
·Basic Research· Current
Issue IF in JCR CiteScore ·Submission· In Press Recent Accepted PMC RSS
Citation: Yan ZX, Luo Y, Liu NF. Blockade of
angiopoietin-2/Tie2 signaling pathway specifically promotes
inflammation-induced angiogenesis in mouse cornea. Int J Ophthalmol
2017;10(8):1187-1194
Blockade of angiopoietin-2/Tie2 signaling pathway
specifically promotes inflammation-induced angiogenesis in mouse cornea
Zhi-Xin Yan1, Yi Luo1,
Ning-Fei Liu2
1Department of Plastic & Burn Surgery, Affiliated Hospital of Jiangsu
University, Zhenjiang 212001, Jiangsu Province, China
2Lymphology Center of Department of Plastic & Reconstructive Surgery,
Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of
Medicine, Shanghai 200011, China
Correspondence to: Ning-Fei Liu. Lymphology Center of Department of Plastic &
Reconstructive Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong
University School of Medicine, 639 Zhi
Zao Ju Road, Shanghai 200011, China. liuningfei@126.com
Received:
2017-01-24
Accepted: 2017-06-01
Abstract
AIM:
To investigate angiopoietin-2 (Ang-2)/Tie2 signaling pathway involving in
inflammatory angiogenesis.
METHODS: Three
interrupted 11-0 nylon sutures were placed into the corneal stroma of BALB/c
mice (6wk old) to induce inflammatory neovascularization. Expression of Ang-2
and Tie2 protein on neovascularization were examined by immunofluorescence. The
dynamic expression of Ang-2 mRNA on neovascularization was examined by
quantitative real-time reverse transcriptase-polymerase chain reaction
(RT-PCR). Finally, the mouse model of suture- induced corneal
neovascularization was used to assess the role of Ang-2/Tie2 signaling pathway
in inflammatory angiogenesis by systemic application of L1-10, an Ang-2
specific inhibitor. Mouse corneal hemangiogenesis were evaluated by whole mount
immunofluorescence.
RESULTS: Both
Ang-2 and Tie2 were expressed on newly generated blood vessels in inflammatory
cornea. Ang-2 expression was gradually upregulated around 2wk following injury,
which was concurrent with an increased number of blood vessels. Blockade of
Ang-2/Tie2 signaling pathway obviously promoted angiogenesis in inflammatory
cornea.
CONCLUSION:
Ang-2/Tie2 signaling pathway seems to play an important role during
angiogenesis in inflammatory cornea. This may open new therapeutic applications
in pathological processes such as corneal graft survival, wound healing and
carcinogenesis.
KEYWORDS:
angiogenesis; angiopoietin-2; Tie2; inflammation
DOI:10.18240/ijo.2017.08.01
Citation: Yan ZX, Luo Y, Liu NF. Blockade of
angiopoietin-2/Tie2 signaling pathway specifically promotes
inflammation-induced angiogenesis in mouse cornea. Int J Ophthalmol
2017;10(8):1187-1194
INTRODUCTION
Angiogenesis,
such as the formation of new blood vessel from preexisting blood vessels, plays
a key role in multiple physiological and pathological processes. Three of these
processes have gained extensive attention in the past decades years: the
involvement of blood vessels in wound healing[1],
proliferation of tumor cells depending on nutrition supply via blood
vessels[2], and the role of blood vessels in
(corneal) graft survival[3]. The outgrowth of
blood vessels is initially induced by the vascular endothelial growth factor
(VEGF) and its receptor (VEGFR). While more evidence indicates that VEGF
signaling often keeps a critical rate-limiting step in pathological
angiogenesis[4], there is consensus that
angiogenesis is very complex and coordinated processes, needing the sequential
activation of a series of signaling [5-7].
Members of VEGFs and angiopoietins are thought to function in a complementary
way during angiogenesis.
Angiopoietin-2
(Ang-2), the second member of the angiopoietin family, is a key molecule for
blood vessel formation, which is known as a secreted protein ligand of the
receptor tyrosine kinase Tie2[8-9].
Ang-2 gene was reported to be expressed in the endothelium and the associated
cells of the arterial vessels, inner stripe of the renal outer medulla[8,10-12], and ocular
blood vessels[13] during embryonic and postnatal
development. Ang-2 is speculated to destabilize blood vessels as a natural Tie2
antagonist[8,14-15].
It is thought to cause blood vessel regression in the absence of VEGF-A,
whereas promote hemangiogenesis in the presence of VEGF-A[14-15]. However, biochemical researches on Ang-2 have
reached disputed results[8-10,16-17]. Ang-2 blocks Ang-1-induced
Tie2 activation in endothelial cells (ECs) but triggers Tie2 phosphorylation
when Tie2 is genetically introduced into NIH3T3 fibroblast cells[8]. While other researches elucidate that high level of
Ang-2 stimulation activates Tie2 in vascular ECs[16]
and triggers vascular tube formation[18-19],
revealing the complexity of Ang2 in angiogenesis. Inflammation is the body’s
physiological reaction to injury or inflammation, but it may also develop
during and involve in multiple pathological processes. Angiogenesis can be
induced by inflammation during various pathological processes, including
grafting, carcinogenesis and wound healing. However, there are limitations and
concerns associated with the function of Ang-2 in the progress of inflammatory
angiogenesis, and underlying mechanisms of Ang-2/Tie2 signaling pathway on
inflammatory angiogenesis in vivo remain also uncharacterized.
Here,
the murine model of combined inflammatory hemangiogenesis and lymphangiogenesis
in the normally avascular cornea was used to investigate the contributions of
Ang-2/Tie2 signaling pathway to the progression of inflammatory angiogenesis.
MATERIALS AND METHODS
Animals The mouse
models of sutured cornea were used to assess inflammatory neovascularization[20]. BALB/c mice (female, aged 6-8wk),weighing
20-25 g, were purchased from the Animal Care Centre of Pudong Shanghai and the
Experimental Animal Centre of Jiangsu University, China. All animals participated
in the research were managed according to the Jiangsu University and Shanghai
Jiao Tong University School of Medicine Administration Office of Laboratory
Animals Guidelines for the Care and Use of Laboratory Animals and the
Association for Research in Vision and Ophthalmology (ARVO) statement for the
use of animals in ophthalmic and vision research[20].
The study follows to the Guide for the Care and Use of Laboratory Animals
published by the US National Institutes of Health (NIH Publication No. 85-23,
revised 1996). Prior to experiment, all mice were confirmed to be free from
corneal diseases by using slit lamp microscope and other disorders.
Mouse
Suture-induced Corneal Model of Inflammation We used
microsurgical techniques to establish the mouse suture-induced corneal model of
inflammation as formerly described[20-22].
Before surgical procedures, the intramuscular injection of ketamine (100 mg/kg)
plus xylazine (10 mg/kg) were used to anesthetize mice which were euthanized at
experimental end points with a lethal dose of CO2 asphyxiation.
Three 11-0 nylon sutures (Jinhua, China) were laid intrastromally, with two
stromal incursions extending over 120° of corneal circumference each. The outer
point of suture placement was selected near the limbus, and the inner suture
point was selected near the corneal centre equidistant from limbus for
obtaining standardized angiogenic responses. Sutures were kept in place until
the end of experiment[23].
Immunofluorescence Staining
protocols of cryosections were standardized as previously described[20,24-26]. Shortly,
indirect immunofluorescence was used to localize Ang-2 and Tie2 in blood
vessels in the pathologically vascularized mouse corneas and the normal
nonvascularized mouse corneas at the limbus. For these experiments, murine eyes
were cryopreserved in optimal cutting temperature embedding medium, and 5 to 7
μm cryosections were harvested. Sections were dried (15min, 37℃) and fixed in acetone for 15min on slides.
After being rinsed with phosphate-buffered saline (PBS) (3×5min), specimens
were permeabilized with 0.2% Triton X-100 in PBS for 5min and incubated with
PBS containing 2% bovine serum albumin (BSA) at room temperature for 1h.
Specimens were incubated with the mixed primary antibody’s fluid in PBS
containing 2% BSA followed overnight at 4℃. To
localize Ang-2 expression on blood vessels, the mixed primary antibody’s fluid
of rabbit anti-mouse Ang-2 antibody (Abcam, United Kingdom, 1:500) and rat
anti-mouse CD31 Biotin antibody (BD Biosciences pharmingen, USA, 1:400) in PBS
containing 2% BSA was used. To localize Tie2 expression on blood vessels, the
mixed primary antibody’s fluid of rabbit anti-mouse Tie2 antibody (Abcam,
1:500) and rat anti-mouse CD31 Biotin antibody (BD Biosciences pharmingen,
1:400) in PBS containing 2% BSA was used. On the second day, the antibodies
were rinsed with PBS (5×5min) and blocked with 2% BSA in PBS for 1h at room
temperature, and then specimens were incubated with the mixed fluid of Alexa
Fluor®555 donkey anti-rabbit antibody (Invitrogen, USA, 1:1000) and
Streptavidin-DylightTM488 (Biolegend, CA, 1:200) in PBS containing
2% BSA for 1h at 37℃ in the
dark. Then, the antibodies were rinsed with PBS (3×15min, on a shaker) in the
dark, and specimens were incubated with 4’,6-diamidino-2-phenylindole (DAPI;
Invitrogen, 1:500) in PBS for 5min at 37℃ in the
dark. All incubations of staining were carried out in a humid chamber. After a
last rinsing step (3×5min PBS), sections were covered using fluorescent
mounting medium (DAKO Corporation, Denmark) and stored at 4℃ in the dark humid chamber. Fluorescence
microscopy and photography was taken with a confocal laser scanning microscope
(Zeiss Confocal LSM 710 microscope, Germany), and digital pictures were done
with Zen 2010 Light Edition (Carl Zeiss, Germany).
RNA
Isolation and Purification Prior to
suture and 1, 7, 11 and 14d after suture, the eyes of mice were removed under
anaesthesia. To each time point, all RNA was extracted from the 6 corneas with
Trizol® Reagent (Invitrogen). RNA was prepared following according
to the manufacturer’s protocol. The RNA pellets were washed with 75% ethanol,
centrifuged and dried. Pellets were dissolved in DEPC-treated water. The
concentration and purity of RNA were determined by measuring optical density at
260 nm and 280 nm using a Beckman Coulter DU 800 UV/Vis spectrophotometer
(Beckman Coulter, USA).
Real-time
Reverse Transcriptase-polymerase Chain Reaction Analysis Complementary
DNA synthesis was carried out by using a 20 μL reaction system. cDNA was
synthesized from 2 μg of total RNA with ThermoScript reverse transcriptase
(Invitrogen) following manufacturer's protocol. Real-time reverse
transcriptase-polymerase chain reaction (RT-PCR) was carried out with
gene-specific primers using a Stratagene Mx3000P qPCR System (Agilent
Technologies Inc. USA). The sequences of primers for RT-PCR were as follows:
β-actin:(sense: 5'-CTGTCCCTGTATGCCTCTG-3', antisense: 5'-TGTCACGCACGATTTCC-3');
Ang-2:(sense: 5'-TCTTCCTCCAGCCCCTACAT-3', antisense: 5'-TCTC
CACCATCTCCTTCTTCATC-3').
All reactions were carried out in triplicate.
The melting curve analyses were carried out to guarantee the specificity of the
quantitative RT-PCR reactions. The data analysis was carried out with the 2-△△ Ct method
depicted previously[27], while β-actin was acted
as reference gene. Values are presented as means±SEM. P<0.05 were
regarded as significant. In the figure, the alphabet a is used to represent a
significant difference between groups (P<0.01). Graph was drawn using
origin7.5 (Originlab Inc., USA).
Neovascularization
Assay of Suture-induced Inflammatory Cornea The mouse
models of sutured cornea were randomly divided into two groups. The treatment
group (n=7) received L1-10 (4 mg/kg), an Ang-2 specific inhibitor (Amgen
Inc., USA), which was dissolved in PBS and injected subcutaneously every other
day beginning with 1d before surgery. The control mice (n=7) received a
same amount of PBS solution. Mice were sacrificed on 14d, morphological
determination of corneal neovascularization was examined by whole mount
immunofluorescence staining.
Whole
Mount Preparations and Immunofluorescence Staining Preparation
was done as previously depicted[20,24].
Briefly, mice were sacrificed under anaesthesia, the sutured eyes were taken
out and the corneas were anatomized from the eyes in the rear of the corneal
limbus. Corneas were washed 3×5min with PBS at room temperature. Fixation was
done with acetone for 30min. After three more washing steps with PBS and
blocking by 2% BSA containing 0.3% Triton X-100 in PBS for 2h at room
temperature, corneas were stained overnight at 4℃ by rabbit anti-mouse LYVE-1 antibody (Abcam 1:500) plus 2% BSA in
PBS. On 2d, after washing 5×5min with PBS, the antibody was blocked by 2% BSA
in PBS for 2h. The secondary antibody Alexa Fluor®488 rat anti-mouse
CD31 (Biolegend, USA), diluted 1:50 by PBS containing 2% BSA, was added to
incubate with corneas overnight at 4℃ in the
dark. On the third day, after washed 5×5min by PBS, 2% BSA in PBS was used to
block the antibody for 2h. The third antibody, Alexa Fluor®555
donkey anti-rabbit antibody (Invitrogen), diluted 1:1000 by 2% BSA in PBS, was
used to incubate with corneas for 45min at room temperature in the dark. As a
last step, antibody was washed 3×15min with PBS. Corneas were moved to
microscope slides, covered with DAKO fluorescent mounting medium and stored at
4℃ in the
dark. Fluorescence microscopy and photography was taken with a confocal laser
scanning microscope (Zeiss Confocal LSM 710 microscope, Germany), and digital
pictures were done with Zen 2010 Light Edition (Carl Zeiss, Germany).
Dynamic
Functional and Statistical Analysis and Graph Quantitative
analysis of neovascularization was carried out in a standardized procedure by
using Image-pro plus 6.0 (soft imaging system, USA) software via
threshold analysis. In order to measure, we used rectangles of a standardized
size (1.1 mm2), aligning along the limbus as previously depicted[21]. The corneal area suffused with newborn vessels
(hemvascularized or lymphvascularized area) was calculated in each rectangle.
The ratio of vessel area was decided by the vascularized area of the treatment
group in correlation to that of the control group. The vascularized areas of
the control groups were regarded as being 100%. Analysis of differences between
two samples was achieved by using a standard two-tailed Student’s t-test
(SPSS 17.0 statistical software, USA). Values are expressed as mean±SEM. P
value of being less than 0.05 was regarded significant. In the figure, the
alphabet ‘a’ is used to represent a significant difference between 2 groups (P<0.01).
Origin 7.5 (Originlab Inc., USA) was used to draw graphs.
RESULTS
Ang-2 Expression on Physiological Blood
Vessels Immunofluorescence
staining was used to investigate whether Ang-2 was expressed on physiological
blood vessels at the limbus (border between vascularized conjunctiva and
nonvascularized cornea) in normal murine eyes. We found that blood vessels were
only found in limbus (Figures 1A and 2A), and a low-level expression of Ang-2
was co-localized on the quiescent CD31-positive blood vessels at the limbal
arcade and adjacent physiological vascularized conjunctiva in normal cornea
(Figure 1D). While, some inside epithelial cells near matrix was found also to
express Ang-2 (Figure 1B).
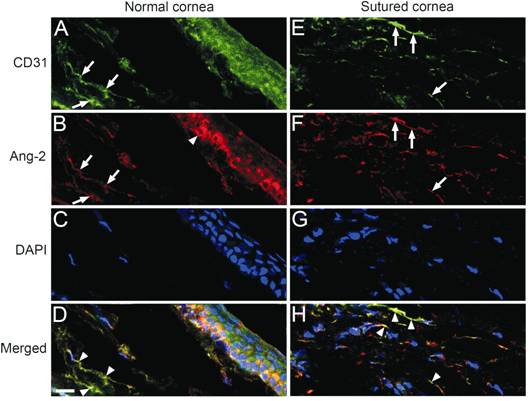
Figure
1 Representative images of Ang-2 expressed on the CD31-positive blood vessels
in the cornea stained by immunofluorescence (A, B, C,
D): Representative images of the immunofluorescence-stained limbus (border
between vascularized conjunctiva and nonvascularized cornea) of normal murine
eyes. A: Stained blood
vessels at limbus (CD31, green, while arrows); B: Stained Ang-2 at limbus (red,
white arrows) and on some epithelial cells (red, white arrowhead); C: Stained
nuclei [4’, 6-diamidino-2-phenylindole (DAPI), blue]; D: Merged images of A, B
and C (arrowheads: Co-location of Ang-2 on quiescent blood vascular
endothelial cells). E, F, G, H: Representative images of the cornea 10d
after suture stained by immunofluorescence; E: Stained blood vessels (CD31,
green, white arrows). F: Stained Ang-2 (red, white arrows); G: Stained nuclei
(DAPI, blue); H: Merged images of E, F and G (arrowheads: Ang-2 is
co-localized with CD31-positive blood vessels). Scale bar (D, bottom, left)=20
μm.
Ang-2
Expression on Pathological Blood Vessels Induced by Inflammation In this study, we used immunofluorescence staining to localize Ang-2 expression in
pathologically vascularized murine corneas and observed that angiogenesis was obviously induced by inflammation
(Figures 1E and 2E), and that the expression of Ang-2 was simultaneously
intensely raised in the sutured cornea (Figure 1F). We also found that the
newly generated blood vessels strongly expressed Ang-2 in the sutured cornea
(Figure 1H).
Tie2
Expression on Physiological Blood Vessels
Immuno-fluorescence staining was used to investigate whether Tie2
was expressed on physiological blood vessels at the limbus in normal murine
eyes. We found that blood vessels were only found in limbus (Figures 1A and
2A), and a low-level expression of Tie2 co-localized on the quiescent
CD31-positive blood vessels at the limbal arcade and adjacent physiological
vascularized conjunctiva in normal cornea (Figure 2D). While, some inside
epithelial cells near matrix was found also to express Tie2 (Figure 2B).
Tie2
Expression on Pathological Blood Vessels Induced by Inflammation In this study, we used immunofluorescence staining to localize Tie2 expression in
pathologically vascularized murine corneas and observed that angiogenesis was obviously induced by inflammation
(Figures 1E and 2E), and that the expression of Tie2 was simultaneously
intensely raised in the sutured cornea (Figure 2F). We also found that the
newly generated blood vessels strongly expressed Tie2 in the sutured cornea
(Figure 2H).
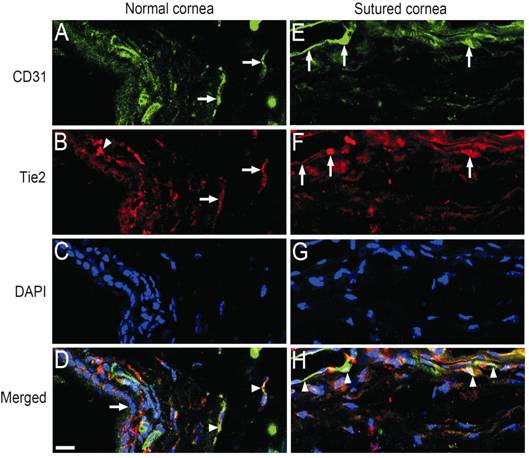
Figure
2 Representative images of Tie2 expressed on the CD31-positive blood vessels in
the cornea stained by immunofluorescence
A, B, C, D: Representative images of the limbus of normal murine
eyes stained by immunofluorescence. A: Stained blood vessels at limbus (CD31,
green, while arrows); B: Stained Tie2 at limbus (red, white arrows) and on some
epithelial cells (red, white arrowhead); C: Stained nuclei (DAPI, blue); D:
Merged images of A, B and C (arrowheads: co-location of Ang-2 on
quiescent blood vascular endothelial cells, arrows: limbus). E, F, G, H:
Representative images of the cornea 7d after suture stained by
immunofluorescence; E: Stained blood vessels (CD31, green, white arrows); F:
Stained Tie2 (red, white arrows); G: Stained nuclei (DAPI, blue); H: Merged
images of E, F and G (arrowheads: Tie2 is co-localized with
CD31-positive activated blood vessels). Scale bar (D, bottom, left)=20 μm.
Expression
of Ang-2 mRNA in Sutured
Corneas We used the murine model of suture-induced corneal neovascularization to
further explore the expression of Ang-2 mRNA in inflammatory angiogenesis. Quantitative
RT-PCR analysis was used to estimate the expression level
of Ang-2 mRNA. Our results show that normal corneas expressed a low-level Ang-2
mRNA, and that the expression of Ang-2 mRNA was triggered by inflammation in
the sutured corneas (Figure 3). Accompanying
inflammatory angiogenesis in inflamed corneas and the raised quantity and
density of inflammatory blood vessels during this time (Figure 4D), the expression of Ang-2 mRNA gradually raised lasting at least 14d.
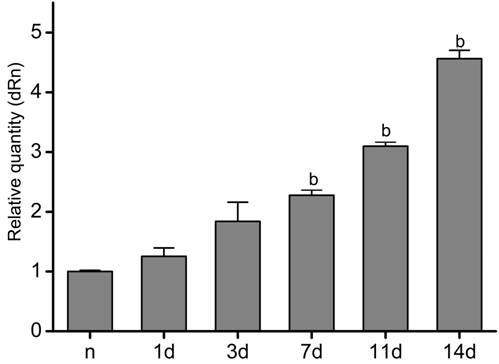
Figure
3 Expression of Ang-2 mRNA in the inflamed murine corneas Untreated
corneas expressed a low-level Ang-2 mRNA. Expression of Ang-2 mRNA was
increased gradually in inflamed murine
corneas after suture lasting about 2wk, as proved by qRT-PCR analysis. n
represents group of untreated corneas; 1, 3, 7, 11, and 14d represent groups of
corneas 1, 3, 7, 11, and 14d after suture, respectively. Data are presented as
the mean±SEM. bP<0.01 vs group of normal corneas.
L1-10,
a Specific Inhibitor of Ang-2, Increases Hemangio-genesis In our
study, whole mount staining indicated that blood and lymphatic vessels were
physiologically existing at the limbus (Figure 4A and 4B), and normal mouse
corneas were lack of blood and lymphatic vessels (Figure 4A and 4B), and that
corneal newborn blood and lymphatic vessels were interspersed through the
stroma after suture about 2wk (Figure 4D and 4E). L1-10, an Ang-2 specific
inhibitor, was used to judge the role of
Ang-2 in inflammatory angiogenesis in murine models of sutured
cornea. L1-10 was injected into mice subcutaneously
every other day starting at one day before surgery. The experiment group
received L1-10 (4 mg/kg), dissolved in PBS, while control group received the
same total of PBS solution. Mice were given the treatment for 14d after suture.
After 14d, the treatment group (n=7) showed significantly increased
blood vessel growth with raised vascular density but slimmer in diameter (P<0.01)
(Figure 4J) and reduced lymphatic growth (P<0.01) (Figure 4K)
compared with the control group (n=7). Therefore, inhibition of the
Ang-2/Tie2 signalling pathway by L1-10 efficiently promoted inflammatory
angiogenesis but obviously blocked inflammatory lymphangiogenesis, indicating
that Ang-2/Tie2 system is an important signaling pathway involving in regulating
inflammatory angiogenesis.
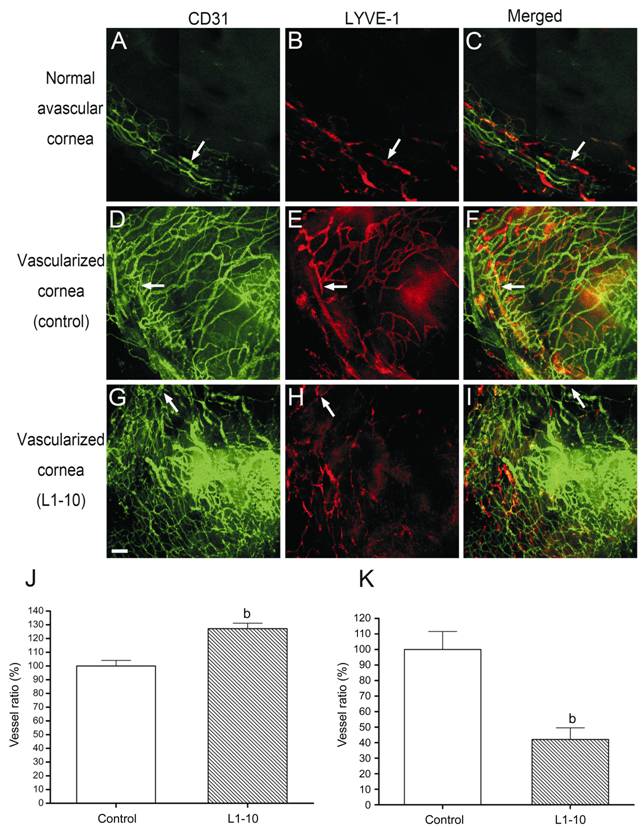
Figure
4 Systemic application of L1-10, an Ang-2 specific inhibitor, for 14d in the
mouse models of suture-induced corneal neovascularization L1-10 obviously promoted hemangiogenesis
in the inflamed murine corneas, but decreased lymphangiogenesis in the inflamed
murine corneas compared with the control group 14d after suture. A-I:
Representative segments of corneal whole mounts (green: blood vessels; red:
lymphatic vessels). Arrows: Limbus. A, D, G: CD31+ blood vessels; B,
E, H: LYVE-1+ lymphatic vessels; C, F, I: Merge of A and B, D and E,
and G and H, respectively. Raising of hemangiogenesis (J) (P<0.01, n=7)
and inhibition of lymphangiogenesis (K) (P<0.01, n=7) 2wk
after treatment with L1-10 in a suture-induced neovascularization assay. Vessel
area ratio: Area covered by blood/lymphatic vessels (%) in correlation to the
control (set to 100%). Data are expressed as the mean±SEM. bP<0.01 comparing
the treatment group with the control group. Scale bar (G, bottom, left)=100 μm.
DISCUSSION
Until
now, the function of Ang-2/Tie2 signaling pathway in inflammatory angiogenesis
remains largely undefined. In this study, we determine that both Ang-2 and Tie2 are merely weakly expressed on quiescent blood
vessel endothelium, however, inflammation induces obvious expression of Ang-2
and Tie2 on newly generated blood vessels. Upregulation of the expression of
Ang-2 and Tie2 occurred simultaneously with inflammatory angiogenesis. The
expression of Ang-2 mRNA was also strongly induced by inflammation in sutured
corneas, which was gradually upregulated for around 2wk following injury, while
the number and density of inflammatory blood vessels also were raised during this
time. These observations clearly demonstrate that Ang-2 expression is consistent,
and strictly controlled and dynamic, and it is
induced and obviously increased by exogenous stimuli which induce the
pathological process of angiogenesis, such as inflammation and hypoxia. For all
we know, this is the first time to report the expression of both Ang-2 and Tie2
on inflammatory blood vessels, suggesting that Ang-2/Tie2
signaling pathway may play an important role in inflammatory angiogenesis.
Previous
study shows Ang-2 plays an intricate role in regulation of vascular remodeling
that leads to either vessel sprouting or regression, depending on its context[8]. To enucleate the direct function of Ang-2/Tie2 signaling
pathway in inflammatory angiogenesis, we treated the murine models of
suture-induced inflammatory corneal neovascularization with the FC-fusion
protein L1-10, which blocks Ang-2 binding to its receptor Tie2 and
inhibits the proliferation of endothelial cells[28]. Current studies have proved that L1-10 is a specific and strong
depressor of Ang-2[29-30]. In
the current study, we showed that blockade
of the Ang-2/Tie2 signaling pathway by using L1-10 obviously promoted
inflammatory angiogenesis. These data suggest that Ang-2/Tie2 signaling pathway is a crucial system which involves in
inflammatory angiogenesis. Because blood vessels show the identical
morphological and functional characteristics in the eye as they do in other
tissues[25], we can guess that the blockade of
Ang-2 might affect angiogenesis in other organs, but the accurate timing and
the molecular mechanisms might be other than in the cornea because of the
different microenvironment.
In transplantation, the blood vessels and lymphatic
vessels have received tremendous attention because of theirs association with
graft survival and rejection[3]. A great amount of
animal and clinical studies have showed that graft survival depends on both
arms of the so-called immune reflex arc. This arc
comprises the lymphatic vessels as the afferent arm and the blood vessels as
the efferent arm. Through the lymphatic vessels antigens and dendritic cells
can get to local lymph nodes and trigger an immune response[30],
while via the blood vessels oxygen and nutrients can reach local tissue.
Therefore, specifically promoting hemangiogenesis could provide the graft with
more nutrients and benefit wound healing, and at the same time blockade of
lymphangiogenesis could result in inhibition of the induction of an immune
response. So it is important to distinguish ways to specifically upregulate
hemangiogenesis to promote wound healing and graft survival. Previous studies
show both inflammatory angiogenesis and inflammatory lymphangiogenesis can be
blocked by inhibiting VEGF-A/VEGFR-2 system[21], and blocking the VEGF-C/-D/VEGFR-3 signaling pathway inhibits the outgrowth of inflammatory lymphatic vessels in
contrast to hemangiogenesis[23]. However, there
are limitations and concerns associated with anti-Ang-2/Tie2 signaling pathway until now. Previous studies also show Ang-2 stimulates
pathologic angiogenesis, and inhibition of Ang-2 promotes neovascular
regression[31-33]. Here, we show for the first time that the different role of Ang-2/Tie2
system in inflammatory angiogenesis. So, we speculate that Ang-2/Tie2 system is
intricately involved in angiogenesis, and Ang-2/Tie2 system acts as accelerator
or inhibitor, which is dependent on tissue microenvironment. Blocking
Ang-2/Tie2 signaling pathway may found a new treatment option to promote
graft survival by boosting inflammatory hemangiogenesis, which is also
important for wound healing.
In
general, inflammatory corneal angiogenesis seems to rely on Ang-2/Tie2 signaling pathway. By
inhibiting this pathway, the ingrowths of blood vessels can be obviously
promoted in inflammatory cornea, which provide new field for therapeutic
exploration. This research effort is uncovering new, important molecular
regulator of inflammatory angiogenesis. Illustrating the role of Ang-2/Tie2 signaling pathway in inflammatory
angiogenesis might broaden our understanding of numerous pathological processes, and the discovering that Ang-2/Tie2 signalling
pathway is participated in the regulation of inflammatory angiogenesis suggests
that blockade of Ang-2/Tie2 system may have important therapeutic applications in some pathological processes
such as (corneal) graft survival, wound healing and carcinogenesis.
Acknowledgements
Foundations: Supported
by National Natural Science Foundation of China (No.81641174; No.30772262).
Conflicts
of Interest: Yan ZX, None; Luo Y, None; Liu NF,
None.
REFERENCES
1 Johnson KE, Wilgus TA. Vascular Endothelial Growth
Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv Wound Care (New Rochelle) 2014;3(10):647-661. [CrossRef]
[PMC free article] [PubMed]
2 Paduch R. The role of lymphangiogenesis and
angiogenesis in tumor metastasis. Cell
Oncol (Dordr) 2016;39(5):397-410.
[CrossRef] [PMC free article] [PubMed]
3 Fasciani R, Mosca L, Giannico MI, Ambrogio SA,
Balestrazzi E. Subconjunctival and/or intrastromal bevacizumab injections as
preconditioning therapy to promote corneal graft survival. Int Ophthalmol 2015;35(2):221-227. [CrossRef]
[PubMed]
4 Kumar VB, Binu S, Soumya SJ, K H, Sudhakaran PR.
Regulation of vascular endothelial growth factor by metabolic context of the
cell. Glycoconj J
2014;31(6-7):427-434. [CrossRef]
[PubMed]
5 Yamazaki Y, Morita T. Molecular and functional
diversity of vascular endothelial growth factors. Mol Divers 2006;10(4):515-527. [CrossRef]
[PubMed]
6 Khan KA, Bicknell R. Anti-angiogenic alternatives to
VEGF blockade. Clin Exp Metastasis
2016;33(2):197-210. [CrossRef]
[PMC free article] [PubMed]
7 Kumar M, Dhatwalia SK, Dhawan DK. Role of angiogenic
factors of herbal origin in regulation of molecular pathways that control tumor
angiogenesis. Tumour Biol 2016;37(11):14341-14354.
[CrossRef] [PubMed]
8 Wang S, Park JK, Duh EJ. Novel targets against
retinal angiogenesis in diabetic retinopathy. Curr Diab Rep 2012;12(4):355-363. [CrossRef]
[PubMed]
9 Isidori AM, Venneri MA, Fiore D. Angiopoietin-1 and
Angiopoietin-2 in metabolic disorders: therapeutic strategies to restore the
highs and lows of angiogenesis in diabetes. J
Endocrinol Invest 2016;39(11):1235-1246. [CrossRef]
[PubMed]
10 Gale NW, Thurston G, Hackett SF, Renard R, Wang Q,
McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA,
Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal
angiogenesis and lymphatic patterning, and only the latter role is rescued by
Angiopoietin-1. Dev Cell
2002;3(3):411-423. [CrossRef]
11 Shimoda H. Immunohistochemical demonstration of
Angiopoietin-2 in lymphatic vascular development. Histochem Cell Biol 2009;131(2):231-238. [CrossRef]
[PubMed]
12 Yuan HT, Suri C, Landon DN, Yancopoulos GD, Woolf
AS. Angiopoietin-2 is a site-specific factor in differentiation of mouse renal
vasculature. J Am Soc Nephrol
2000;11(6):1055-1066. [PubMed]
13 Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA.
Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol 2002; 192(2):182-187. [CrossRef]
[PubMed]
14 Visconti RP, Richardson CD, Sato TN. Orchestration
of angiogenesis and arteriovenous contribution by angiopoietins and vascular
endothelial growth factor (VEGF). Proc
Natl Acad Sci U S A 2002;99(12):8219-8224. [CrossRef]
[PMC free article] [PubMed]
15 Eklund L, Olsen BR. Tie receptors and their
angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res 2006;312(5):630-641. [CrossRef]
[PubMed]
16 Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY.
Angiopoietin-2 at high concentration can enhance endothelial cell survival
through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Oncogene 2000;19(39):4549-4552. [CrossRef]
[PubMed]
17 Bogdanovic E, Nguyen VP, Dumont DJ. Activation of
Tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor
internalization. J Cell Sci
2006;119(Pt 17):3551-3560. [CrossRef]
[PubMed]
18 Mochizuki Y, Nakamura T, Kanetake H, Kanda S.
Angiopoietin 2 stimulates migration and tube-like structure formation of murine
brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci 2002;115(Pt 1): 175-183. [PubMed]
19 Chen JX, Chen Y, DeBusk L, Lin W, Lin PC. Dual functional
roles of Tie-2/angiopoietin in TNF-alpha-mediated angiogenesis. Am J Physiol Heart Circ Physiol
2004;287(1):H187-H195. [CrossRef]
[PubMed]
20 Yan ZX, Jiang ZH, Liu NF. Angiopoietin-2 promotes
inflammatory lymphangiogenesis and its effect can be blocked by the specific
inhibitor L1-10. Am J Physiol Heart Circ
Physiol 2012;302(1):H215-H223. [CrossRef]
[PubMed]
21 Bock F, Onderka J, Dietrich T, Bachmann B, Kruse
FE, Paschke M, Zahn G, Cursiefen C. Bevacizumab as a potent inhibitor of
inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci 2007;48(6):2545-2552. [CrossRef]
[PubMed]
22 Cursiefen C, Maruyama K, Jackson DG, Streilein JW,
Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal
inflammation. Cornea
2006;25(4):443-447. [CrossRef] [PubMed]
23 Bock F, Onderka J, Dietrich T, Bachmann B, Pytowski
B, Cursiefen C. Blockade of VEGFR3-signalling specifically inhibits
lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch Clin Exp Ophthalmol
2008;246(1):115-119. [CrossRef]
[PubMed]
24 Cursiefen C, Chen L, Borges LP, Jackson D, Cao J,
Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A
stimulates lymphangiogenesis and hemangiogenesis in inflammatory
neovascularization via macrophage recruitment. J Clin Invest 2004;113(7): 1040-1050. [CrossRef]
[PMC free article] [PubMed]
25 Cursiefen C, Schlötzer-Schrehardt U, Küchle M,
Sorokin L, Breiteneder-Geleff S, Alitalo K, Jackson D. Lymphatic vessels in
vascularized human corneas: immunohistochemical investigation using LYVE-1 and
podoplanin. Invest Ophthalmol Vis Sci
2002;43(7): 2127-2135. [PubMed]
26 Dietrich T, Onderka J, Bock F, Kruse FE, Vossmeyer
D, Stragies R, Zahn G, Cursiefen C. Inhibition of inflammatory
lymphangiogenesis by integrin α5 blockade. Am
J Pathol 2007;171(1):361-372. [CrossRef]
27 Livak KJ, Schmittgen TD. Analysis of relative gene
expression data using real-time quantitative PCR and the 2(-Delta Delta C(T))
Method. Methods 2001;25(4):402-408. [CrossRef]
[PubMed]
28 Oliner J, Min H, Leal J, et al. Suppression of angiogenesis and tumor growth by selective
inhibition of angiopoietin-2. Cancer Cell
2004;6(5):507-516. [CrossRef]
[PubMed]
29 Tressel SL, Kim H, Ni CW, Chang K,
Velasquez-Castano JC, Taylor WR, Yoon YS, Jo H. Angiopoietin-2 stimulates blood
flow recovery after femoral artery occlusion by inducing inflammation and
arteriogenesis. Arterioscler Thromb Vasc
Biol 2008;28(11):1989-1995. [CrossRef]
[PMC free article] [PubMed]
30 Fang SC, Zhang HT, Hu HD, Wang CY, Zhang YM. Effect
of Endostar combined with angiopoietin-2 inhibitor on malignant pleural
effusion in mice. Med Oncol
2015;32(1):410. [CrossRef] [PubMed]
31 Carmeliet P, Jain RK. Molecular mechanisms and
clinical applications of angiogenesis. Nature
2011;473(7347):298-307. [CrossRef]
[PMC free article] [PubMed]
32 Rennel ES, Regula JT, Harper SJ, Thomas M, Klein C,
Bates DO. A human neutralizing antibody specific to Ang-2 inhibits ocular
angiogenesis. Microcirculation
2011;18(7):598-607. [CrossRef]
[PubMed]
33 Palmer GM, Tiran Z, Zhou Z, Capozzi ME, Park W,
Coletta C, Pyriochou A, Kliger Y, Levy O, Borukhov I, Dewhirst MW, Rotman G,
Penn JS, Papapetropoulos A. A novel angiopoietin-derived peptide displays
anti-angiogenic activity and inhibits tumour-induced and retinal
neovascularization. Br J Pharmacol
2012;165(6):1891-1903. [CrossRef]
[PMC free article] [PubMed]