
·Clinical
Research· Current
Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
Ocular surface evaluation in eyes with chronic
glaucoma on long term topical antiglaucoma therapy
Manu Saini,
Murugesan Vanathi, Tanuj Dada, Tushar Agarwal, Rebika Dhiman, Sudarshan Khokhar
Cornea & Ocular Surface Services, Dr R P Centre
for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi
110029, India
Correspondence to: Murugesan Vanathi.
Cornea & Ocular Surface, Cataract & Refractive Services, Dr R P Centre
for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi
110029, India. vanathi_g@yahoo.com
Received: 2015-06-12
Accepted: 2016-06-20
Abstract
AIM: To evaluate ocular surface changes
and its correlation with the central corneal subbasal nerve fibre layer in
chronic glaucoma patients.
METHODS: A prospective comparative study
of ocular surface evaluation was performed in 50 eyes of 25 patients using two
or more antiglaucoma medications for at least 6mo and 50 eyes of 25 normal
subjects without any ocular problems as controls. The study parameters
evaluated included visual acuity, intraocular pressure, ocular surface
evaluation parameters [fluorescein break-up time (FTBUT), Schirmer’s I test,
ocular surface staining scores and ocular surface disease index score (OSDI)],
central corneal sensation (Cochet Bonnett aesthesiometer), central subbasal
nerve fiber layer density (SBNFLD) by confocal microscopy.
RESULTS: The mean values in the glaucoma
cases and control groups respectively were as follows: OSDI score
(35.89±16.07/6.02±3.84; P=0.001), Schirmer’s I test score (7.63±2.64
mm/12.86±1.93 mm; P=0.001), FTBUT (9.44±2.76s/11.8±1.88s; P=0.001),
corneal (5.7±2.33/ 1.1±0.58; P=0.001) and conjunctival staining score
(5.06±1.94/0.84±0.46; P=0.001), corneal sensitivity
(4.68±0.44/5.07±0.37; P=0.076), mean subbasal nerve fiber number
(3.58±0.99/5.40±1.70; P=0.001), SBNFL length (1101.44±287.56 μm/1963.70±562.56
μm; P=0.001)
and density (6883.94±1798.03 μm/mm2/12
273.15±3516.04 μm/mm2;
P=0.001). Dry eye severity of level 2 and 3 was seen in 66% of glaucoma
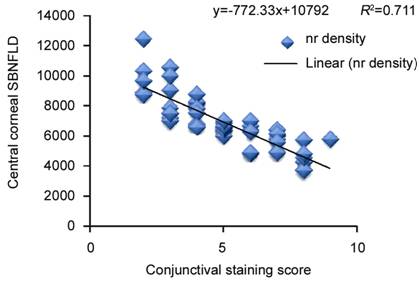
group. Corneal (R²=0.86) and conjunctival staining (R²=0.71) and
OSDI score (R²=0.67) showed statistically significant negative
correlation with central corneal SBNFLD while FTBUT (R²=0.84), corneal
sensitivity (R²=0.52) showed positive correlation to central corneal
SBNFLD in the long term topical antiglaucoma medication group.
CONCLUSION: Ocular surface changes and
antiglaucoma therapy induced dry eye is found to be associated with decreased
SBNFLD in eyes on long term topical antiglaucoma medications.
KEYWORDS: confocal
microscopy; glaucoma; ocular surface disease; subbasal nerve fiber layer;
therapy
DOI:10.18240/ijo.2017.06.16
Citation: Saini M. Vanathi M, Dada T, Agarwal T, Dhiman R, Khokhar S. Ocular
surface evaluation in eyes with chronic glaucoma on long term topical
antiglaucoma therapy. Int J Ophthalmol 2017;10(6):931-938
Article Outline
INTRODUCTION
Ocular surface side effects
occur due to chronic, long term use of antiglaucoma medications. Instillation
of topical antiglaucoma drops for a period of three or more months has been
found to cause significant subclinical inflammation, which has been detected as
increased expression of HLA-DR on conjunctival epithelial cells[1]. Pro-inflammatory cytokine secretion by conjunctival
cells has been noted to occur as a result of instillation of antiglaucoma eye
drops[2-4].
Topical medication related
ocular surface disease (OSD) results in worse symptoms, poorer compliance to
treatment, poor surgical results, and decreases the quality of life in glaucoma
patients[5-7]. The major side
effects include local allergic reactions, chronic conjunctival inflammation,
tear film abnormalities, corneal epitheliopathy, punctate epitheliopathy,
medically resistant herpetic keratitis, disruption of epithelial function,
chronic inflammatory infiltration, expression of inflammatory markers, impaired
wound healing, squamous metaplasia[8-9].
The most commonly used
antiglaucoma medications like timolol and latanoprost when used for a long term
lead to chronic ocular surface disease. Noted ocular adverse effects of
timolol-included corneal punctate erosions, burning sensation, hyperemia, tear film
alterations and corneal anesthesia. Topical latanoprost causes increased
pigmentation of the iris, hypertrichosis, hyperemia, allergic contact
dermatitis and cystoid macular edema[10].
Benzalkonium chloride (BAC,
quaternary ammonium compound), the most commonly used preservative in topical
antiglaucoma preparations has a slow turnover and the quaternary ammonium
molecules may be retained in the ocular tissues for as long as 168h after
application[11]. BAC promotes the activation of
lipooxygenases, synthesis and secretion of eicosanoids, inflammatory mediators
and many cytokines such as interleukin (IL)-1a, tumor necrosis factor, IL-8,
IL-10, resulting in irritation, delayed hypersensitivity and allergic reactions[12].
Delayed and prolonged effect
of BAC is because of incorporation and persistence of BAC molecules in cell
membranes[13]. This affects the lipid layer of
the tear film causing it’s instability[14]
thereby predisposing to inflammation of the ocular surface and conjunctival
metaplasia. In addition, preservatives have direct destructive effects on the
mucous gland, reducing the number of goblet cells and production of the
protective mucus layer[15]. Hence, the three
mechanisms of BAC toxicity include a detergent effect causing loss of tear film
stability, direct damage to the corneal/conjunctival epithelium and immune
allergic reaction[13].
Corneal innervation is vital
for the maintenance of corneal epithelial integrity, proliferation function and
in corneal wound healing after injury[15-16].
The subbasal nerve plexus along with stromal keratocytes secrete a number of
neuropeptides. These diffusible factors are believed to stimulate the
epithelial growth, proliferation, differentiation, the production of collagen
type VII, DNA synthesis, neurite survival and keratocyte proliferation[17-19]. Alterations in corneal
innervations impairs the wound healing ability of the epithelium and results in
dry eye[18-20].
Nerve degeneration that occurs
in the scenario of chronic ocular surface inflammation, as in cases of dry eye,
has been described to alter the subbasal nerve fiber layer (SBNFL)morphology[21]. The role of in vivo confocal microscopy in
ocular surface analysis of dry eye and glaucomatous patients has been
elucidated[20,22-27].
The rationale for the current study was to evaluate the correlation between the
ocular surface changes and central corneal subbasal nerve fibre layer changes
in cases of chronic glaucoma on long term medical control.
SUBJECTS
AND METHODS
A prospective comparative open
label study of ocular surface evaluation in 50 eyes of 25 patients using two or
more antiglaucoma medications for at least 6mo and 50 eyes of 25 normal
subjects without any ocular problems as controls was done. Patients on
follow-up with the glaucoma clinic (during the period of November 2011 to
November 2013) with chronic glaucoma on combination therapy with two or more
topical antiglaucoma medications with preservatives (timolol 0.5%, brimonidine
0.1%, latanoprost 0.005%) for at least six months or more and consenting to
participate in the study were included in the study. Patients with history of
intraocular surgery, laser treatment in recent six months, contact lens use,
autoimmune disease, recent ocular inflammation/injection, eyes with
trachomatous changes, dry eye related to other causes, previous or current use
of other ocular medications such as artificial tear therapy were excluded from
the study. Informed consent from
all the enrolled patients was taken and institute Ethics Committee approval was
sought and obtained.
Demographic
characteristics including age, gender, duration of therapy, and study
parameters data were noted on a predesigned proforma. Comprehensive ocular
examination, aided Snellen’s visual acuity, intraocular pressure (Goldman
applanation tonometry), ocular surface evaluation tests[28]
[tear break-up time (TBUT), Schirmer’s I test, ocular surface staining score[29], ocular surface disease index (OSDI), central corneal
sensation and in vivo scanning slit confocal microscopy of the central cornea],
dry eye severity (DEWS classification[30]) and
OSDI[31] were done. Corneal sensation threshold
measurement was done using Cochett Bonnet Anesthsiometer (CBA, Luneau, Paris,
France)[32] and measurement in both cases and
control groups was taken in morning hours, between temperature 20℃ and 25℃ on
the basis of out door patient services to avoid temperature variation and
diurnal bias. We didn’t asses any association in diabetic patients with loss of
corneal sensitivity, as our cases and control groups did not have retinopathy
and neuropathy clinical features. None of the enrolled subjects in our study
had neurodegenerative diseases, ruled out after complete systemic evaluation
In vivo
slit scanning confocal microscopy (ConfoScan 4, NIDEK Technologies, Padova,
Italy) of the central cornea was done in automatic gain mode using a standard
setting of 4 passes, with a scanning range of 200 µm to image the anterior
layers of the cornea i.e. epithelium, SBNFL, stromal keratocytes at 40×
magnifications[33]. If satisfactory images were
not obtained, procedure was repeated to get the desired images.
Each eye was scanned three
times through its entire depth and the two best images were selected for
analysis, of which the best one containing maximum number of SBNFL nerves
imaged was selected for analysis. SBNFL image analysis was done in a masked
manner using free downloadable custom NIH Image J software
(http://www.imagescience.org/meijering/software/neuronj/). The tracing of
subbasal nerves were performed using Neuron J, a semi-automatic Image J plugin
to facilitate the tracing and quantification of elongated image structures.
Then the total nerve number, total length/frame of subbasal nerves was measured
automatically (Figures 1 and 2).
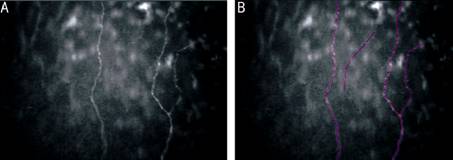
Figure
1 In vivo slit scanning confocal microscopy imaging of central cornea of
eye on long term antiglaucoma therapy A:
Subbasal nerve fiber layer; B: Tracing of the same using Neuron J (pink). Total
nerve number, length/frame of subbasal nerves were measured automatically by
Neuron J.
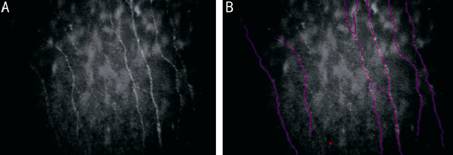
Figure
2 In vivo
slit scanning confocal microscopy imaging of central cornea of control eye A: Subbasal nerve fiber layer; B:
Tracing of the same using Neuron J (Pink). Neuron J, a semi-automatic Image J
plugin facilitates the quantification of these elongated nerve structures.
Nerve branches longer than 50 µm in length were
counted as separated nerves. The total number of subbasal nerves was recorded.
The mean subbasal nerve fiber layer density (SBNFLD) was calculated as total
length of all main nerves and their branches divided by area of standard frame
size containing images (460 µm×345 µm, area=0.16 mm²)[34]. Analysis of SNFL using custom software Neuron J was
repeated on the same images at one week interval to measure the repeatability
of the central corneal subbasal nerve layer parameters by the same observer
(Saini M) to calculate the intraclass correlation coefficient (ICC).
The basal cells in the
confocal images were identified in the scans manually and the density was
calculated using the inbuilt software (all cells that intersected the edges of
the frame of the image were not included in manual counting to avoid biasing
the results with poorly illuminated or poorly defined cells)[35].
Statistical
Analysis Statistical
analysis was done using the program SPSS version 15. Quantitative variables
(expressed as mean, standard deviation, range) were compared between
antiglaucoma medication group and control groups using two sample t-test
with P value <0.05 considered statistically significant. Correlation
between ocular surface evaluation parameters and SBNFLD was assessed using
Pearson correlation coefficient. ICC was calculated to estimate repeatability
of measurements between two occasions by the same observer at one week interval
(ICC for reproducibility was defined as: ≤0.4, poor; 0.4 to 0.75, fair to good;
≥0.75, excellent[36]). Bland Altman plot
summarized the agreement between the 2 data sets.
RESULTS
The demographic
characteristics of enrolled eyes are shown in Table 1.
Table 1
Demoraphic characteristics of eyes on antiglaucoma therapy and controls
|
Demographic data |
Antiglaucoma therapy
group |
Controls |
P |
|
No. of eyes |
50 |
50 |
- |
|
Gender (M/F) |
29/21 |
38/12 |
0.056 |
|
Mean age (mean±SD, range) |
49.42±16.98 (22-75)a |
40.68±13.73 (26-65)a |
0.031 |
|
Treatement duration (mean±SD,
range) |
3.61±2.88 (0.6-12)a |
- |
- |
Of the 50 eyes on long term
topical antiglaucoma therapy enrolled, 10 eyes were on combination of BAC
preservative containing timolol 0.5% (one drop twice daily) and brimonidine
0.2% (one drop twice daily), 8 eyes on latanoprost 0.005% and brimonidine 0.2%
(one drop twice daily) and 32 eyes on combination of timolol 0.5% (one drop
twice daily) and latanoprost 0.005%. The 26 eyes had primary open angle
glaucoma (POAG), 7 eyes had primary angle closure glaucoma (PACG), 6 eyes had
juvenile open angle glaucoma (JOAG), 4 eyes had ocular hypertension, 4 eyes had
normal tension glaucoma (NTG) and 3 eyes had mixed mechanism glaucoma. The
control group comprised of 50 normal eyes of 25 subjects (age and sex matched)
with normal eyes.
The mean values of the ocular
surface evaluation tests (OSDI score, Schirmer’s I test, fluorescein breakup
time, conjunctival and corneal staining score) showed statistical significant
difference between the antiglaucoma group and controls (Table 2).
Table 2 Depicting results of ocular
surface evaluation tests analysed in long term antiglaucoma medication group vs
control group
mean±SD
(range)
|
Ocular surface evaluation tests |
Antiglaucoma eyes |
Control eyes |
P |
|
Mean OSDI (score) |
35.89±16.07 (5.54-67) |
6.02±3.84 (2.21-9.85) |
0.001 |
|
Schirmer’s I test (mm/5min) |
7.63±2.64 (3-12) |
12.86±1.93 (10-16) |
0.001 |
|
FTBUT (s) |
9.44±2.76 (4.3-15.72) |
11.80±1.88 (9-15.26 ) |
0.001 |
|
Corneal staining score |
5.7±2.33 (2-9) |
1.1±0.58 (0-2) |
0.001 |
|
Conjunctival staining score |
5.06±1.94 (2-9) |
0.84±0.46 (0-2) |
0.001 |
Dry eye disease (as per DEWS
classification[29]) of level 1 severity in 34% (n=17
eyes); levels 2 and 3 severity in 66% (n=33 eyes) was in the
antiglaucoma therapy group. Level 1 severity 42% (n=21) was seen in
control eyes.
Density of basal epithelial
cells was found to be increased in antiglaucoma therapy group 4796.619±647.1526
cells/mm² compared to that of the control group 3926.819±571.8765 cells/mm² (P=0.0004).
SBNFL number, length and density showed significant decrease in chronic
glaucoma eyes compared to that of the controls (Table 3). Central corneal
sensation threshold in antiglaucoma eyes was found to be decreased as compared
to the controls (P=0.076).
Table 3 Mean values of central
corneal SBNFL parameters in eyes on long term antiglaucoma therapy and controls
mean±SD
(range)
|
SBNFLD |
Antiglaucoma eyes |
Control eyes |
P |
|
Nerve number |
3.58±0.99 (2-6) |
5.40±1.70 (3-10) |
0.001 |
|
Nerve length |
1101.44±287.64
(599.09-1990) |
1963.70±562.56
(739.36-2697.74) |
0.001 |
|
Nerve density |
6883.94±1798.03
(3700-8757.5) |
12 273.15±3516.04
(4621-19 351.96) |
0.001 |
|
Corneal sensitivity |
4.68±0.44 (4-5.5) |
5.07±0.37 (4-5.5) |
0.076 |
The OSDI score and ocular surface staining scores
showed a statistically significant negative correlation with central corneal
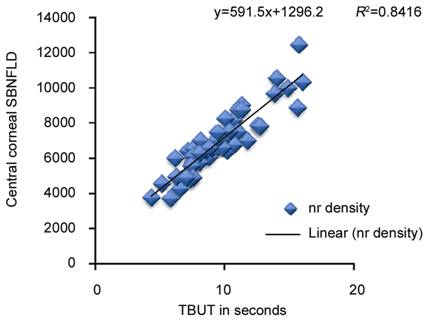
SBNFL in the antiglaucoma medication eyes (Figures 3-5). TBUT and central
corneal SBNFLD showed a strong, statistically significant positive correlation
(Figure 6), central corneal sensitivity also showed a good positive correlation
with SBNFLD (Figure 7). Schirmer’s I values did not show a significant
correlation (Figure 8).
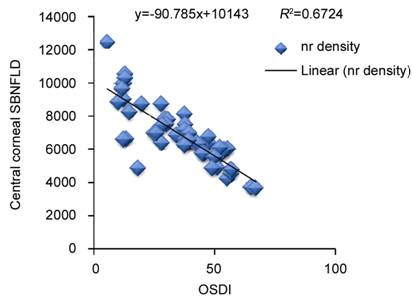
Figure
3 Correlation of OSDI scores with central corneal subbasal nerve (nr) fiber
density in eyes on longterm antiglaucoma therapy.
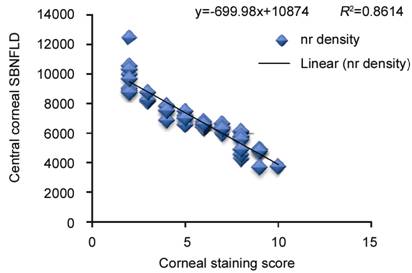
Figure
4 Correlation of corneal staining score with central corneal subbasal nerve
(nr) fiber density in eyes on longterm antiglaucoma therapy.
Figure
5 Correlation of conjunctival staining score with central corneal subbasal
nerve (nr) fiber density in eyes on longterm antiglaucoma therapy.
Figure
6 Correlation of TBUT with central corneal subbasal nerve (nr) fiber layer
density in eyes on longterm antiglaucoma medication therapy.
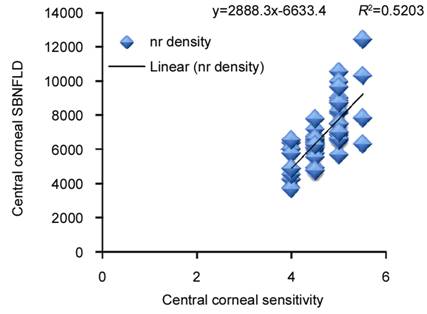
Figure
7 Correlation of central corneal sensation threshold with subbasal nerve (nr)
fiber density in eyes on longterm antiglaucoma therapy.
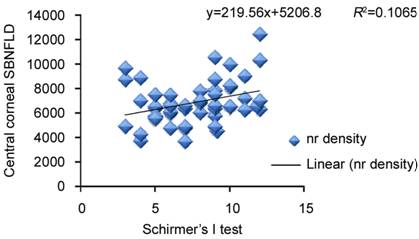
Figure
8 Correlation of Schirmer’s I test with central corneal sub-basal nerve (nr)
fiber density in eyes on longterm antiglaucoma therapy.
Intraobserver repeatability for in vivo
confocal microscopy analysis of the SBNFL (nerve number, nerve length, nerve
density) and ICC values given in Table 4 was calculated for both groups to
measures the reproducibility of SBNFL measurements.
Table 4 Intraclass correlation
coefficient (ICC) for single image analysis in antiglaucoma medication group
and control group
|
Variables |
Control group |
Antiglaucoma medication
group |
||||||
|
Observer 1 |
Observer 2 |
ICC |
P |
Observer 1 |
Observer 2 |
ICC |
P |
|
|
Nerve number |
5.40±1.70 |
5.32±1.54 |
0.98 |
0.15 |
3.58±0.99 |
3.88±0.76 |
0.63 |
0.02 |
|
Nerve length |
1963.70±562.56 |
1964.01±562.26 |
0.99 |
0.43 |
1101.44±287.64 |
1099.24±281.91 |
0.99 |
0.68 |
|
Nerve density |
12 273.15±3516.04 |
12 275.29±3514.274 |
0.99 |
0.39 |
6883.94±1798.03 |
6870.24±1761.94 |
0.99 |
0.69 |
P value was found to be insignificant
for subbasal nerve fiber length and density indicating good repeatability of
measurements at two separate occasions.
The mean values were plotted against the differences
between the measurements and the upper and lower limits of agreements (limits
of agreement 1.96±SD) were obtained by Bland and Altman[37]
to appreciate the between occasion agreement as depicted in Figures 9 and 10.
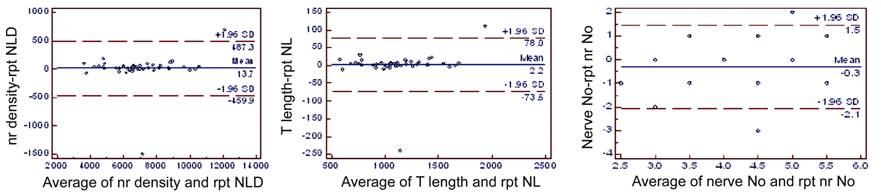
Figure
9 Bland-Altman plots for subbasal fiber layer density, length and number in
eyes on antiglaucoma treatmemt indicating agreement between two separate
measurements at different instances of the same eye, by same observer rpt: Repeat.

Figure
10 Bland-Altman plots for subbasal nerve fiber layer density, length, nerve
number in eyes in the control group, indicating agreement between two seperate
measurements at different instances of the same eye, by same observer rpt: Repeat.
DISCUSSION
Altered epithelial barrier function leads to exposure
of corneal nerve ending to environment stimuli causing irritation, unstable
tear film resulting in ocular surface epithelial changes, compromised visual
function[37-40]. The
prevalence of ocular surface disease of 59% has been reported in glaucoma cases
with higher prevalence in patients using BAC containing antiglaucoma
medications [41]. The frequency of eye symptoms
and signs of ocular surface irritation are higher in patients treated with
preserved than preservative-free eye drops[42].
Our study also found a similar prevalence of dry eye in 66% (levels 2 and 3) in
our cases. Patients enrolled in our study were using combination therapy for
mean duration of 0.61±2.88y (range 0.6-12y).
Corneal epithelial cells and stromal innervations
influence corneal trophism and contribute to the maintenance of a healthy
corneal surface. Alteration in corneal innervation will affect epithelial
healing abilities and results in development of dry eye[17].
A complex relationship seems to exist between ocular surface changes, dry eye
disease and decreased SBNFL in eyes on chronic ocular hypotensive treatment. Our evaluation
of ocular surface changes in eyes with chronic glaucoma on long term topical
antiglaucoma medications showed statistical significant differences in ocular
surface evaluation parameters. Central corneal in vivo confocal
microscopic examination showed a statistical significant decrease in central
corneal SBNFL nerve number, length and density and corresponding decrease in
corneal sensitivity. Density of the basal epithelial cells was significantly
increased in the eyes with chronic ocular hypotensive medications as compared
to that in the control group. Central corneal sensation threshold was observed
to be decreased in antiglaucoma medication group with corresponding decrease in
SBNFLD (not statistically significant). This can probably be attributed to the
inherent practical difficulty in the placement, positioning and force
application of the nylon filament of the the Cochet Bonnet anesthesiometer.
The eyes on long term antiglaucoma therapy with
reduced SBNFLD can probably still appreciate corneal sensations, but at higher
stimulus intensity. The SBNFLD at which the threshold of corneal sensitivity
become significantly decreased is not known.
Normal central cornea SBNFLD observed with NIDEK
ConfoScan 4 in our study was 12 273.15±3516.04 µm/mm². Patel et al[43] reported a SBNFLD of 14 731±6056 µm/mm². The
variation in central corneal SBNFLD noted in different studies was because of
difference in the methodologies adopted in computing the corneal nerve length.
Subbasal corneal nerve layer, keratocyte density and
endothelial characteristics in ocular hypertensive patients with and without
therapy has been studied earlier[27], in which
the medication group were found to have lower SBNFL nerve number and density.
Similar results were also noted in other studies[20,44]. Our study results also concur with their
observations.
Our study also analyzed the correlation between the
ocular surface changes in the antiglaucoma therapy eyes with their central
corneal SBNFLD. We observed a strong negative correlation of FTBUT, OSDI score
and ocular surface staining scores with decreased central corneal confocal
SBNFLD, indicating that ocular surface changes due to chronic antiglaucoma
therapy with preservative containing ocular hypotensives does result in a
proportionate damage to the SBNFL of the cornea. As all patients of the
antiglaucoma treatment group in our study were asymptomatic, further molecular
level analysis can perhaps help to establish the cause effect relationship. A
large sample size would have helped to establish the relation of decreased
SBNFLD with the duration.
In conclusion our study shows that long term
antiglaucoma medication with preservatives results in significant alteration of
ocular surface parameters producing ocular surface morbidity. SBNFLD is
decreased in these eyes due to long term preservative containing antiglaucoma
therapy with significant correlation to ocular surface staining scores, FTBUT
and OSDI values. Concurrent topical lubricant and anti-inflammatory treatment
may be considered in cases of chronic preservative containing antiglaucoma
therapy showing ocular surface changes.
ACKNOWLEDGEMENTS
Foundation: Supported by the
Institute Research Grant of All India Institute of Medical Sciences, New Delhi
110029, India.
Conflicts of Interest: Saini M, None; Vanathi M, None;
Dada T, None; Agarwal T, None; Dhiman R, None; Khokhar S, None.
REFERENCES
1 Arici MK, Arici DS, Topalkara A, Guler C. Adverse effects of
topical antiglaucoma drugs on the ocular surface. <ii>Clin Exp Ophthalmol
</ii>2000;28(2):113-117. [CrossRef] [PubMed]
2 Malvitte L,
Montange T, Vejux A, Baudouin C, Bron AM, Creuzot-Garcher C, Lizard G.
Measurement of inflammatory cytokines by multicytokine assay in tears of
patients with glaucoma topically treated with chronic drugs<ii>. Br J
Ophthalmol </ii>2007;91(1):29-32. [CrossRef] [PMC free article]
[PubMed]
3 Baudouin C,
Pisella PJ, Fillacier K, Goldschild M, Bacquet F, De Saint Jean M, Bechetoille
A. Ocular surface inflammatory changes induced by topical antiglaucoma drugs.
<ii>Ophthalmology </ii>1999;106(3):556-563. [CrossRef]
<no>4
Ariturk N, Oge I, Baris S, Erkan D, Sullu Y, Koc F. The effects of
antiglaucomatous agents on conjunctiva used for various durations.
<ii>Int Ophthalmol</ii> 1996-1997;20(1-3):57-62.</no>
5 Pisella PJ,
Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved
and preservative-free glaucoma medication<ii>. Br J Ophthalmol
</ii>2002;86(4):418-423. [CrossRef]
6 Nordmann JP,
Auzanneau N, Ricard S, Berdeaux G. Vision related quality of life and topical
glaucoma treatment side effects<ii>. Health Qual Life Outcomes
</ii>2003;1:75. [CrossRef]
[PMC free article]
[PubMed]
7 Broadway DC,
Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma
medication. The outcome of filtration surgery. <ii>Arch Ophthalmol</ii>
1994;112(11):1446-1454. [CrossRef] [PubMed]
<no>8
Kuppens EV, Stolwijk TR, de Keizer RJ, van Best JA. Basal tear turnover and
topical timolol in glaucoma patients and healthy controls by fluorophotometry.
<ii>Invest Ophthalmol Vis Sci
</ii>1992;33(12):3442-3448.</no>
9 Reidy JJ,
Zarzour J, Thompson HW, Beuerman RW. Effect of topical beta-blockers on corneal
epithelial wound healing in the rabbit<ii>. Br J Ophthalmol
</ii>1994;78(5):377-380. [CrossRef]
<no>10
Pandey AN, Sujata S. Study of long term structural and functional changes in
medically controlled glaucoma. <ii>Int J Ophthalmol </ii>2014;7(1):
128-132.</no>
<no>11
Champeau EJ, Edelhauser HF. Effect of ophthalmic preservatives on the ocular surface:
conjunctival and corneal uptake and distribution of benzalkonium chloride and
chlorhexidine digluconate. In: Holly F, Lamberts D, Mac Keen D, editors.
<ii>The preocular tear film in health, disease, and contact lens
wear</ii>. Lubbock, Texas: Dry Eye Institute Inc. 998:292-302.</no>
12 De Saint
Jean M, Debbasch C, Brignole F, Rat P, Warnet JM, Baudouin C. Toxicity of
preserved and unpreserved antiglaucoma topical drugs in an in vitro model of
conjunctival cells. <ii>Curr Eye Res </ii>2000;20(2):85-94. [CrossRef]
13 Yee RW. The
effect of drop vehicle on the efficacy and side effects of topical glaucoma
therapy: a review. <ii>Curr Opin Ophthalmol </ii>2007;18(2):
134-139. [CrossRef]
[PubMed]
<no>14
Detry-Morel M. Side effects of glaucoma medications. <ii>Bull Soc Belge
Ophtalmol</ii> 2006;299:27-40.</no>
15 Herreras
JM, Pastor JC, Calonge M, Asensio VM. Ocular surface alteration after long-term
treatment with an antiglaucomatous drug. <ii>Ophthalmology</ii>
1992;99(7):1082-1088. [CrossRef]
16 Muller LJ,
Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structures, contents and
function. <ii>Exp Eye Res</ii> 2003;76(5):521-542. [CrossRef]
17 Benitez del
Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal
masked study on corneal epithelium and subbasal nerves in patients with dry
eye. <ii>Invest Ophthalmol Vis Sci </ii>2004;45(9):3030-3035. [CrossRef] [PubMed]
18
Garcia-Hirschfeld J, Lopez-Briones L, Belmonte C. Neurotrophic influences on
corneal epithelial cells. <ii>Exp Eye Res </ii> 1994;59(5):597-605.
[CrossRef] [PubMed]
<no>19
Baker KS, Anderson SC, Romanowski EG, Thoft RA, SundarRaj N. Trigeminal
ganglion neurons affect corneal epithelial phenotype: influence on type VII
collagen expression in vitro. <ii>Invest Ophthalmol Vis Sci
</ii>1993;34 (1):137-144.</no>
20 Martone G,
Frezzotti P, Tosi GM, Traversi C, Mittica V, Malandrini A, Pichierri P,
Balestrazzi A, Motolese PA, Motolese I, Motolese E. An in vivo confocal
microscopy analysis of effects of topical antiglaucoma therapy with
preservative on corneal innervation and morphology. <ii>Am J
Ophthalmol</ii> 2009;147(4):725-735. [CrossRef] [PubMed]
21
Benítez-Del-Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D, Gegúndez JA,
Fernandez C, García-Sánchez J. Relation between corneal innervation with
confocal microscopy and corneal sensitivity with noncontact esthesiometry in
patients with dry eye. <ii>Invest Ophthalmol Vis Sci</ii>
2007;48(1):173-181. [CrossRef]
[PubMed]
22 Qazi Y,
Aggarwal S, Hamrah P. Image-guided evaluation and monitoring of treatment
response in patients with dry eye disease. <ii>Graefes Arch Clin Exp
Ophthalmol</ii> 2014;252(6):857-872. [CrossRef] [PMC free article]
[PubMed]
23 Villani E,
Baudouin C, Efron N, Hamrah P, Kojima T, Patel SV, Pflugfelder SC, Zhivov A,
Dogru M. In vivo confocal microscopy of the ocular surface: from bench to
bedside. <ii>Curr Eye Res</ii> 2014;39(3):213-231. [CrossRef] [PMC free article]
[PubMed]
24
Mastropasqua L, Agnifili L, Mastropasqua R, Fasanella V, Nubile M, Toto L,
Carpineto P, Ciancaglini M. In vivo laser scanning confocal microscopy of the
ocular surface in glaucoma. <ii>Microsc Microanal</ii>
2014;20(3):879-894. [CrossRef]
[PubMed]
25
Mastropasqua L, Agnifili L, Fasanella V, Curcio C, Ciabattoni C, Mastropasqua
R, Toto L, Ciancaglini M. Conjunctival goblet cells density and
preservative-free tafluprost therapy for glaucoma: an in vivo confocal
microscopy and impression cytology study<ii>. Acta Ophthalmol </ii>
2013;91(5):e397-e405. [CrossRef]
[PubMed]
26 Agnifili L,
Fasanella V, Costagliola C, Ciabattoni C, Mastropasqua R, Frezzotti P,
Mastropasqua L. In vivo confocal microscopy of meibomian glands in
glaucoma<ii>. Br J Ophthalmol</ii> 2013;97(3):343-349. [CrossRef] [PubMed]
27 Baratz KH,
Nau CB, Winter EJ, McLaren JW, Hodge DO, Herman DC, Bourne WM. Effects of
glaucoma medications on corneal endothelium, keratocytes and subbasal nerves
among participants in the ocular hypertension treatment study<ii>. Cornea
</ii>2006;25(9):1046-1052. [CrossRef] [PubMed]
<no>28
Asbell PA, Lemp MA. <ii>Dry eye disease: The clinician’s guide to
diagnosis and treatment </ii>2009:39-44.</no>
<no>29
Lemp MA. National eye institute/industry workshop on clinical trials in dry
eyes. <ii>CLAO </ii>1995;21(4):221-232.</no>
30 Behrens A,
Doyle J, Stern L, Chuck RS, Mc Donnell PJ, Azar DT, Dua HS, Hom M, Karpecki PM,
Laibson PR, Lemp MA, Meisler DM, Del Castillo JM, O'Brien TP, Pflugfelder SC,
Rolando M, Schein OD, Seitz B, Tseng SC, van Setten G, Wilson SE, Yiu SC.
Dysfunctional tear syndrome: a Delphi approach to treatment recommendations.
<ii>Cornea </ii>2006;25(8):900-907. [CrossRef] [PubMed]
31 Schiffman
RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity
of the Ocular Surface Disease Index. <ii>Arch Ophthalmol
</ii>2000;118(5):615-621. [CrossRef]
32 Murphy PJ,
Lawrenson JG, Patel S, Marshall J. Reliability of the non-contact corneal
aesthesiometer and its comparison with the Cochet-Bonnet aesthesiometer.
<ii>Ophthalmic Physiol Opt</ii> 1998;18(6):532-539. [CrossRef]
<no>33
Vanathi M, Tandon R, Sharma N, Titiyal JS, Pandey RM, Vajpayee RB. In-vivo slit
scanning confocal microscopy of normal corneas in Indian eyes. <ii>Indian
J Ophthalmol </ii>2003;51(3):225-230.</no>
34
Oliveira-Soto LM, Efron N. Morphology of cornea nerves using confocal
microscopy. <ii>Cornea </ii>2001;20(4):374-384. [CrossRef]
35 Eckard A,
Stave J, Guthoff RF. In vivo investigations of the corneal epithelium with the
confocalrostock laser scanning microscope. <ii>Cornea
</ii>2006;25(2):127-131. [CrossRef]
36 Kim G,
Singleton JR, Mifflin MD, Digre KB, Porzio MT, Smith AG. Assessing the
reproducibility of quantitative in vivo confocal microscopy of corneal nerves
in different corneal locations. <ii>Cornea </ii>2013;
32(10):1331-1338. [CrossRef]
[PubMed]
37 Bland JM,
Altman DG. A note on the use of the intraclass correlation coefficient in the
evaluation of agreement between two methods of measurement. <ii>Comput
Biol Med</ii> 1990;20(5):337-340. [CrossRef]
38 Rolando M,
Iester M, Macrí A, Calabria G. Low spatial-contrast sensitivity in dry eyes.
<ii>Cornea</ii> 1998;17(4):376-379. [CrossRef]
<no>39
Tutt R, Bradley A, Begley C, Thibos LN. Optical and visual impact of tear
break-up in human eyes. <ii>Invest Ophthalmol Vis Sci</ii>
2000;41(13): 4117-4123.</no>
40 Montés-Micó
R, Alió JL, Charman WN. Dynamic changes in the tear film in dry eyes.
<ii>Invest Ophthalmol Vis Sci</ii> 2005;46(5):1615-1619. [CrossRef] [PubMed]
41 Leung EA,
Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma
patients. <ii>J Glaucoma</ii> 2008;17(5):350-355. [CrossRef] [PubMed]
42 Baudouin C.
Allergic reaction to topical eye drops. <ii>Curr Opin Allergy Clin
Immunol</ii> 2005;5(5):459-463. [CrossRef]
43 Patel DV,
Tavakoli M, Craig JP, Efron N, McGhee CN. Corneal sensitivity and slit scanning
in vivo confocal microscopy of the subbasal nerve plexus of the normal central
and peripheral human cornea. <ii>Cornea </ii>2009;28:735-740. [CrossRef] [PubMed]
44 Ranno S,
Fogagnolo P, Rossetti L, Orzalesi N, Nucci P. Changes in corneal parameters in
treated glaucoma patients. <ii>Clin Ophthalmol </ii>2011;5:
1037-1042. [CrossRef] [PMC free article]
[PubMed]