
·Clinical
Research·Current
Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
Viscocanalostomy combined with
trabeculotomy and mitomycin C in the treatment of primary congenital glaucoma
Chao-Xu Qian1, Yuan Zong2, Qin Chen3,
Zhi-Lan Yuan3
1Department of
Ophthalmology, the Third People’s Hospital of Changzhou, Changzhou 213001,
Jiangsu Province, China
2Department of
Ophthalmology, Eye, Ear, Nose & Throat Hospital of Fudan University,
Shanghai 200031, China
3Department of
Ophthalmology, the First Affiliated Hospital of Nanjing Medical University,
Nanjing 210029, Jiangsu Province, China
Correspondence
to:
Zhi-Lan Yuan. Department of Ophthalmology, the First Affiliated Hospital of
Nanjing Medical University, 300 Guangzhou Road, Nanjing 210029, Jiangsu
Province, China. zhilanyuan@vip.sina.com
Received:
2016-10-10
Accepted: 2017-07-06
Abstract
AIM: To evaluate the
long-term outcome of viscocanalostomy combined with trabeculotomy and mitomycin
C in the treatment of primary congenital glaucoma.
METHODS: This is a retrospective
study. Forty-two eyes of 26 patients with primary congenital glaucoma were
enrolled. Intraocular pressure (IOP), corneal diameter (mm) and cup/disc (C/D)
were measured before and after the surgery respectively. Follow-up period was
30mo.
RESULTS: The mean
preoperative IOP was 30.6±7.35 mm Hg. Of the 42 eyes, 2 eyes were required
conversion to trabeculectomy for the absence of Schlemm’s canal. Of remained 40
eyes, 38 eyes (95%) achieved successful IOP control. The average postoperative
IOP was 11.69±4.18 mm Hg at 12mo. The mean reduction was 18.91 mm Hg (P<0.0001).
Eighteen (75%) eyes presented a reduction in corneal diameter, and 25 (62.5%)
eyes presented a C/D ratio reversal after the surgery. There was no serious
complication in any patients over the follow-up period.
CONCLUSION: Viscocanalostomy
combined with trabeculotomy and mitomycin C is useful in the management of
primary congenital glaucoma.
KEYWORDS: glaucoma; treatment
surgery; intraocular pressure; congenital eye disorders
DOI:10.18240/ijo.2017.06.14
Citation:Qian CX,
Zong Y, Chen Q, Yuan ZL. Viscocanalostomy combined with trabeculotomy and
mitomycin C in the treatment of primary congenital glaucoma. Int J
Ophthalmol 2017;10(6):919-924
Article Outline
INTRODUCTION
Primary
congenital glaucoma (PCG) is characterized by significantly increased
intraocular pressure (IOP) with accompanied symptoms and signs. Recently,
surgical intervention remains the main option for the initial treatment of PCG.
The conventional therapies include goniotomy, trabeculotomy, combined
trabeculotomy-trabeculectomy and trabeculectomy. Non-penetrating filtering
surgery (NPFS) began in 1962 with the first sinusotomy performed by Kraznov[1]. Viscocanalostomy was one of non-penetrating
filtering firstly described by Stegmann in 1999. This surgery has been
considered to be quite effective in lowering IOP, with less risks of
complication[2]. The operator did
viscocanalostomy, and combined with trabeculotomy and mitomycin C for the PCG’s
treatment. The aim of this study was to demonstrate the IOP lowering effects
and the potential complications of viscocanalostomy combined with trabeculotomy
(VCT) and mitomycin C in patients with PCG.
SUBJECTS AND METHODS
Patients This study has been approved by the
Ethics Committee of the First Affiliated Hospital of Nanjing Medical
University. The study was conducted on the patients’ records. Twenty-six Chinese
patients (20 males, 6 females), 42 eyes with uncontrolled PCG were enrolled in
this study. Patient's age ranged from 3 to 36mo (mean 10.7mo). All the relevant
data were collected from 2012 to 2015, and every child’s parents had signed the
informed consent.
The inclusion
criteria were: 1) the IOP is greater than 21 mm Hg in one eye as measured by
applanation tonometry on at least two occasions is considered abnormally
elevated; 2) enlargement of the globe (axial length); 3) increased corneal
diameter; 4) anomalously cup/disc (C/D) ratio.
The exclusion
criteria were: 1) patients who had previous surgery; 2) secondary glaucoma; 3)
Axenfeld-Rieger syndrome; 4) Aniridia or Sturge-Weber syndrome.
Baseline
examinations: at the baseline visit, all patients had a full ocular examination
under anesthesia by ketamine to confirm the diagnosis.
Methods The following examinations were
performed at the baseline visit: IOP (TONO-Pen AVIA, Reichert, NY, USA),
corneal diameters (with Caliphers), the presence of corneal opacification,
Habb's striae, the depth of the anterior chamber, iris structure, lens transparency, pupil reactivity and shape, optic disc
evaluation, the C/D ratio measurement. The gonioscopy and biomicroscopy were
also performed. All the examinations were conducted by the same glaucoma
specialist under anesthesia before the surgery and every child’s parents had
signed the informed consent for it. As the patients were too young, the authors
did not do visual acuity on patients.
Surgical
Procedures The operations were
started under general anesthesia and also that there's an informed consent
process each time. All surgeries were performed by 1 experienced senior surgeon
follow standard techniques: a corneal traction suture (8-0 nylon suture) was
placed in the nasal cornea to enhance exposure. The conjunctiva and Tenon's
capsule was opened at the limbus to expose sclera. A 5×4 mm tongue-shaped,
one-third thickness superficial scleral flap was prepared at the 12th
and 1st clock where there were no large penetrating vessels and
dissected 1.5-2 mm into clear cornea. At this stage, 0.2 mg/mL of mitomycin-C
was applied with a sponge under the scleral flap for three minutes before
dissecting the deeper scleral flap[3]. This area
was then flushed with balanced salt solution (BSS®). A deeper
scleral flap was fashioned 0.5 mm inside the border of superficial scleral flap,
about two-thirds of scleral thickness, leaving a thin translucent layer over
the choroids. Cut apart the deeper scleral flap from its base (Figure 1A, 1B).
High molecular weight sodium hyaluronate was injected into the ostia of
Schlemm’s canal each for five times
(Figure 1C, 1D), and then peel away the internal layer of Schlemm’s
canal and juxtacanalicular connective tissue carefully (Figure 1E, 1F). In the
cases when the aqueous humor outflow was not sufficient, the surgery was
converted to a trabeculectomy by cutting apart a 1 mm× 3 mm trabecular block
and creating a peripheral iridectomy[4]. Before thetrabeculotomy, a “side port” incision
was made to inject some high molecular weight sodium hyaluronate. The
trabeculotome (Sourdille-Paufique, Moria®) was inserted into the
ostia of Schlemm’s canal, checking for obstacles to the advancement into the
canal (Figure 1G, 1H). Then rotated carefully towards the anterior chamber,
crossed the internal side of Schlemm’s canal, and breaked the angle’s
embrionary tissue, including the trabecular mesh. With a cut of 120°-150°,
Healon GV (Healon GV solution for intraocular use) was injected beneath the
first flap, the wound was seamed tightly with 10-0 nylon sutures[5]. The conjunctival flap was seamed with 10-0 nylon
sutures, too.
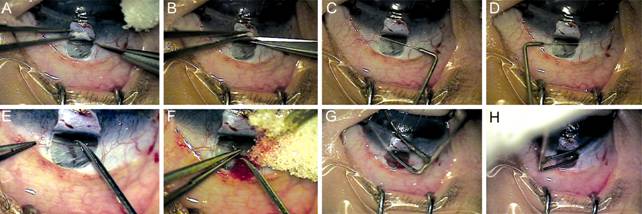
Figure 1 The main procedures of viscocanalostomy A, B: A deeper scleral flap was fashioned 0.5 mm inside the border of superficial scleral flap, about two-thirds of scleral thickness, leaving a thin translucent layer over the choroids. Cut apart the deeper scleral flap from its base. C, D: High molecular weight sodium hyaluronate was injected into the ostia of Schlemm’s canal each for five times. E, F: Peel away the internal layer of Schlemm’s canal and juxtacanalicular connective tissue carefully. G, H: The trabeculotome (Sourdille-Paufique, Moria®) was inserted into the ostia of Schlemm’s canal, checking for obstacles to the advancement into the canal.
After surgery,
patients were prescribed to tobramycin and dexamethasone (Tobradex,
Alcon-Couvreur, Puurs, Belgium) 0.1% eye drops four times daily for 4wk.
Postoperative
patients were measured IOP at 1wk, 3, 6mo and thereafter every 6mo. Corneal
diameter (mm), C/D ratio, however, was only measured at 1wk, 6mo and at the
last reported follow-up.
Statistical
Analysis Paired-samples t-test
was used to analyze the results using SPSS V 16.0 (SPSS Inc., Chicago,
Illinois, USA) and P values equal or less than 0.05 were considered as
statistically significant. Cumulative probabilities of success were determined
in accordance with Kaplan-Meier survival analysis.
RESULTS
Forty-two eyes
from 26 patients were enrolled into the study. Two eyes were required
conversion to trabeculectomy the absence of Schlemm’s canal. This patient’s data was excluded. Total of 40 eyes were
analyzed from 26 patients including 20 males (76.9%) and 6 females (23.1%). The
mean (SD) age at the time of undergoing VCT for each eye was 10.7 (9.70)mo,
with a range of 3 to 36mo. The disease was bilateral in 16 children (61.5%) and
unilateral in 10 children (38.5%). In unilateral cases the left eye accounted
for 66.7%. The mean (SD) number of glaucoma medications used prior VCT was 1.1
(0.56). One patient (two eyes) had glaucoma family history, his mother also
suffered from congenital glaucoma (Table 1).
Table 1
Patients demographics
|
Characteristics |
Value |
|
Patients, n |
26 |
|
Sex, n
(%) |
|
|
M |
20 (76.9) |
|
F |
6 (23.1) |
|
Eyes, n
(%) |
40 |
|
R |
18 (45) |
|
L |
22 (55) |
|
Age at
surgery, mo |
|
|
Mean (SD) |
10.7 (9.70) |
|
Range |
3-36 |
|
Median |
6 |
|
Preoperative
IOP, mm Hg |
|
|
Mean (SD) |
30.6±7.35 |
|
Range |
21.0-55.9 |
|
Preoperative
medications used, mean±SD |
1.1±0.56 |
|
Family
history |
2 (5%) |
Suspicion of
congenital glaucoma was based on enlarged corneal size (24 eyes; 60.0%),
corneal edema (26 eyes; 65%), tearing (8 eyes; 20.0%) (Figure 2). Follow-up
time lasted from 3 to 30mo, with a mean value of 11.79mo.
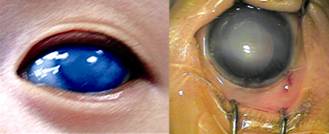
Figure 2
The two children’s cornea was enlarged obviously and the edema was severe that
the iris was hardly to be seen.
Intraocular
Pressure The preoperative and postoperative
IOP recordings are listed in Table 2, Figure 3. The mean preoperative IOP (±SD)
under glaucoma medication was 30.6±7.35 mm Hg. The mean postoperative IOP was
8.83±2.82 mm Hg at the first week, 12.55±7.26 mm Hg at 3mo, 12.43±7.17 mm Hg at
6mo, 11.69±4.18 mm Hg at 12mo, 13.44±4.49 mm Hg at 24mo, and 14.48±2.10 mm Hg
at 30mo. The mean deduction of IOP at week 1 postoperation compared to
preoperation was 21.77 mm Hg.
Table 2
Preoperative and postoperative IOP undergoing VCT
|
Characteristic |
No. of eyes |
Mean IOP (SD) (mm Hg) |
Mean IOP decrease (mm Hg) |
aP |
|
Preoperative |
40 |
30.6 (7.35) |
|
|
|
Postoperative |
|
|
|
|
|
1wk |
40 |
8.83 (2.82) |
21.77 |
<0.001 |
|
3mo |
35 |
12.55 (7.26) |
18.05 |
<0.001 |
|
6mo |
39 |
12.43 (7.17) |
18.17 |
<0.001 |
|
12mo |
30 |
11.69 (4.18) |
18.91 |
<0.001 |
|
18mo |
26 |
12.05 (3.08) |
18.55 |
<0.001 |
|
24mo |
21 |
13.44 (4.49) |
17.16 |
<0.001 |
|
30mo |
6 |
14.48 (2.10) |
16.12 |
<0.001 |
aPaired-samples Student’s t-test.
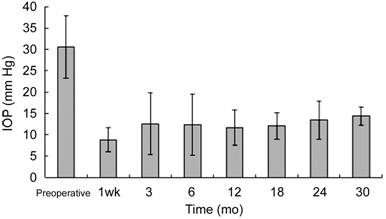
Figure 3 Mean IOP over time in
eyes having viscocanalostomy combined with trabeculotomy The decline of the IOP at each follow-up
point was statistically obvious.
Success
Probabilities Qualified success was
defined through the following criteria: alleviation of corneal edema,
stabilisation or reduction in horizontal corneal diameter, reversal of disc
cupping, an IOP measurement equal or less than 21 mm Hg. When medications were
not required, it was defined as a complete success. When IOP was >21 mm
Hg with three kinds of glaucoma medications, the operation was considered as a
failure.
Our results
presented a 100% qualified success rate at 12mo, 95.5% at 24mo, 68.6% at 30mo.
The success was complete in 97.4% of cases at 6mo, in 88.5% at 12mo, and in
70.4% at 24mo and 53.8% at 30mo. The cumulative probability curves for complete
and qualified success were depicted in Figures 4 and 5[6].
However, only 6 patients had completed 30mo of follow-up. There were three
patients (four eyes) who failure with VCT and required a secondary surgery to
achieve pressure control.
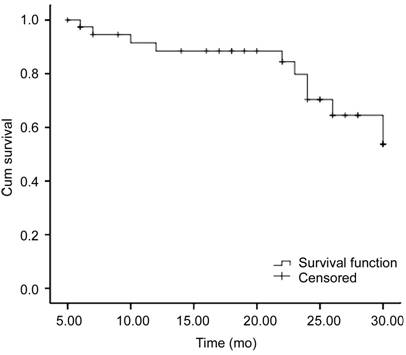
Figure 4 The
cumulative probability curves for complete success The complete success rate was 97.4%
at 6mo, 88.5% at 12mo, 70.4% at 24mo and 53.8% at 30mo.
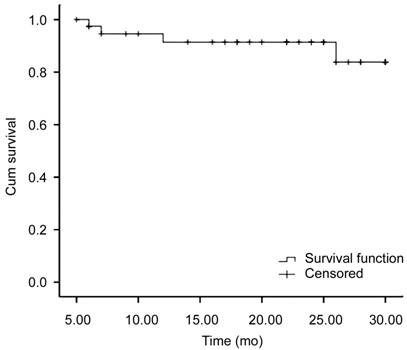
Figure 5
The cumulative probability curves for qualified success The qualified success rate was 100%
at 12mo, 95.5% at 24mo, 68.6% at 30mo.
Corneal
Diameter and Cup/Disc Ratio The corneal diameter and
C/D ratio did not change significantly in the first week after postoperative[6]. However, a significant reversal was observed after 6mo
(Table 3). There were 18 eyes presented a reduction in corneal diameter, and 25
eyes presented a C/D ratio reversal. The mean corneal diameter was 13.81±0.77
mm preoperative and 12.59±0.78 postoperative (t=7.60, P=0.000).
The mean C/D ratio was 0.75±0.12 preoperative and 0.55±0.17 postoperative (t=6.70,
P=0.000).
Table 3
Preoperative and postoperative corneal diameter and C/D ratio undergoing VCT
|
Characteristic |
Preoperative |
Post-6mo |
n |
t |
aP |
|
Corneal
diameter (mm) |
13.81±0.77 |
12.59±0.78 |
27 |
7.6 |
<0.05 |
|
C/D ratio |
0.75±0.12 |
0.55±0.17 |
35 |
6.7 |
<0.05 |
C/D: Cup/disc.
aPaired-samples Student’s t-test.
Complications There was no sight threatening intraoperative
and postoperative complication. The shallow anterior chambers were found in 2
(5.0%) eyes and recovered spontaneously. Four (10.0%) eyes developed hyphema, and 2 (5.0%) eyes had
an inadvertent small hole in the Descemet’s membrane. There were no severe complications
such as retinal detachment, choroidal haemorrhage, late bleb leakage, blebitis
and endophthalmitis in any patient intraoperatively and postoperatively.
DISCUSSION
As medical therapy for PCG is hardly effective, PCG is almost
managed surgically[7]. Although the conventional
operation therapy for congenital glaucoma such as goniotomy, trabeculotomy,
trabeculectomy, effective pressure control can be acquired in up to 90%[8-11]. However, all of them have
disadvantages. Goniotomy which need transparent corneas is the main choice for
the western surgeons, because patients may not have serve corneal edema at the
time for treatment[12]. While in China, quite a
few patients present with clouding because of limited medical conditions and
goniotomy is technically impossible[13]. In our
study, there are 26 (65%) eyes present with cornea edema. External
trabeculotomy’s main advantage over goniotomy is its relatively ignore the
transparency of the cornea[14]. As an initial
procedure, goniotomy has lower success rate than external trabeculotomy in
China[15]. But for locating Schlemm’s canal
exactly is difficult, the complications of external trabeculotomy hardly can be
avoided: hyphema, peripheral anterior synechia of iris, Descemet’s detachment,
marginal ulcer corneal, prolapse of iris. Tube drainage surgery is another
choice for PCG because it offers the best opportunity of long-term IOP control[16]. While people often didn’t choose this surgery as the
initial treatment for the reason of the complications like tube exposure,
infectious endophthalmitis, retinal detachment and high costs which couldn’t be
afford by many rural patients in China[17-18].
In primary
open angle glaucoma, viscocanalostomy is said to be rather effective in lowering
IOP, with less risks of complication than full-thickness filtering surgery[19]. After someone used it to treat infantile glaucoma
and proved that it could maintain a considerably lower IOP during a long-time
follow-up period[1]. Some of the results have
indicated that when the child’s angle was poorly developed, viscocanalostomy
without breaking the trabecular mesh and the angle’s embrionary tissue is
insufficient. So we also combined trabeculotomy for infantile glaucoma[20]. As the use of mitomycin C is a safe and effective
therapy during nonpenetrating filtering surgery[21].
VCT with mitomycin C provided a significant reduction of IOP in congenital
glaucoma in this study. It demonstrated a 100% overall success rate at 12mo,
95.5% at 24mo, 68.6% at 30mo.
VCT included two steps, the first step was viscocanalostomy,
shaped a scleral lake separated from the anterior chamber only by Descemet’s
membrane, dilated the Schlemm’s canal with high-molecular-weight viscoelastic
substance to keep its open, aqueous humor then permeated into a scleral lake
from the Descemet’s window, and finally into the Schlemm’s canal[22-23]. The second step was
trabeculotomy, breaked the angle’s embrionary tissue, sliced the whole
thickness of trabecular mesh, made the anterior chamber and Schlemm’s canal be
linked together. Aqueous could flow into Schlemm’s canal directly even the
child’s angle was poorly developed. So VCT creates two exit ways for aqueous
humor. And that’s why our researches have high success rates in IOP control.
Circle aqueous humor goes through the permeable trabeculo-descemetic membrane
to the intrascleral space, and then it’s absorbed by several pathways.
The main cause
of failure of infantile glaucoma filtration surgery is postoperative scar
formation[3]. The operator had took some measures
to inhibiting wound healing. First, mitomycin C was being applied to the
scleral bed for three minutes[20]. In addition,
we don’t use cautery during this surgery. Moreover, some high molecular weight
sodium hyaluronate should be injected under the first scleral flap, which can
decrease the risk of wound’s fibrosis after operation. That’s why this surgery
has an acute effect in reducing IOP and works quite well in infantile glaucoma.
Shallow filtration blebs were observed postoperatively in 10 cases, however,
bleb formation wasn’t crucial factors of IOP control. In this study, we found
that VCT is at least as successful in reducing pressure as multiple standard
procedures. After viscocanalostomy was being done, the baseline IOP (±SD) had
significantly decreased at the last follow-up visit for each patient
(paired-samples t-test, P<0.001). Our results presented a 100%
qualified success rate at 12mo, 68.6% at 30mo. Because of the non-compliant
patients, only 6 patients have completed 30mo of follow-up, and that’s why a
large drop in the success rate was noted from 24 to 30mo.
The advantage
of the procedures include that when viscocanalostomy was being done, we could
confirm the Schlemm’s canal’s locating. Second, high molecular weight sodium
hyaluronate was injected into the ostia of Schlemm’s canal each for five times
during the surgery so that the trabeculotome can be inserted into it smoothly.
Also some high molecular weight sodium hyaluronate should be injected into the
anterior chamber before trabeculotomy. Thus, it doesn’t lead to sharp decrease
of IOP, which can cause choroids detachment, shallow anterior chamber,
expulsive choroidal haemorrhage and some other severe complications[24].
However, there
are certain disadvantages. It may be that more time will be cost to do the
operation. More likely, VCT is more difficult than either goniotomy or
trabeculotomy ab externo in technique. The main difficulty encountered in VCT
is dissection of the scleral flap. Although we can dissect the superficial
scleral flap easily by an experienced doctor, but it’s difficult to grasp the
deeper flap’s thickness accurately. These infantile eyes’ limbal sclera is
relatively thinner than adults have. So two-thirds of scleral thickness was
hardly decided, we must dissect it carefully. So surgeon should invest time and
effort to overcome the relatively long learning curve associated with it.
Complications may also be higher when surgeons have no previous experience. The
most common intraoperative complication is trabeculo-Descemet's membrane
perforation, which essentially converts viscocanalostomy to a trabeculectomy.
Other studies
have reported delayed-onset hyphema, retinal detachment and endophthalmitis occured
in the surgery for pediatric glaucoma[25-26].
In contrast, our study showed that after taking VCT, only four cases developed
a spontaneously reabsorbed (within 48h) microhyphema, two cases had shallow
anterior chamber and recovered in 48h, two cases had an inadvertent small hole
in the Descemet’s membrane, others all had no complications. So it seems to be
a safer procedure for a child who has infantile glaucoma. Because of the less
risk of surgical complications, viscocanalostomy has gained more interests from
ophthalmologists.
In summary,
our study has demonstrated that VCT is effective and safe in controlling
infantile glaucoma, and it could be an alternative choice for infantile
glaucoma procedure in the future although the controlled studies with large
subject numbers and long follow-up period are needed.
ACKNOWLEDGEMENTS
Conflicts
of Interest: Qian CX, None; Zong Y, None; Chen Q, None; Yuan
ZL, None.
REFERENCES
1 Noureddin BN, El-Haibi CP, Cheikha A, Bashshur ZF.
Viscocan-alostomy versus trabeculotomyabexterno in primary congenital glaucoma:
1-year follow-up of a prospective controlled pilot study. Br J Ophthalmol 2006;90(10):1281-1285. [CrossRef]
2 Grieshaber MC, Peckar C, Pienaar A, Koerber N,
Stegmann R. Long-term results of up to 12 years of over 700 cases of
viscocanalostomy for open-angle glaucoma. Acta
Ophthalmol 2015;93(4):362-367. [CrossRef]
3 Al-Obeidan SA, Osman Eel D, Dewedar AS, Kestelyn P,
Mousa A. Efficacy and safety of deep sclerectomy in childhood glaucoma in Saudi
Arabia. Acta Ophthalmol 2014;92(1):65-70.
[CrossRef]
6 Cillino S, Casuccio A, Di Pace F, Cagini C, Ferraro
LL, Cillino G. Biodegradable collagen matrix implant versus mitomycin-C in
trabeculectomy: five-year follow-up. BMC
Ophthalmol 2016;16:24. [CrossRef]
7 Mandal AK, Chakrabarti D. Update on congenital
glaucoma. Indian J Ophthalmol 2011;59 Suppl:S148-157. [CrossRef]
8 Ozawa H, Yamane M, Inoue E, Yoshida-Uemura T,
Katagiri S, Yokoi T, Nishina S, Azuma N. Long-term surgical outcome of
conventional trabeculotomy for childhood glaucoma. Jpn J Ophthalmol 2017;61(3):237-244. [CrossRef]
9 Temkar S, Gupta S, Sihota R, Sharma R, Angmo D,
Pujari A, Dada T. Illuminated microcatheter circumferential trabeculotomy
versus combined trabeculotomy-trabeculectomy for primary congenital glaucoma: a
randomized controlled trial. Am J
Ophthalmol 2015;159(3):490-497.e2. [CrossRef]
10 Luntz MH, Livingston DG. Trabeculotomy ab externo
and trabeculectomy in congenital and adult-onset glaucoma. Am J Ophthalmol 1977;83(2):174-179. [CrossRef]
11 Draeger J. Surgical measures in congenital
glaucoma. Klin Monbl Augenheilkd 1993;202(5):425-427. [CrossRef]
12 Beck AD. Primary congenital glaucoma in the
developing world. Ophthalmology
2011;118(2):229-230. [CrossRef]
13 Papadopoulos M, Edmunds B, Fenerty C, Khaw PT.
Childhood glaucoma surgery in the 21st century. Eye (Lond) 2014;28(8):931-943. [CrossRef]
14 Shi Y, Wang H, Yin J, Zhang X, Li M, Xin C, Chen X,
Wang N. Outcomes of microcatheter-assisted trabeculotomy following failed angle
surgeries in primary congenital glaucoma. Eye
(Lond) 2017;31(1):132-139. [CrossRef]
15 Ishida K, Mandal AK, Netland PA. Glaucoma drainage
implants in pediatric patients. Ophthalmol
Clin North Am 2005;18(3):431-442. [CrossRef]
16 Yu Chan JY, Choy BN, Ng AL, Shum JW. Review on the
Management of Primary Congenital Glaucoma. J
Curr Glaucoma Pract 2015;9(3):92-99. [CrossRef]
17 Ben-Zion I, Tomkins O, Moore DB, Helveston EM.
Surgical results in the management of advanced primary congenital glaucoma in a
rural pediatric population. Ophthalmology
2011;118(2):231-235. [CrossRef]
20 Cheng JW, Cai JP, Li Y, Wei RL. Intraoperative
mitomycin C for nonpenetrating glaucoma surgery: a systematic review and
meta-analysis. J Glaucoma 2011;20(5):322-326.
[CrossRef]
21 Mendrinos E, Mermoud A, Shaarawy T. Nonpenetrating
glaucoma surgery. Surv Ophthalmol 2008;53(6):592-630. [CrossRef]
22 Nguyen C, Boldea RC, Roy S, Shaarawy T, Uffer S,
Mermoud A. Outflow mechanisms after deep sclerectomy with two different designs
of collagen implant in an animal model. Graefes
Arch Clin Exp Ophthalmol 2006;244(12):1659-1667. [CrossRef]
23 d'Epinay SL, Reme C. Histopathological aspects of
the surgical treatment of congenital glaucoma. Klin Monbl Augenheilkd 1980;176(4):
566-568. [CrossRef]
24 Coleman AL, Hill R, Wilson MR, Choplin N,
Kotas-Neumann R, Tam M, Bacharach J, Panek WC. Initial clinical experience with
the Ahmed glaucoma valve implant. Am J Ophthalmol
1995;120(1):23-31. [CrossRef]
25 Olayanju JA, Hassan MB, Hodge DO, Khanna CL.
Trabeculectomy-related complications in Olmsted County, Minnesota, 1985 through
2010. JAMA Ophthalmol 2015;133(5):574-580.
[CrossRef]
26 Medina CA, Butler MR, Deobhakta AA, Banitt MR,
Albini TA, Smiddy WE, Berrocal AM, Gedde SJ, Flynn HW Jr. Endophthalmitis
associated with glaucoma drainage implants. Ophthalmic
Surg Lasers Imaging Retina 2016;47(6):563-569. [CrossRef]