Vet Sci 2012;13(4):413-417.
[CrossRef]
30 Morimoto N, Takemoto S, Kanda N,
Ayvazyan A, Taira MT, Suzuki S. The utilization of animal product-free media
and autologous serum in an autologous dermal substitute culture. J Surg Res 2011;171(1):339-346. [CrossRef]
31 Takeda A, Yamazaki Y, Baba K,
Ishiguro M, Aoyagi K, Ikemoto S, Osteogenic potential of human bone marrow-derived
mesenchymal stromal cells cultured in autologous serum: a preliminary study. J Oral Maxillofac Surg 2012;70(8):e469-e476.
[CrossRef]
32 Josh F, Kobe K, Tobita M, Tanaka R,
Suzuki K, Ono K, Hyakusoku H, Mizuno H. Accelerated and safe proliferation of
human adipose-derived stem cells in medium supplemented with human serum. J Nippon Med Sch 2012;79(6):444-452. [CrossRef]
33 Shumiya T, Shibata R, Shimizu Y,
Ishii M, Kubota R, Shintani S, Murohara T. Evidence for the therapeutic
potential of ex vivo expanded human endothelial progenitor cells using
autologous serum. Circ J 2010;74(5):1006-1013.
[CrossRef]
34 Mahelkova G, Vesela V, Seidler
Stangova P, Zidlicka A, Dotrelova D, Fales I, Skalicka P, Jirsova K. Tear
osmolarity in patients with severe dry eye syndrome before and after autologous
serum treatment: a comparison with tear osmolarity in healthy volunteers. Cesk Slov Oftalmol 2015;71(4):184-188. [PubMed]
35 Hu C, Ding Y, Chen J, Liu D, Zhang Y,
Ding M, Wang G. Basic fibroblast growth factor stimulates epithelial cell
growth and epithelial wound healing in canine corneas. Vet Ophthalmol 2009;12(3):170-175. [CrossRef]
36 Yan L, Wu W, Wang Z, Li C, Lu X, Duan
H, Zhou J, Wang X, Wan P, Song Y, Tang J, Han Y. Comparative study of the
effects of recombinant human epidermal growth factor and basic fibroblast
growth factor on corneal epithelial wound healing and neovascularization in
vivo and in vitro. Ophthalmic Res 2013;49(3):150-160.
[CrossRef]
37 Zhang J, Upadhya D, Lu L, Reneker LW.
Fibroblast growth factor receptor 2 (FGFR2) is required for corneal epithelial
cell proliferation and differentiation during embryonic development. PLoS One 2015;10(1): e0117089. [CrossRef]
38 Zelenka PS, Arpitha P. Coordinating
cell proliferation and migration in the lens and cornea. Semin Cell Dev Biol 2008;19(2):113-124. [CrossRef]