
·Basic Research· Current
Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
Citation: Chang CK, Lin JT,
Zhang Y. Human eye ocular component analysis for refractive state and
refractive surgery. Int J Ophthalmol
2017;10(7):1076-1080
Human eye ocular component analysis for refractive state and refractive surgery
Chao-Kai Chang1, Jui-Teng Lin2,3,
Yong Zhang4
1Nobel Eye Institute, Taipei 101, Taiwan, China
2New Vision Inc., Taipei 103, Taiwan, China
3Gong-Rui Medical Technology, Xiamen 361000, Fujian Province, China
4Department of Ophthalmology, Shandong Provincial Hospital, Jinan
250021, Shandong Province, China
Correspondence
to: Jui-Teng Lin. New Vision Inc., Taipei 103, Taiwan, China.
jtlin55@gmail.com; Yong Zhang. Department of Ophthalmology, Shandong Provincial
Hospital, Jinan 250021, Shandong Province, China. yzhangmd@hotmail.com
Received:
2016-08-26
Accepted: 2016-12-27
Abstract
AIM: To
analyze the clinical factors influencing the human vision corrections via
the changing of ocular components of human eye in various applications; and to
analyze refractive state via a new effective axial length.
METHODS: An
effective eye model was introduced by the ocular components of human eye
including refractive indexes, surface radius (r1, r2, R1, R2) and thickness (t,
T) of the cornea and lens, the anterior chamber depth (S1) and the vitreous
length (S2). Gaussian optics was used to calculate the change rate of
refractive error per unit amount of ocular components of a human eye (the rate
function M). A new criterion of myopia was presented via an effective
axial length.
RESULTS: For
typical corneal and lens power of 42 and 21.9 diopters, the rate function Mj
(j=1 to 6) were calculated for a 1% change of r1, r2, R1, R2, t, T (in
diopters) M1=+0.485, M2=-0.063, M3=+0.053, M4=+0.091, M5=+0.012, and M6=-0.021
diopters. For 1.0 mm increase of S1 and S2, the rate functions were M7=+1.35,
and M8=-2.67 diopter/mm, respectively. These rate functions were used to
analyze the clinical outcomes in various applications including laser in
situ keratomileusis surgery, corneal cross linking procedure, femtosecond
laser surgery and scleral ablation for accommodation.
CONCLUSION: Using
Gaussian optics, analytic formulas are presented for the change of refractive
power due to various ocular parameter changes. These formulas provide useful
clinical guidance in refractive surgery and other related procedures.
KEYWORDS: Gaussian optics;
human eye ocular components; refractive errors; vision correction laser in
situ keratomileusis; corneal collagen crosslinking
DOI:10.18240/ijo.2017.07.09
Citation: Chang CK, Lin JT, Zhang Y. Human eye ocular
component analysis for refractive state and refractive surgery. Int J
Ophthalmol 2017;10(7):1076-1080
INTRODUCTION
A
complete optical description of a human eye should include its 12 ocular
parameters including 4 refractive indexes, 4 surface radius and 2 thickness
(for cornea and lens), the anterior chamber depth (ACD) and the vitreous length
(or axial length). Gaussian optics[1-2]
has been used for the calculations of intraocular lens (IOL) power,
accommodation amplitude in IOL and human natural lens and the refractive state
of human eyes[3-4]. Conventional
refractive state is defined solely by the axial length (L) which could not
apply to all eyes, although it is true for averaged eyes. Base on an effective
eye model, a new standard for refractive state will be presented based on a
relative axial length of (L-L’), rather than its absolute axial length (L),
where L’ is the effective axial length of the emmetropic state. The roles of
ocular components on the refractive power have been reported only partially[2-3]. Derivation of the rate
function (M) defined by the change rate of refractive error per unit amount of
ocular components will be presented else where. This study will focus upon
their clinical applications including laser in situ keratomileusis
(LASIK) surgery, corneal cross linking (CXL) procedure, femtosecond laser
surgery and laser scleral ablation for accommodation.
MATERIALS AND METHODS
Effective Eye Model By Gaussian optics theory (or paraxial ray approximation along the axial
axis), the refractive error (De) is given by[1,3] De=1000 [n1/(L-L2)-n1/F] (1), where n1 is the
refractive index of the aqueous humor, L is the axial length, L2 is position of
the system second principal plane and F is the system effective focal length
(EFL). The system total power is given by D=1000n1/F (D in diopter, F in mm)
which is determined by the corneal (D1) and lens power (D2) as follows[3] D=D1+D2-S(D1D2)/(1000n1) (2a), D1=1000
[(n3-1)/r1-(n3-n1)/r2]+bt (2b), D2=1000 [(n4-n1)/R1+(n4-n2)/R2]-aT (2c), where
nj (j=1, 2, 3, 4) are the refractive index for the aqueous, vitreous, cornea
and lens, respectively. The anterior and posterior radius of curvatures (mm) of
the cornea and lens are given by (r1, r2) and (R1, R2), respectively, where the
only concave surface R2 is taken as its absolute value in this study. Finally,
S is the effective ACD, related to the ACD, S1, by S=S1+P11+0.05 (mm), where
P11 is the distance between the lens anterior surface and its first principal
plane, and 0.05 mm is a correction amount to include the effect of corneal
thickness (assumed to be 0.55 mm)[2-3].
The thickness terms in equation (Eq.) (2b) and (2c) are given by b=11.3/(r1r2),
a=4.97/(R1R2) for refractive indexes of n1=n2=1.336, n3=1.377 and n4=1.42; and
t and T are the thickness of the cornea and lens, respectively.
As
shown in Figure 1, using L-L2=X+SF/f, with X=L-S-aT+0.05, and aT and 0.05 are
the correction factors for the lens and cornea thickness, Eq. (1) may be
rewritten in an effective eye model Eq.[3], De=Z2[1336/X–D1/Z–D2]
(3a), Z=1-S/f (3b) where f (in
mm) is the EFL of the cornea given by f=1336/D1, and the nonlinear term k is
about 0.003 calculated from the second-order approximation of SF/(1336f). The
nonlinear term may also be derived from the IOL power formula[5].
We note that in Eq. (3), X, Z, S and f are in the unit of mm; D1, D2 and De are
in the unit of diopter; and the 1336 is from 1000×1.366 in our converted units.
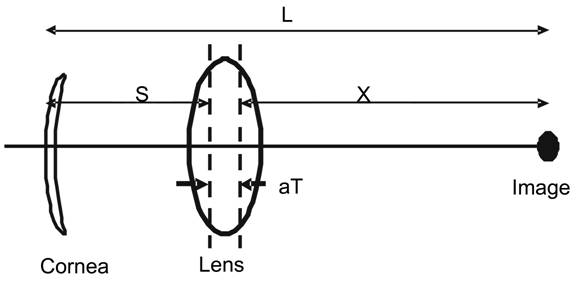
Figure 1 An effective eye model[3] defined
by the power of the cornea and lens
Also shown are the parameters of S and X
which is related to the axial length by L=S+X+aT-0.05 (mm).
A
New Standard of Refractive State The
emmetropic state (“E-state”, when De=0) can be described by a simple formula
reduced from Eq. (3a) when 1336/X=D1/Z+D2, or as shown by Figure 1, when the
effective axial length at E-state (L’) is given by[3]
L’=X+S+aT-0.05, which also define the refractive states for hyperopia De>0
(L<L’), and myopia De<0 (L>L’). We may also easily see that at
emmetropia De=0, or when L=L’, therefore, a new standard for E-state is governed
by the relative axial length of (L-L’), rather than its absolute axial length
(L). A large L’ may be due to flat cornea or lens (i.e. small D1 or D2)
or deep ACD (S), or thick lens (T). The commonly accepted concept of long axial
length resulting myopia is only true under statistical “mean”. The refractive
state of a specific subject shall be defined by our new criterion as described
above. For example, a subject with L=26 mm will have about 2.7 diopter myopia
when L’=25 mm, whereas it becomes about 1.4 diopter of hyperopia, when L’=27
mm. The above new standard for E-state was first introduced by Lin[3] in 2006. Using the referenced parameter set of (f1, f2,
So, T, L’)=(31, 60, 3.3, 4.0, 24) mm, an ocular system deviating from this
referenced-set, its emmetropic state is governed by[3]
L’=24.0+0.36 (43.1-D1)+0.23 (22.3-D2)+0.5 (So-3.3)+0.35 (T-4.0) (4).
Rate
Functions To find the
change of refractive error (De) due to the change of Qj, we further define
Qj=(r1, r2, R1, R2, t, T, S1, S2) with j=(1 to 8), respectively. The ACD (S1)
and vitreous length (S2) are related to the axial length by L=S1+S2+T. The
derivative of the refractive error (De) with respect to these ocular parameter
change (Qj) given by Mj=dDe/dQj, defines the rate function, or the change of De
per unit amount change of Qj, where the standard notation “d” for “derivative”
is used in this study.
In
general, under the second-order approximation including the contributions from
both n1/(L-L2) and (n1/F) in Eq. (1), one shall rigorously calculate the
derivative dDe=Mj (dQj) based on Eq. (1). The complexity of this method is due
to the nonlinear dependence of L2 on the ocular parameters.
Using
Eq. (2) and (3) analytic formulas for the rate function for the surface
curvatures and thickness of the cornea and lens may be derived (to be presented
else where) by Mj=dDe/dQj, with Qj (j=1 to 4, for r1, r2, R1 and R2,
respectively), and Q5=t, Q6=T as follows. M1=+378/r12 (5a), M2=-41/r22
(5b), M3=+82.75 C2/R12 (5c), M4=+82.75 C2/R22
(5d), M5=11.3/(r1r2) (5e), M6=+4.97 C2/(R1R2) (5f). Where we had
used the refractive indexes nj=(1.336, 1.336, 1.3371, 1.42) for the aqueous,
vitreous, cornea and lens, respectively, and a lens conversion function C2=(dDe/dD2)=Z2.
The rate function for S1 and S2, defined by M7=dDe/dS1 and M8=dDe/dS2, were
previously derived and given by[4-6]
M7=1336 (1/F2–1/f2) (6a), M8=-1336/F2 (6b),
where f and F (both in mm) are the corneal and system EFL given by f=1336/D1
and F=1336/D. For Mj=dDe/dQj, with Q (j=9, 10, 11, 2) for nj (j=1, 2, 3, 4),
respectively, we derive (to be presented else where) M9=1000 (1/r2-C2/R1)
(7a), M10=-1000C2/R2 (7b), M11=-1000 (1/r2- 1/r1) (7c), M12=-1000 C2
(1/R1+1/R2) (7d).
RESULTS
Rate Functions
By using a set of typical ocular parameters[2]:
refractive indexes nj (i=1 to 4)=(1.336, 1.336, 1.3771, 1.42), (r1, r2)=(7.8,
6.5) mm, (R1, R2)=(10.2, 6.0) mm, thickness (t, T)=(0.55, 4.0) mm and S=6.0,
S1=3.5 and S2=16.0 mm, or an axial length of L=3.5+16+4=23.5 mm, the corneal
and lens power are calculated D1=42 diopter, D2=21.9 diopter and total power,
from Eq. (2a), D=D1+0.811D2=59.8 diopter, The rate function Mj (j=1 to 6) are
calculated for a 1% change of r1, r2, R1, R2, t, T (in diopters) M1=+0.485,
M2=-0.063, M3=+0.053, M4=+0.091, M5=+0.012, and M6=-0.021 diopters.
For
1.0 mm increase of S1 and S2, the rate functions are: M7=+1.35, and M8=-2.67 diopter/mm.
Furthermore, for each 1.0 diopter increase of corneal and lens power, the rate
functions are 1.0 and 0.66 diopter, respectively, for a typical value of
effective ACD, S=6.0 mm and corneal power of 43 diopters. We shall note that
the above values of Mj depend on the choices of the ocular parameters and may
vary 10%-15% from the typical values chosen. Our calculated data are consistent
with that of Atchison[2].
Effects
of Cornea and Lens Curvatures The increase
of radius of curvature of the cornea and lens (r1, r2, R1, R2) all result in
hyperopic shift, except the change of the posterior surface of the lens (R2)
having a myopia shift, since it is the only concave surface and all other three
surfaces (r2, R1, R2) are convex surfaces. Furthermore, the effect due to
anterior corneal surface change is the dominant one, where M1 is about 8 times
of M2 and M3, and 5 times of M4, as shown by Eq. (5). This may be easily
realized from Eq. (2b) that (n3-1) is much higher than the other terms, such as
(n3-n1) and (n4-n1). Therefore reshaping of lens surface is much less efficient
than that of cornea. We will discuss more later in femtosecond laser procedure.
Effects
of S1 and S2 The increase
of S1 results in a hyperopia shift (HS), whereas S2 results in a myopia shift
(MS), where M8 is about two times of M7 which has two competing terms as shown
by Eq. (6). The rather high change rate M8=-2.67 (D/mm) has significant impact
on the onset of emmetropization and myopia which are governed by the
correlation among the growth of axial length (L=S1+S2+T) and the power decrease
of the cornea and lens when an eye grows[3]. The
change rate M7 having a lower value than M8 can be analyzed as follows.
The
competing between the MS (due to the increase of ACD, S1) and the HS (due to
the associate decrease of S2 for a fixed axial length L=S1+S2+T) results in a
net hyperopic-shift, because the hyperopic component is always the dominant
one, since the corneal power (D1) is always less than the total system power
(D) or F<f in Eq. (3a). This new finding based on the analytic formula of
Eq. (5) has not been explored before.
The
hyperopic shift due to the increase of S1 is equivalent to a myopic-shift when
S1 decreases, or a forward movement of the lens. This feature is important for
presbyopia accommodation which is contributed by two components: the lens
curvature decrease and the lens forward movement[3-4]. The lens forward movement is also the main feature in
an accommodative IOL and our formulas, Eq. (6) for M7 and M8 provide the amount
of accommodation.
Effects of Refractive Index The
refractive error change (dDe) is extremely sensitive to the refractive indexes,
about 0.3 to 2.5 diopters per 1% change. The increase of n1 and n4 result in a
myopic-shift (MS), whereas the increase of n2 and n3 result in a
hyperopic-shift (HS). These opposite behavior may be readily observed from Eq.
(7). One may also find from Eq. (8a) the reason why m2 is larger than m1. This
is due to the minus term C2/R1 in Eq. (7a) and r2<R1, in general,
which results in an MS. The HS of m2 is given by Eq. (8b), where R2 is defined
as the absolute value of lens posterior radius in this study. Eq. (7c) clearly
shows that m3 has an MS due to the fact that r2 is always smaller than r1,
without exceptions in all human eyes. Finally, the increase of lens refractive
index (n4) always results in an MS, or becomes more power as expected from Eq.
(5d) and n4=1.42 is always larger than n1 and n2 in Eq. (2c).
It
should be emphasized that the new feature of m1, based on Eq. (7a), is not
obvious due to the contribution of the second term C2/R1 involving a
rather complex mathematics to derive the formula for C2 which has
been ignored in most textbook formulas[2]. Another
interesting situation is when both n1 and n2 increase the same amount of 1%
(the most likely case, since the aqueous and vitreous humor are circulated, the
net effect will be dDe=-1.19+1.46=+0.27, a hyperopic-shift only about 18% of
dDe due to the change of n2 alone and shows a much less effect than that is due
to the lens index change M12=2.47.
DISCUSSION
Clinical Applications We will
present various applications related to the formulas presented in this paper,
including: LASIK surgery, CXL procedure, femtosecond laser surgery and
accommodative IOL. Greater details are described as follows.
Laser
in Situ Keratomileusis Surgery A procedure
called LASIK, where one diopter
correction only requires an ablation depth about 8 to 11 microns of the corneal
central thickness[6] or a corresponding change of
r1 about 0.16 mm based on Eq. (5a). It is important to know that the corneal power
change is 100% converted to the system power or refractive error change, as
demonstrated by our cornea conversion factor C1. We should also note
that the refractive error (De) defined on the corneal plan is the same as that
of a contact lens. However, a conversion formula is needed when it is
translated to a spectacle power Ds, given by De= Ds/ [1-V Ds], where V is a
vertex distance about 12 mm. The central ablation depth for a 3-zone myopic
correction is given by[7] H’(3-zone)=RH
(single-zone) (8a), H (single-zone)=(DW2/3) (1+C) (8b), where W is
the diameter of the outer ablation zone having a typical value of 6.5 to 7.5
mm; C is a nonlinear correction term given by C=0.19 (W/r1)2, r1 is
the corneal anterior radius of curvature. For examples, for r1=7.8 mm (or a
K-reading of K=337.R1=43.2 D), C=(11.2, 13.2, 16.5)% for W=(6.0, 6.5, 7.00) mm.
The reduction factor R=(0.70 to 0.85) depending on the algorithms used. For
example, comparing to a single zone with W=6.5 mm, a 3-zone depth will reduces
to 71.6% (or R=0.716) when an inner zone 5.5 mm and an outer zone 6.5 mm are
used. Furthermore, in a LASIK system, the input pre-operative parameter of the
treated eye must include the K values which affect the laser ablation depth via
the nonlinear term of Eq. (8b).
Age
Dependent Lens Power
It was reported that the change in the refractive index gradient of the
lens cortex has a substantial factor in the contribution to the onset and
progress of presbyopia[8], where an age-dependent
Eq. for an equivalent lens index neff=1.441-0.00039 ‘x’ Age (in year) was
proposed to explain the lens paradox[9]. Lens
index decreases from 1.434 to 1.416 (about 1.25% decrease) between 20 and 65
years of age to compensate the more convex shape of aged-lens, given by
R1=12.9-0.05 ‘x’ Age and R2=6.2-0.012 ‘x’ Age[10],
which would have caused a myopia rather than presbyopia, if neff would not be
age-dependent. Above statements have been known, but only qualitatively. The
formula Eq. (7d) provides the quantitative argument that a HS of 2.47 ‘x’
1.25%=3.1 diopter is associated to this proposed index decrease of 1.2%. The
commonly accepted estimation of dDe due to the change of lens index was based
on a conversion factor (C2) of 80% which ignored the contribution
from the second principal plane, the first term of Eq. (1) in comparing to the
new value of CF=(65% to 75%) in this study which includes both terms.
Accommodative
Intraocular Lens in Aphakic Eye For patient
after cataract, an accommodative intraocular lens (AIOL) may be implanted for
vision correction to see both near and far. The accomondation formulas for M7
and M8 can be used to calculate the accomondation amplitude of the AIOL. Our
calculations show the typical values of M7=+1.35, and M8=-2.67 diopter/mm.
These formulas can also be used to calculate the power error of the piggy-back
IOL due to mis-position. Our formulas based on the Gaussian optics are
consistent with that of raytracing methods[11-12].
Femtosecond
Laser Surgery One may use
a femtosecond laser to ablate or remove a small portion of the lens and change
its curvature (R1), where each 1% reduction may cause a 0.05 to 0.06 diopter
change, based on our formula for M3, see Eq. (5c). This procedure is not as
effective as that of corneal ablation (LASIK) given by M1 in Eq. (5a). However,
ablation of the lens has no thickness limitation like a cornea. Therefore one
may ablate the lens to restore a 40% change of R1 resulting 2.0 to 2.4 diopter
accommodation. The current femtosecond laser has a very low average power and
therefore lens ablation could take a much longer time than a corneal surface
ablation in LASIK.
Scleral
Ablation for Presbyopia Treatment Scleral
laser ablation and band expansion have been used to increase the space of the
ciliary-body and zonus such that accomondation is improved by two components[8]: the lens translation and the lens shaping which are
given by, respectively, M7 and M3. For older and/or harder lens, the
accommodation is mainly attributed by the lens translation (or S1 change),
whereas lens shaping dominates the power change in young or soft lens. It was
known that change of the rear surface of the lens is about one-third of the
front surface during accommodation[12], our
formulas Eq. (5c) and (5d) shows that the contribution from R2 is about the
same as that of R1, because of R2 (6.0 mm)<R1 (10.2 mm), and M4=2.9 M3, for
the same change of curvature, dR1=dR2.
Cornea
Cross Linking Depending on
the ocular location of the CXL procedure, the new applications of CXL include
examples shown as follows: 1) for CXL applied inside the corneal stroma,
correction of low myopia is possible and may be measured by the K-value (or
thickness) reduction after CXL; where 2% reduction of K-value may cause a 0.9
to 1.1 diopter myopic correction, based on the formula for M1, see Eq. (5a),
where K=337/r1. We shall note that the refractive power change based on M1
calculated by the K-value change may be underestimated, because the CXL could
change both the front and back surface of the cornea resulted by the thickness
reduction after the CXL. A more accurate calculation should include both M1 and
M2 shown by Eq. (5); 2) for CXL applied to the orbital scleral tissue, one may
stop or reduce the abnormal axial length (L) growth rate in high myopic eyes,
where each 1.0 mm increases of L may cause 2.2 to 2.8 diopter change, based on
our formula for M8, see Eq. (6b), assuming that the axial grow is dominated by
S2; 3) for CXL applied to the corneal stroma postoperatively for procedures
such as conduction keratoplasty, diode laser thermal keratoplasty, the
postoperative regression due to unstable thermal shrinkage may be stabilized by
CXL process. Eq. (5a) for M1 may be used to estimate the amount of
postoperative regression reduced by CXL[13-20].
Using
Gaussian optics, we have presented analytic formulas for the change of
refractive power due to various ocular parameter changes. These formulas
provide useful clinical guidance in various applications including LASIK
Surgery, CXL procedure, femtosecond laser surgery and scleral ablation for
accommodation. Accuracy of our formulas for human eyes would depend on
individual ocular parameters, which were taken as their averaged values in our
calculations. Moreover, we have assumed a simplified paraxial approximation eye
mode (along the optical axis, z) which does not include the (x, y) off axis
surface effects. Therefore the formulas developed in this article would only
provide a general trend for clinical guidance, rather than accurate prediction
for refractive surgeries in human eyes, in which a full 3-dimensiotinal model
is required and only numerical simulation are available. Our intent of this
article is to present comprehensive model with analytic formulas.
ACKNOWLEDGEMENTS
Supported
by an Internal Research of New Vision Inc., Taipei, Taiwan.
Conflicts of Interest: Chang CK, None; Lin JT, the CEO of New Vision Inc. and has financial
interest; Zhang Y, None.
REFERENCES
1 Pedrotti LS, Pedrotti F. Optics and vision. Prentice
Hall 1998.
2 Atchison DA, Smith G. Optics of the human eye. Woburn, USA, Butterworth Heinemann; 2000.
3 Lin JT. Analysis of refractive state ratios and the
onset of myopia. Ophthalmic Physiol Opt
2006;26(1):97-105. [CrossRef] [PubMed]
4 Lin JT, Jiang M, Chang CL, Hong YL, Ren Q. Analysis
and applications of accommodative lenses for vision corrections. J Biomed Optics 2011;16(1):018002. [CrossRef] [PubMed]
6 Lin JT. New formulas comparing the accommodation in
human lens and intraocular lens. J
Refract Surg 2005;21(2):200-201. [PubMed]
7 Garg A, Lin JT. Mastering
the advanced surface ablation techniques. India, Jaypee Brothers; 2008. [CrossRef]
8 Lin JT, Mallo O. Treatment of presbyopia by infrared
laser radial sclerectomy. J Refract Surg
2003;19(4):465-467. [PubMed]
9 Rosen AM, Denham DB, Fernandez V, Borja D, Ho A,
Manns F, Parel JM, Augusteyn RC. In vitro dimensions and curvatures of human
lenses. Vision Res
2006;46(6-7):1002-1009. [CrossRef] [PubMed]
10 Garner LF, Yap MK. Changes in ocular dimensions and
refraction with accommodation. Ophthalmic
Physiol Opt 1997;17(1):12-17.
11 Nawa Y, Ueda T, Nakatsuka M, Tsuji H, Marutani H, Hara Y, Uozato H.
Accommodation obtained per 1.0 mm forward movement of a posterior chamber
intraocular lens. J Cataract Refract Surg
2003;29(11):2069-2072. [CrossRef]
12 Ho A, Manns F, Therese, Parel JM. Predicting the
performance of accommodating intraocular lenses using ray tracing. J Cataract Refract Surg 2006;
32(1):129-136. [CrossRef] [PubMed]
13 Hafezi F, Randleman JB. Corneal collagen cross-linking. Thorofare (NJ):SLACK;2013.
14 Sorkin N, Varssano D. Corneal collagen
crosslinking: a systematic review. Ophthalmologica
2014;232(1):10-27. [CrossRef] [PubMed]
17 Lin JT, Wang KC. Analytic formulas and numerical simulations
for the dynamics of thick and non-uniform polymerization by a UV light. J Polymer Research 2016;23:53. [CrossRef]
18 Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety
of UVA-riboflavin cross-linking of the cornea. Cornea 2007;26(4):385-389.
19 Lanchares E, del Buey MA, Cristobal JA, Lavilla L,
Calvo B. Biomechanical property analysis after corneal collagen cross-linking
in relation to ultraviolet A irradiation time. Graefes Arch Clin Exp Ophthalmol 2011; 249(8):1223-1227. [CrossRef] [PubMed]
20 Schumacher S, Mrochen M, Wernli J, Bueeler M,
Seiler T. Optimization model for UV-riboflavin corneal cross-linking. Invest Ophthalmol Vis Sci
2012;53(2):762-769. [CrossRef] [PubMed]