
·Letter to the Editor·Current Issue·
·Achieve·
·Search Articles· ·Online Submission· ·About IJO· PMC
Citation: Lee SM, Jung JW, Park SW, Lee JE, Byon IS.
Retinal injury following intravitreal injection of a dexamethasone implant in a
vitrectomized eye. Int J Ophthalmol 2017;10(6):1019-1020
Retinal
injury following intravitreal injection of a dexamethasone implant in a
vitrectomized eye
Seung
Min Lee1,2, Jae Woo Jung1,2, Sung Who Park2,3,
Ji Eun Lee2,3, Ik Soo Byon1,2
1Research
Institute for Convergence of Biomedical Science and Technology, Pusan National
University Yangsan Hospital, Yangsan 50612, Korea
2Department
of Ophthalmology, College of Medicine, Pusan National University, Yangsan
50612, Korea
3Biomedical
Research Institute, Pusan National University Hospital, Busan 49241, Korea
Correspondence
to: Ik Soo Byon. Pusan National University Yangsan Hospital, 20,
Geumo-ro, Mulgeum-eup, Yangsan 50612, Korea. isbyon@naver.com
Received: 2016-05-02
Accepted: 2016-09-05
DOI:10.18240/ijo.2017.06.31
Citation: Lee SM, Jung JW, Park SW, Lee JE, Byon IS.
Retinal injury following intravitreal injection of a dexamethasone implant in a
vitrectomized eye. Int J Ophthalmol 2017;10(6):1019-1020
Dear Editor,
Ozurdex® (Allergan,
Inc, Irvine, CA, USA) is a sustained- release dexamethasone implant and is an
approved therapy for several types of macular edema (ME) and for treatment of
inflammation associated with non-infectious uveitis. Common adverse effects
from Ozurdex® insertion include increased intraocular pressure and
cataract progression[1-2]; however other ocular complications, such
as crystalline lens trauma, could develop[3-4]. In this case report,
we describe the accidental retinal injury following intravitreal injection of
Ozurdex® into a vitrectomized eye with branch retinal vein occlusion
(BRVO) and ME.
CASE PRESENTATION
A 73-year-old woman with
controlled hypertension presented with sudden visual loss in the right eye.
Right eye visual acuity was counting fingers and examination of the fundus
revealed vitreous hemorrhage and asteroid hyalosis. Par plana vitrectomy was
performed, with concurrent cataract surgery and laser photocoagulation, because
of the discovery during the operation of an attenuated retinal vein and
neovascular membrane in the mid-peripheral retina (Figure 1). The patient was
diagnosed with vitreous hemorrhage secondary to BRVO. Her vision had improved
to 20/25 3mo after surgery. At five months post-surgery, the patient complained
of gradual vision loss in the vitrectomized eye and a decline in vision to
20/50. Fundus photography revealed attenuation of retinal vasculature and a
laser photocoagulation scar in the superotemporal quadrant area. No evidence of
vitreous hemorrhage was seen (Figure 2A), but ME was detected. Swept source
optical coherence tomography (SS-OCT, Atlantis-OCT, Topcon, Tokyo, Japan)
established that macular thickness had increased to 401 μm because of the
presence of intraretinal fluid (Figure 2B). The patient was diagnosed with ME
resulting from BRVO. Ozurdex® was delivered by intravitreal
injection through the superotemporal sclera, 3.5 mm away from the limbus, and
positioned in the peripheral retina anterior to the equator. Fundus examination
determined that the Ozurdex® implant had lodged in the retinal
tissue (Figure 2C). Prophylactic laser photocoagulation was performed to
prevent retinal detachment. One month later, the patient’s vision had improved
to 20/32. Macular thickness had decreased to 304 μm and intraretinal fluid had
disappeared. Three months after Ozurdex® injection, the implant had
disappeared and a laser scar around bare sclera was seen (Figure 2D). The
retina had not detached.
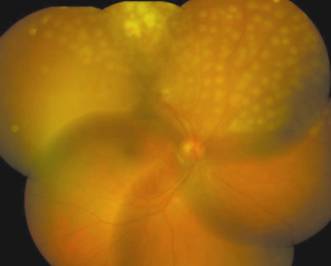
Figure 1
Three days after surgery, the vitreous hemorrhage had resolved Removed
neovascular membrane, coagulated retinal vessel and retinal burns from laser
photocoagulation were observed.
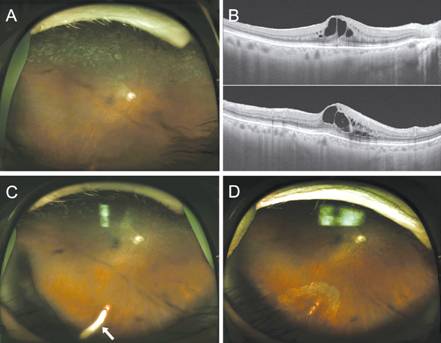
Figure 2
Before and after Ozurdex® implant injection A: A wide-field fundus photograph
showing photocoagulation scars in the area of the superior BRVO; B:
Swept-source optical coherence tomography image showing cystoid ME; C:
Following injection, the Ozurdex® implant was observed lodged in the
inferior retina (white arrow); D: Three months later, implant had disappeared
and a barrier photocoagulation scar around the bare sclera was seen. Retinal
detachment did not occur.
DISCUSSION
Accidental injection
of an Ozurdex® implant into retinal tissue is a rare and unexpected
complication. Physicians normally inject intraocular drugs or implants into the
central vitreous cavity for the treatment of vitreoretinal diseases, as these
products are designed not to reach the retinal tissue. Injected Ozurdex®
implant is generally settled on the inferior vitreous cavity even in
vitrectomized eye. However, in this case, retinal injury did occur following
intravitreal Ozurdex® injection in a patient presenting with BRVO
and ME. It is possible that prior vitrectomy contributed to the retinal injury
observed. Vitreous humor, a transparent, gelatinous tissue that fills the eye
cavity, is composed of 98%-99% water and factors that contribute to its viscous
nature, including collagen fiber, hyaluronin, and opticin[5]. Its
viscosity is higher than that of water and balanced salt solution, and is
affected by a number of factors, including age, eye axial length, and prior
intraocular surgery. Lee et al[6] reported that the viscosity
of human vitreous fluid was 300-2000 centipoise (cP), compared to a value of 1
cP for water. The viscosity of vitreous humor provides resistance against the
projectile velocity of an intraocular injection. Upon injection of an Ozurdex®
implant into a vitrectomized eye, intraocular resistance may be lower than
expected, and injection velocity may be higher, which may cause the Ozurdex®
implant to lodge in the retinal tissue. It is possible in this instance that
the Ozurdex® implant was injected too anteriorly, rather than into
the central vitreous cavity. It has been reported previously that injection of
an Ozurdex® implant too anteriorly may cause complications such as
crystalline lens trauma[3-4]. It is also possible that the
peripheral retinal damage, rather than lens trauma, occurred because the
patient had a pseudophakic eye.
To prevent the
accidental retinal damage following intravitreal injection of Ozurdex®
implant, it should be injected posteriorly to secure over 15 mm distance in
vitreous cavity. Panjaphongse et al[7] reported that Ozurdex®
implant could travel 15 mm in the vitrectomized eyes. In case of performing
vitrectomy, it could be helpful to save the anterior vitreous for the patients
receiving Ozurdex® implant later.
Prompt ocular examination using
indirect ophthalmoscopy is crucial for post-injection management. Although
retinal injury did develop following the Ozurdex® injection, severe
ocular complications, such as retinal detachment, were avoided because of
immediate ocular examination and prophylactic laser photocoagulation treatment.
In summary, intraocular Ozurdex®
injection into a vitrectomized eye can result in accidental retinal injury. The
ignorance for the kinematics of Ozurdex® injection in the
vitrectomized eyes as well as the design of injectable drug delivery device may
contribute to this accidental retinal injury. Prompt ocular examination and
laser photocoagulation following the intravitreal injection are important for
preventing subsequent ocular complications.
ACKNOWLEDGEMENTS
Conflicts of
Interest: Lee SM, None; Jung JW, None; Park SW, None;
Lee JE, None; Byon IS, None.
REFERENCES
1 Haller JA, Bandello
F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH,
Jacques ML, Jiao J, Li XY, Whitcup SM; OZURDEX GENEVA Study Group. Randomized, sham-controlled
trial of dexamethasone intravitreal implant in patients with macular edema due
to retinal vein occlusion. <ii>Ophthalmology</ii>
2010;117(6):1134-1146. [CrossRef]
[PubMed]
2 Heng LZ, Sivaprasad
S, Crosby-Nwaobi R, Saihan Z, Karampelas M, Bunce C, Peto T, Hykin PG. A
prospective randomised controlled clinical trial comparing a combination of
repeated intravitreal Ozurdex and macular laser therapy versus macular laser
only in centre-involving diabetic macular oedema (OZLASE study). <ii>Br J
Ophthalmol </ii> 2016;100(6):802-807. [CrossRef] [PubMed]
3 Berarducci A, Sian
IS, Ling R. Inadvertent dexamethasone implant injection into the lens body
management. <ii>Eur J Ophthalmol </ii> 2014;24(4): 620-622. [CrossRef] [PubMed]
4 Coca-Robinot J,
Casco-Silva B, Armadá-Maresca F, García-Martínez J. Accidental injections of
dexamethasone intravitreal implant (Ozurdex) into the crystalline
lens.<ii> Eur J Ophthalmol </ii> 2014;24(4):633-636. [CrossRef] [PubMed]
5 Bishop P. The
biochemical structure of the mammalian vitreous.<ii> Eye(Lond)
</ii> 1996;10(Pt 6):664-670. [CrossRef] [PubMed]
6 Lee B, Litt M,
Buchsbaum G. Rheology of the vitreous body. Part I: Viscoelasticity of human
vitreous. <ii>Biorheology </ii>1992;29(5-6):521-533. [PubMed]
7 Panjaphongse R, Liu
W, Pongsachareonnont P, Stewart JM. Kinematic study of ozurdex injection in
balanced salt solution: modeling the behavior of an injectable drug delivery
device in vitrectomized eyes. <ii>J Ocul Pharmacol Ther
</ii>2015;31(3):174-178. [CrossRef]
[PubMed]