
·Basic Research· Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
Comparative analysis of
different feeder layers with 3T3 fibroblasts for culturing rabbits limbal stem
cells
Hui-Xian Wang1,2,
Xiao-Wei Gao2, Bing Ren2, Yan Cai2, Wen-Jing
Li2, Yu-Li Yang3, Yi-Jian Li3
1Medical College of Shihezi University, Shihezi 832000, Xinjiang Uygur
Autonomous Region, China
2Ophthalmic Center, No.474 Hospital of Chinese PLA, Urumqi 830013,
Xinjiang Uygur Autonomous Region, China
3Southwest Hospital, Third Military Medical University, Chongqing
400038, China
Correspondence to: Xiao-Wei Gao. Ophthalmic Center, No.474 Hospital of Chinese PLA,
Urumqi 830013, Xinjiang Uygur Autonomous Region, China. gxwgaoxw@263.net
Received: 2016-08-10
Accepted: 2017-03-31
Abstract
AIM: To explore
the possibility of human umbilical cord mesenchymal stem cells (hUCMSCs), human
umbilical vein endothelial cells (hUVECs), human dental pulp stem cells
(hDPSCs) and human periodontal ligament stem cells (hPDLSCs) serving as feeder
cells in co-culture systems for the cultivation of limbal stem cells.
METHODS:
Different feeder layers were cultured in Dulbecco’s modified Eagle’s medium
(DMEM)/F12 and were treated with mitomycin C. Rabbits limbal stem cells (LSCs)
were co-cultured on hUCMSCs, hUVECs, hDPSCs, hPDLSCs and NIH-3T3, and then
comparative analysis were made between each group to see their respective
colony-forming efficiency (CFE) assay and immunofluorescence (IPO13,CK3/12).
RESULTS: The
efficiency of the four type cells in supporting the LSCs morphology and its
cellular differentiation was similar to that of NIH-3T3 fibroblasts as
demonstrated by the immunostaining properties analysis, with each group
exhibiting a similar strong expression pattern of IPO13, but lacking CK3 and
CK12 expression in terms of immunostaining. But hUCMSCs, hDPSCs and hPDLSCs
feeder layers were superior in promoting colony formation potential of cells
when compared to hUVECs and feeder-cell-free culture.
CONCLUSION: hUCMSCs,
hDPSCs and hPDLSCs can be a suitable alternative to conventional mouse NIH-3T3
feeder cells, so that risk of zoonotic infection can be diminished.
KEYWORDS: limbal stem cells; feeder layers; umbilical cord mesenchymal stem
cells; umbilical vein endothelial cells; dental pulp stem cells; periodontal
ligament stem cells
DOI:10.18240/ijo.2017.07.01
Citation: Wang HX, Gao XW, Ren B, Cai Y, Li WJ, Yang YL, Li YJ. Comparative
analysis of different feeder layers with 3T3 fibroblasts for culturing rabbits
limbal stem cells. Int J Ophthalmol 2017;10(7):1021-1027
Article Outline
INTRODUCTION
Limbal stem cells (LSCs) are of great significance
as a regenerative source of corneal epithelial cells in maintaining of corneal
transparency and repairing damaged corneal surface[1-2]. Damage to the limbal area by chemical burns,
autoimmune diseases, infections or hereditary conditions may cause limbal stem
cell deficiency (LSCD), which may further result in poor corneal
epithelialization, corneal neovascularization, persistent epithelial defects,
corneal conjunctivalization, corneal scarring and so on. In turn, these
problems may lead to blepharospasm, photophobia, pain, redness, tearing,
decreased vision and even corneal blindness[3-5]. LSCD affects millions of people worldwide[6]. The number of corneal LSCs determines the success of
tissue engineering cornea transplantion. Using mouse embryonic 3T3 feeder layer
can greatly increase the number of stem cells and it’s useful in supporting of
the tagert cells[7]. However, the mouse feeder
cells contains N-glycolylneuraminic acid and nonhuman sialic acid (Neu5Gc), an
immune response could be induced when we use the cells for transplantion, since
most people have Neu5Gc circulating antibodies[8-9]. In this study, in order to overcome the potential
risks of possible immunological rejection caused by using such cells in
clinical xenotransplantation, we used different human-derived feeder layers for
the ex vivo expansion of LSCs and cultivated LSCs in a coculture system
using human umbilical cord mesenchymal stem cells (hUCMSCs), human umbilical
vein endothelial cells (hUVECs), human dental pulp stem cells (hDPSCs), human
periodontal ligament stem cells (hPDLSCs) and NIH-3T3 fibroblasts. Then their
ability of expanding limbal cells and maintaining undifferentiated state were
compared to evaluate whether these four cells can be used as feeder cells that
could avoid zoonotic hazards.
MATERIALS AND METHODS
Materials Fetal bovine serum (FBS),
Dulbecco’s modified Eagle’s medium (DMEM)/F12 were purchased from Hyclone
(Logan, UT, USA); Minimum Essential α-Minimum (α-MEM), M199 Medium, penicillin,
streptomycin and trypsin-EDTA were purchased from Invitrogen-Gibco (Grand
Island, NY, USA); 4,6-diamidino-2-phenylindole (DAPI) was purchased from Roche
Life Science (Indianapolis, IN, USA); Mitomycin C and dispase were purchased
from Sigma-Aldrich Corp (St. Louis, MO, USA); The secondary antibodies were
obtained from Abcam (Cambridge, UK), anti-CD31, CD45 and CD90 antibodies were
purchased from BD Pharmingen (1:1000; BD Pharmingen, San Diego, CA, USA),
respectively. The NIH-3T3 fibroblast used in the study were contributed by the
Laboratory of Ophthalmology, Third Military Medical University of University.
All the experimental animals were treated in accordance with the principle
listed in ARVO Statement and with the approval of the Xinjiang Medical
University Ethics Committee, and the experimental platform was provided by
Xinjiang Medical University Institute of Clinical Medicine.
Methods
hUCMSCs, hUVECs culture and identification A total of 10 healthy human
umbilical cords were collected from healthy mothers at the No.474 Hospital of
Chinese PLA following their informed consent according to the Declaration of
Helsinki. All experimental procedures were approved by the Institutional Ethics
Committee, No.474 Hospital of Chinese PLA in China. And all tissues were tested
for HIV and hepatitis Isolation of mesenchymal stem cells (MSCs) from cord
tissue. Approximately 5-cm long pieces of human umbilical cords were collected
and cut into smaller pieces and washed in phosphate-buffered saline (PBS). Then
they were put into dishes containing an appropriate volume of collagenase type
I that allowed the Wharton’s jelly to come into contact with the enzymes. The
dishes were then incubated in water bath at 37℃ for 40min to allow loosening and separation of the Wharton’s jelly.
The hUCMSCs were cultured for expansion in DMEM/F12, containing 10% FBS and 1%
penicillin/streptomycin (pen/strep), at 37℃ in a 5% CO2 incubator. When the MSCs at P3-P5 to appraise the
multipotent differentiation capacity, the cells were treated with adipogenic
supplements medium (1 mmol/L dexamethasone, 10 μg/mL insulin, 1 mmol/L
3-isobutyl-1-methylxanthine, 50 μmol/L indomethacin) and osteogenic induction
medium (0.1 mmol/L dexamethasone, 10 mmol/L β-glycerophosphate, 0.05 mmol/L
ascorbic acid) for 10 to 21d as described previously[10].
After the differentiation process was completed, cells were dyed with Oil-red O
for adipocytes and Alizarin red for osteoblasts. A part of MSCs were incubated
with FITC‑conjugated mouse anti‑human antibodies for CD45 and CD90 (1:1000; BD
Pharmingen, San Diego, CA, USA) for 10min at room temperature. The umbilical
veins were carefully excised with 0.1% collagenase treatment and were cultured
in endothelial cell growth media (M199) at 37℃ for 0.5h as previously described[11].
Replace M199 medium into DMEM/F12 medium step by step after subculturing. The purity
of extracted HUVECs was identified by flow cytometric analysis to compare the
expression of the endothelial marker CD31.
hPDLSCs, hDPSCs culture and identification Periodontal ligament and dental pulp tissues were obtained from 12 to
24-year old patients undergoing impacted mandibular third molar extraction of
Stomatology Department of No.474 Hospital of Chinese PLA. Donors signed an
informed consent according to the Ethics Committee of our Institution. The
periodontal ligament and attached gingival tissue were scraped from the root
surface of healthy extracted impacted tooth and extraction of the pulp from
tooth that both were sterilised with PBS. The tissue was chopped with a
ophthalmic scissors into small sections, and the suspension was incubated in
α-MEM containing 10% FBS and 1% antibiotics at 37℃ in incubator for 3-5d. When the cells stretched out from the tissue
sections were re-plated, the media started to replace every 3d until the cells
had grown to the appropriate confluency. Then
replace α-MEM medium into DMEM/F12 medium step by step. hPDLSCs and hDPSCs
identification were the same as the description of hUCMSCs identification
above.
Feeder Cell Preparation Cultivation of hUCMSCs, hUVECs, hDPSCs, hPDLSCs and NIH-3T3 were
maintained in DMEM/F12 with 10% FBS eventually. To determine the minimum
effective concentration of mitomycin C (Sigma) for the three cells, treated
with 0, 1, 2, 4, 6 and 8 μg/mL of mitomycin C and plated in 96-well plates. Use
MTT assay to test the cellular proliferation activity of growth arrested feeder
cells then to confirm the minimum concentration of inhibition of cell
proliferation. When the cells of P3 at 80% confluence, all the types of cells
were treated with the minimal effective concentrations of mitomycin C for 2.5h
at incubator to arrest cell growth. After incubation, the cells were washed
with PBS twice for 3min each, the cells were then disassociated with 0.25%
trypsin-EDTA and replated at a count of 5×104 cells seeded per well
in 6-well plates and incubated at 37℃ overnight[6].
Cultivation of Limbal Stem Cells The limbal rims of New
Zealand white rabbits weighing 1.5-2 kg were purchased from the Xinjiang
Medical University Animal Center (Xinjiang, China). Superior limbal tissue was extensively
washed with 1% pen/strep in PBS three times, then the limbal rims were exposed
to 2.4 U/mL dispase II and incubated at 37℃ thermostat water bath for 1.5h. Then the epithelial sheet was removed
by scraping and was separated into single cells by 0.25% trypsin-0.02 % EDTA
for 15min. Limbal cells were plated at 1×103 cells in
cell culture dishes containing mitomycin C-treated hUCMSCs, hUVECs, hPDLSCs and
NIH-3T3 feeder cells.
Colony Forming Efficiency Compare colony forming ability of cultured LSCs with the four
feeder layers. About 8d we could count the formed LSCs colonies, then the
cultures were fixed with 4% paraformaldehyde for 15min and stained with 0.1%
crystal violet-solution for 10min. Then colony formation was assessed under a
dissecting microscope. We counted a group of more than 40 contiguous cells that
was defined as a colony and the colony-forming efficiency (CFE) was calculated.
Immunocytochemistry Limbal cells cultivated
with the four feeders were fixed in 4% paraformaldehyde for 1h at room
temperature before blocking and permeabilizing with 3% BSA in PBS with 1%
Triton X-100. The specimens were incubated in mouse anti-karyopherin-13 (1:300,
Santa Cruz, Biotechnology, CA, USA) and mouse anti-cytokeratin-3/12 (1:150,
Abcam, Cambridge, UK) in 1% BSA overnight at 4℃. After being washed with PBS, cells were incubated with the
fluorophore conjugated secondary antibodies (1:400, Alexa Fluor, 647) for 1h at
room temperature and counterstained with DAPI contained in the mounting medium.
All experiments were carried out in triplicate.
Statistical Analysis All analyses were
performed using SPSS 17.0. The quantification data were expressed as means±
standard deviation (SD). Comparisons between the mean variables of multiple
groups were performed using one-way ANOVAs and least significant difference
test (LSD-test) was adopted further for comparisons between each dose group and
the vehicle group. The P-value less than 0.05 was considered to be
statistically significant.
RESULTS
Biological Characterization of hUCMSCs, hUVECs,
hPDLSCs and hPDLSCs The isolated hUCMSCs, hDPSCs and hPDLSCs
demonstrated a fibroblast-like phenotype and hUVECs displayed a
short-fibroblast-like morphology (Figure 1). After the cells were cultured in
diffrerentitation mediums as described previously, we could observe adipogenic
differentiation by Oil Red O-positive cells and osteogenic differentiation by
Alizarin Red S-positive cells[12-16].
Flow cytometric analysis showed that hUCMSCs, hDPSCs and hPDLSCs had high
expression levels of mesenchymal stem cell markers CD90 and low level
expression for hematopoietic cells surface markers CD45 (Table 1). And high
expression (94.3%) of specific endothelial marker CD31 was tested in hUVECs.

Figure 1 Morphology of hUCMSCs, hUVECs, hDPSCs and hPDLSCs A: P4 of hUCMSCs (×50); B: P2 of hUVECs
(×100); C: P3 of hDPSCs (×100); D: P3 of hPDLSCs (×100).
Table 1 Flow cytometry showing the cells
characteristics
%
|
Groups |
Blank |
CD45 |
CD90 |
|
hUCMSCs group |
0.6 |
0.2 |
88.2 |
|
hDPSCs group |
0.2 |
0.4 |
89.0 |
|
hPDLSCs group |
1.7 |
1.4 |
94.6 |
Optimum Concentration Mitomycin C Act on the
Feeder Layers The 4 μg/mL mitomycin C acting on
different cells for 2.5h was the minimum concentration for inhibiting cell
proliferation and keeping the optimum cell viability (Figure 2). All feeder
cells were treated with 4 μg/mL mitomycin C in 10% FBS DMEM/F12 for 2.5h, rinsed
with PBS, trypsinized and resuspended in the medium for later use.

Figure 2 MTT assay test the influence of different concentration of mitomycin
C for the cell proliferation rates
A: Influence on the hUCMSCs; B: Influence on the hPDLSCs.
Ligament Stem Cell Proliferation in Different
Cultures Limbal cells grew as colonies with distinct boundaries for 8d, and the
cells have a regular cuboidal shape similarity for all types of feeder cells.
All the colonies could maintain small, compact and uniform cell morphology
particularly from PDLSCs, which was most similar to that of 3T3 feeder layer.
In this context all the feeder layers had a similar growth pattern of limbal
cells that could support ex vivo culture of them as well as 3T3 feeder
layer (Figure 3).

Figure 3 Morphology and growth profile of freshly collected LSCs (P0) The LSCs cultivated with hUCMSCs
(A), hUVECs (B), hDPSCs (C), hPDLSCs (D) and NIH-3T3 (E) on day 8 (×100).
Colony-forming Efficiency This detection aimed to compare the capacity of the three types of
cells with 3T3 cells as feeder layer to aid colony formation. Based simply upon
the number of discrete islands, the hPDLSCs feeder cells displayed the best CFE
with an average of 4.90%±0.96%,higher than other groups of clone formation rates. Overall difference
between the four groups was statistically significant (F=18.293, P<0.01).
Compared hUCMSCs, hDPSCs, hPDLSCs group respectively with NIH-3T3 group, their
CFE had no significant difference statistically (t=1.09, 1.16, 0.45, P>0.05).
The CFE of the hUVECs groups were lower than that of cells grown on 3T3 feeder
(t=3.09, P<0.01). The four feeder layer were different with no
feerder cells (t=6.31, 4.31, 6.24, 7.16, P<0.01) (Table 2).
Table 2 No. of LSCs colony forming efficiency of
different feeder layers mean±SD
|
Groups |
CFE (%) |
|
A hUCMSCs group |
4.10±0.56 |
|
B hUVECs group |
3.37±0.50 |
|
C hDPSCs group |
4.07±0.65 |
|
D hPDLSCs group |
4.90±0.96 |
|
E NIH-3T3 group |
4.67±0.76 |
|
F No feeder group |
0.83±0.35 |
F=15.08
(F-test for the single-factor ANOVA), P<0.01.
Expression of Putative Stem Cell Markers in the
Clones of Limbal Cells The remarkable phenotype was consisted of
small and compact cells with high nuclear-cytoplasmic ratio and showed positive
expression for IPO13 (Figure 4) and negative expression for CK3/12 (Figure 5),
all of which accorded with a more immature progenitor phenotype.

Figure 4 Cellular localization of LSC markers by fluorescent immunostaining LSCs on three types of feeder layer were
subjected to immunostaining against IPO13 (A-E). DAPI depicts cell nuclei
(F-J). Merged images were shown in (K-O). A, F, K: LSCs on hUCMSCs; B, G, L:
LSCs on hUVECs; C, H, M: LSCs on hDPSCs; D, I, N: LSCs on hPDLSCs; E, J, O:
LSCs on 3T3 fibroblasts.
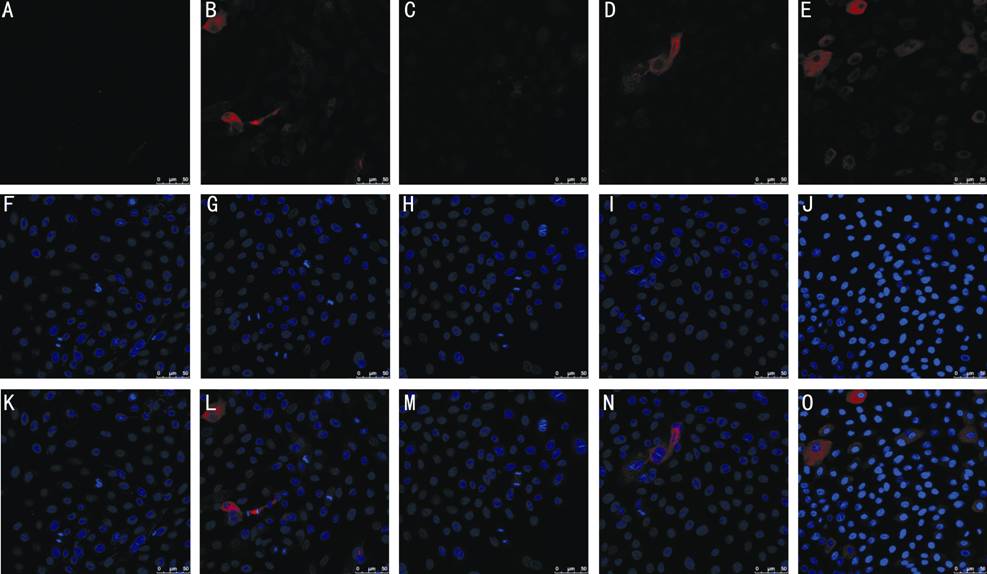
Figure 5 Cellular localization of LSC markers by fluorescent immunostaining LSCs on three types of feeder layer were subjected
to immunostaining against CK3/12 (A-E). DAPI depicts cell nuclei (F-J). Merged
images are shown in (K-O). A, F, K: LSCs on hUCMSCs; B, G, L: LSCs on hUVECs;
C, H, M: LSCs on hDPSCs; D, I, N: LSCs on hPDLSCs; E, J, O: LSCs on 3T3
fibroblasts.
DISCUSSION
In this study, the four kinds of feeder layers we
used have the following significant advantages. Firstly, the selected four
types of cells were derived from abandoned organization that they enjoyed
absolute advantage in source. hDPSCs and hPDLSCs hold excellent promise as
sources of adult stem cells potential for utilization in regenerative medicine[17-18], and they even can be derived
from patients themselves. So we take for that they may become the better
potential breeding source layer. Secondly, the immunogenicity of the hUCMSCs
and hUVECs is very weak. Recent animal experiments have shown that our body
doesn’t reject hUCMSCs[19]. Thirdly, compared
with bone marrow-derived mesenchymal stromal cells[20] and
skin fibroblasts[21-22], the
obtain of umbilical cord and teeth does not impose certain degree of damage to
the organizational structure of the human body.
By far, there wasn’t any research that has
involved so many cells to see their comparative effectiveness with 3T3 feeder
layer. In this study, hPDLSCs had a powerful ability of inducing osteoblastic
differentiation and cell proliferation activity, the clone groups of LSCs
formed were closer to 3T3 feeder layer than others. hPDLSCs had similar
characteristics with MSCs that both could express CD73, CD90, CD105 without
expressing hematopoietic cells surface markers of CD14, CD20, CD34 and CD4[15,23], and both could be
differentiated into fat, osteogenesis and chondrocytes in vitro[24-25]. Therefore, we conclude that the
efficient cell activity is a prerequisite for the cell to be a high quality
feeder layer.
Although many alternative techniques have been
proposed such as amniotic membranes scaffolds and feeder-free explant cultures
and recent 2D and 3D[26-27],
using feeder layers are still considered to be the best method. Chen et al[28] found that hUVEC coculture with hUCMSCs, hiPSC-MSCs,
hESC-MSCs and human bone mesenchymal stem cells (hBMSCs) in calcium phophate
cement scaffold could achieve excellent osteogenic and angiogenic capability in
vivo as a result of their ability to preserve some critical properties of
LSCs such as cell growth viability and stemness phenotype[29].
Various alternative methods to culture LSCs are being explored currently. Ang et
al[30] showed that mucin-expressing
cord lining epithelial cells could substitute for 3T3 fibroblasts as feeder
layer to culture LSCs. In addition, Scafetta et al[31]
showed that human Tenon’s fibroblasts could replace both 3T3 and human dermal
fibroblasts (DF), potentially providing a appropriate microenvironment for LSCs
culture. There are other feeder layers such as human limbal mesenchymal cells,
hBMSCs, human DF and no feeder cells, but currently there is still no clinical
trials employing human feeder layers for LSCs expansion.
No specific marker for the LSCs has been found
yet. But IPO13, newest member of importin-β family is believed to be the
putative one. IPO13 is uniquely expressed by human limbal basal epithelial
cells and plays an important role in maintaining the phenotype, high
proliferative potential and diminishing differentiation of corneal epithelial
progenitor cells[32]. Limbal and cornea tissues
revealed that CK3 was not expressed in basal limbal epithelial cells while it
could be strongly expressed in the whole corneal epithelium and suprabasal
limbal epithelial cells[33]. The co-cultured LSCs
were turned out to be able to express the putative stem cell marker of IPO13
but did not express the differentiation related markers of CK3/CK12. In order
to make sure that the sells we cultured were LSCs, we also observed the cell
morphology[34-38]. Our
research showed that the efficiency of the four types cells in supporting the
cellular differentiation of LSCs was similar to that of NIH-3T3 fibroblasts through
immunostaining properties analysis. It also showed that hUCMSCs, hDPSCs and
hPDLSCs feeder layers were superior in promoting colony formation potential of
cells compared with hUVECs and feeder-free culture. And the colonies on the
three types of feeder layers especially LSCs clone colonies formed by PDLSCs
share the similar characteristic with that of 3T3, this is, maintaining small,
compact and uniform cell morphology. Moreover, for the adults, PDLSCs is
undoubtedly the better choice when using autologous cells for tranplant because
it’s difficult for us to preserve our own hUCMSCs and hUVECs.
In summary, the study showed that the three feeder
cells of hUCMSCs, hDPSCs and PDLSCs could substitute for 3T3 fibroblasts to be
feeder layer for LSCs culturing. And it is worth mentioning that, in
perspective of the ability of LSCs amplication, PDLSCs could be the idealest
substitutes for feeder layer among the three to reduce the potential risk of
xenogenic contamination.
ACKNOWLEDGEMENTS
Foundation: Supported by the Project Plan of Science and Technology Assistance in
Xinjiang Autonomous Region (No.201491171).
Conflicts of Interest: Wang HX, None; Gao XW, None; Ren B, None; Cai Y, None;
Li WJ, None; Yang YL, None; Li YJ, None.
REFERENCES
1 Cauchi PA, Ang GS,
Azuara-Blanco A, Burr JM. A systematic literature review of surgical
interventions for limbal stem cell deficiency in humans. Am J Ophthalmol 2008;146(2):251-259. [CrossRef] [PubMed]
2
Chen YT, Li W, Hayashida Y, He H, Chen SY, Tseng DY, Kheirkhah A, Tseng SC.
Human amniotic epithelial cells as novel feeder layers for promoting ex vivo
expansion of limbal epithelial progenitor cells. Stem Cells 2007;25(8):1995-2005. [CrossRef] [PMC free article] [PubMed]
3
Sareen D, Saghizadeh M, Ornelas L, Winkler MA, Narwani K, Sahabian A, Funari
VA, Tang J, Spurka L, Punj V, Maguen E, Rabinowitz YS, Svendsen CN, Ljubimov
AV. Differentiation of human limbal-derived induced pluripotent stem cells into
limbal-like epithelium. Stem Cells Transl
Med 2014;3(9):1002-1012. [CrossRef] [PMC free article] [PubMed]
4
He H, Yiu SC. Stem cell-based therapy for treating limbal stem cells
deficiency: a review of different strategies. Saudi J Ophthalmol 2014; 28(3):188-194. [CrossRef] [PMC free article] [PubMed]
5
Chan EH, Chen L, Rao JY, Yu F, Deng SX. Limbal basal cell density decreases in
limbal stem cell deficiency. Am J
Ophthalmol 2015;160(4): 678-684.e4. [CrossRef] [PMC free article] [PubMed]
7
Chugh RM, Chaturvedi M, Yerneni LK. Occurrence and control of sporadic
proliferation in growth arrested Swiss 3T3 feeder cells. PLoS One 2015,10(3):e122056. [CrossRef] [PMC free article] [PubMed]
8
Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an
immunogenic nonhuman sialic acid. Nat Med
2005;11(2): 228-232. [CrossRef] [PubMed]
9
Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E.
Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A 2003;100(21): 12045-12050. [CrossRef] [PMC free article] [PubMed]
10
Kita K, Gauglitz GG, Phan TT, Herndon DN, Jeschke MG. Isolation and
characterization of mesenchymal stem cells from the sub-amniotic human
umbilical cord lining membrane. Stem
Cells Dev 2010;19(4):491-502. [CrossRef] [PubMed]
12
Guo ZY, Sun X, Xu XL, Zhao Q, Peng J, Wang Y. Human umbilical cord mesenchymal
stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen Res 2015;10(4):651-658. [CrossRef] [PMC free article] [PubMed]
13
Kumar A, Bhattacharyya S, Rattan V. Effect of uncontrolled freezing on
biological characteristics of human dental pulp stem cells. Cell Tissue Bank 2015;16(4):513-522. [CrossRef] [PubMed]
14
Kadekar D, Kale V, Limaye L. Differential ability of MSCs isolated from
placenta and cord as feeders for supporting ex vivo expansion of umbilical cord
blood derived CD34(+) cells. Stem Cell
Res Ther 2015;6:201. [CrossRef] [PMC free article] [PubMed]
15
Tran Hle B, Doan VN, Le HT, Ngo LT. Various methods for isolation of
multipotent human periodontal ligament cells for regenerative medicine. In Vitro Cell Dev Biol Anim
2014;50(7):597-602. [CrossRef] [PubMed]
16
Ji K, Liu Y, Lu W, Yang F, Yu J, Wang X, Ma Q, Yang Z, Wen L, Xuan K.
Periodontal tissue engineering with stem cells from the periodontal ligament of
human retained deciduous teeth. J
Periodont Res 2013;48(1):105-116. [CrossRef] [PubMed]
17
Yan M, Yu Y, Zhang G, Tang C, Yu J. A journey from dental pulp stem cells to a
bio-tooth. Stem Cell Rev 2011;7(1):161-171.
[CrossRef] [PubMed]
19
Liao W, Xie J, Zhong J, Liu Y, Du L, Zhou B, Xu J, Liu P, Yang S, Wang J, Han
Z, Han ZC. Therapeutic effect of human umbilical cord multipotent mesenchymal
stromal cells in a rat model of stroke. Transplantation
2009;87(3):350-359. [CrossRef] [PubMed]
20
Markel TA, Crafts TD, Jensen AR, Hunsberger EB, Yoder MC. Human mesenchymal
stromal cells decrease mortality after intestinal ischemia and reperfusion
injury. J Surg Res 2015;199(1):56-66.
[CrossRef] [PubMed]
21
Quan C, Cho MK, Shao Y, Mianecki LE, Liao E, Perry D, Quan T. Dermal fibroblast
expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal
keratinocyte proliferation in normal and diseased skin. Protein Cell 2015;6(12):890-903. [CrossRef] [PMC free article] [PubMed]
22
Oie Y, Hayashi R, Takagi R, Yamato M, Takayanagi H, Tano Y, Nishida K. A novel
method of culturing human oral mucosal epithelial cell sheet using post-mitotic
human dermal fibroblast feeder cells and modified keratinocyte culture medium
for ocular surface reconstruction. Br J
Ophthalmol 2010;94(9):1244-1250. [CrossRef] [PubMed]
23
Rodriguez-Lozano FJ, Garcia-Bernal D, Aznar-Cervantes S, Ros-Roca MA, Alguero
MC, Atucha NM, Lozano-Garcia AA, Moraleda JM, Cenis JL. Effects of composite
films of silk fibroin and graphene oxide on the proliferation, cell viability
and mesenchymal phenotype of periodontal ligament stem cells. J Mater Sci Mater Med 2014;
25(12):2731-2741. [CrossRef] [PubMed]
24
Fujii S, Maeda H, Wada N, Tomokiyo A, Saito M, Akamine A. Investigating a
clonal human periodontal ligament progenitor/stem cell line in vitro and in
vivo. J Cell Physiol 2008;215(3):743-749.
[CrossRef] [PubMed]
25
Tomokiyo A, Maeda H, Fujii S, Wada N, Shima K, Akamine A. Development of a
multipotent clonal human periodontal ligament cell line. Differentiation 2008;76(4):337-347. [CrossRef] [PubMed]
26
Utheim TP, Lyberg T, Raeder S. The culture of limbal epithelial cells. Methods Mol Biol 2013;1014:103-129. [CrossRef] [PubMed]
27
Dziasko MA, Tuft SJ, Daniels JT. Limbal melanocytes support limbal epithelial
stem cells in 2D and 3D microenvironments.
Exp Eye Res 2015;138:70-79. [CrossRef] [PubMed]
29
Scafetta G, Siciliano C, Frati G, De Falco E. Culture of human limbal
epithelial stem cells on tenon's fibroblast feeder-layers: a translational
approach. Methods Mol Biol 2015;1283:187-198.
[CrossRef] [PubMed]
30
Ang LP, Jain P, Phan TT, Reza HM. Human umbilical cord lining cells as novel
feeder layer for ex vivo cultivation of limbal epithelial cells. Invest Ophthalmol Vis Sci
2015;56(8):4697-4704. [CrossRef] [PubMed]
31
Scafetta G, Tricoli E, Siciliano C, Napoletano C, Puca R, Vingolo EM, Cavallaro
G, Polistena A, Frati G, De Falco E. Suitability of human Tenon's fibroblasts as
feeder cells for culturing human limbal epithelial stem cells. Stem Cell Rev 2013;9(6):847-857. [CrossRef] [PubMed]
32
Wang H, Tao T, Tang J, Mao YH, Li W, Peng J, Tan G,Zhou YP, Zhong JX, Tseng SC,
Kawakita T, Zhao YX, Liu ZG. Importin 13 serves as a potential marker for
corneal epithelial progenitor cells. Stem
Cells 2009;27(10):2516-2526. [CrossRef] [PMC free article] [PubMed]
34
Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal
stem cells. Exp Eye Res 2005;81(3):247-264.
[CrossRef] [PubMed]
35
Wolosin JM, Budak MT, Akinci MA. Ocular surface epithelial and stem cell
development. Int J Dev Biol 2004;48(8-9):981-991.
[CrossRef] [PubMed]
36
Arpitha P, Prajna NV, Srinivasan M, Muthukkaruppan V. High expression of p63
combined with a large N/C ratio defines a subset of human limbal epithelial
cells: implications on epithelial stem cells. Invest Ophthalmol Vis Sci 2005;46(10):3631-3636. [CrossRef] [PubMed]
37
Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, McGuire SP, Gregory
MS, Vincent WJ, Perez VL, Cruz-Guilloty F, Kao WW, Call MK, Tucker BA, Zhan Q,
Murphy GF, Lathrop KL, Alt C, Mortensen LJ, Lin CP, Zieske JD, Frank MH, Frank
NY. ABCB5 is a limbal stem cell gene required for corneal development and
repair. Nature 2014;511(7509):353-357.
[CrossRef] [PMC free article] [PubMed]
38
Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem
cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol 2003;48(6):631-646. [CrossRef]