
·Basic Research· Current
Issue IF in JCR CiteScore Rank ·Online Submission· Articles in Press PMC RSS
Citation: Shao XL, Chen Y, Gao L. MiR-200c suppresses the migration of
retinoblastoma cells by reversing epithelial mesenchymal transition. Int J
Ophthalmol 2017;10(8):1195-1202
MiR-200c suppresses the migration of
retinoblastoma cells by reversing epithelial mesenchymal transition
Xiao-Lei Shao1,2, Yao Chen1,
Ling Gao1
1Department of Ophthalmology, the Second Xiangya Hospital, Central
South University, Changsha 410011, Hunan Province, China
2Shenzhen Eye Hospital, Affiliated Shenzhen Eye Hospital of Jinan
University, Joint College of Optometry, Shenzhen Universtiy, Shenzhen Key
Laboratory of Ophthalmology, Ocular Trauma Treatment and Stem Cell
Differentiation Public Service Platform of Shenzhen, Shenzhen 518040, Guangdong
Province, China
Correspondence
to: Ling Gao. Department of Ophthalmology, The Second Xiangya
Hospital, Central South University, Changsha 410011, Hunan Province, China.
gaoling6287@163.com
Received:
2016-10-09
Accepted: 2017-04-17
Abstract
AIM: To
analyze the relationship between clinical features and epithelial mesenchymal
transition (EMT) in retinoblastoma (RB), further to investigate whether
miR-200c regulates the EMT and migration of RB cells.
METHODS: Expression
of EMT-related markers and tumor-related factors were detected by
immuno-histochemistry analysis in RB tissue from 29 cases. Correlations between
their expression and clinical characteristics were analyzed. The regulation
effects of miR-200c on EMT-related markers, tumor-related factors were observed
in mRNA level and protein level by real-time polymerase chain reaction (PCR)
and Western blot, respectively, in Y79 and Weri-rb1 cells. Its effects on
migration force of these RB cell lines were also detected with Transwell test.
RESULTS: Lower
expression of E-cadherin was present in the cases with malignant prognosis.
MiR-200c promoted the expression of E-cadherin and decreased the expression of
Vimentin and N-cadherin in Y79 and Weri-rb1 cells. Migration force of RB cells
could be inhibited by miR-200c.
CONCLUSION: EMT
might be associated with bad prognosis in RB. MiR-200c suppresses the migration
of retinoblastomatous cells by reverse EMT.
KEYWORDS: retinoblastoma;
epithelial mesenchymal transition; miR-200c; E-cadherin
DOI:10.18240/ijo.2017.08.02
Citation: Shao XL, Chen Y, Gao L. MiR-200c suppresses the migration of
retinoblastoma cells by reversing epithelial mesenchymal transition. Int J
Ophthalmol 2017;10(8):1195-1202
INTRODUCTION
As
a key developmental program, epithelial to mesenchymal transition (EMT) occurs
in many important biological processes[1-2]. Moreover, it plays
important functions in cancer invasion and metastasis that lead to malignant
prognosis in many tumors[2-4]. The symbol of EMT is the loss of
epithelial phenotypes such as expression of specific marker E-cadherin and
intercellular tight adhesion. By this procedure, the relative differentiated
epithelial carcinoma cells transit
to mesenchymal cells with more invasive dedifferentiated characteristics. A
direct link between EMT and the generation of cancer stem cells had been
verified[5]. Moreover, EMT enables the transmitted cells with
capacities of colonization to build distant metastases[4-5].
MicroRNAs
(miRNAs) are small noncoding single-stranded RNAs (18-25 nucleotides) that
widely exist in mammalian cells and have essential regulating effects in many
cell processes, such as differentiation, proliferation, and apoptosis of cancer[6-7].
They are essential regulators of cancer progressions. Some miRNAs had been
demonstrated to act as oncogenes[8-9], while the others were cancer
suppressors[10-12]. MiR-200c had been verified to suppress the EMT
program and therefore down-regulate the metastasis by targeting Zeb family, the
repressor of E-cadherin, in several malignant tumors[7,13-14].
Retinoblastoma
(RB) is the most common malignant intraocular tumor in children[15].
It originates from the inner nuclear layer of retina and is directly caused by
the mutations of tumor repressor-RB1 gene[6,16]. Intracranial
infiltration and the secondary metastatic tumors are the major causes of death[17].
In clinical practices, we find that not all the tumors at late stage will
definitely lead to bad outcomes, while some patients with early stage tumors
even step to death. EMT has been showed correlation with the prognosis in many
tumors, including lung[18], colorectal[19], cervical[20],
tongue[21] carcinoma and so on. One of the most critical hallmarks
of EMT is the loss of E-cadherin[22]. The expression of E-cadherin
has been demonstrated to be correlated inversely with prognosis in some
epithelial-derived cancers[18,20,23]. However, as far as we know, no
related research had been reported on RB. In order to detect which EMT factor
can predict prognosis of RB, in addition to EMT factors including E-cadherin,
Vimentin and N-cadherin, we also selected EMT-related genes such as cancer stem
cell factor CD133 and drug resistant factor ABCG2 as candidate factors. In our
study, for the first time we investigate the correlation of these target
factors and clinical features in the RB specimens.
It
has been demonstrated that miR-200c up-regulates the expression of E-cadherin
by targeting its transcription repressor-zeb1, a double negative feedback loop
between miR-200c and zeb1 regulates EMT in several tumor cell lines[7,14,24].
However, there is no such research had been done on RB cell lines. In order to
investigate whether the EMT changes the features of tumor cells and whether the
negative feedback loop between miR-200c and zeb1 regulates the EMT process in
RB cell lines, Weri-RB1 and Y79 were cultured for in vitro studies.
SUBJECTS AND METHODS
Tumor Specimens Clinical data and tissue specimens from 29 RB cases were collected from
the Second Xiangya Hospital of Central South University between 2005 and 2011.
All the cases were diagnosed and classified according to the Internatioanl
Classification of Retinoblastoma (ICRB)[25]. Diagnosis of RB was
confirmed by histopathological examination. The procedure of this study was
approved by the Ethics Committee of the Second Xiangya Hospital.
Immunohistochemistry All
specimens were fixed in 10% paraformaldehyde and embedded in paraffin, then
sectioned in 5 µm, and stained with hematoxylin and eosin. The RB specimens
were divided into differentiated and non-differentiated groups according to the
presence or absence of rosettes. The specimens were also divided into optic
nerve-infiltrated and non-infiltrated groups according to tumor infiltration.
The paraffin-embedded sections were dewaxed, dehydrated, and heated in pressure
cooker for antigen retrieval (2min after reaching full pressure), then
incubated with 3% peroxidase for 10min at room temperature. After incubated in
primary antibodies overnight at 4℃ the sections were incubated with
Polymer Helper reagent (KIT-5020, MaxVisionTM, China) for 20min at
37℃ and rinsed with phosphate buffer saline (PBS). Primary antibody
information: E-cadherin (#3195 1:400 Cell Signaling Technology); N-cadherin
(ab12221, 1:300, Abcam); Vimentin (sc-66002, 1:200, Santa Cruz); ABCG2
(BXP-21, 1:40, Abcam); CD133 (AC133, 1:100, Miltenyi Biotec, Germany).
Afterwards, the samples were incubated with poly peroxidase-anti-rabbit IgG for
20min at room temperature. After PBS washing, the sections were stained with
3,3'-diaminobenzidine (DAB) solution
(Beijing Zhongshan, China), counterstained with hematoxylin, routinely
dehydrated, and then mounted. PBS replaced the antibodies as a negative
control, and a known positive section was used as a positive control.
Scoring In the preliminary observation, we found the clue to association with
E-cadherin and outcomes, then H score of the immunochemistry staining of
E-cadherin was done[26-27]. The stain intensity was scored as: 0, 1,
2, or 3. A score of 0+ for negative, less than 10% discernible membranous
staining; 1+ for weak and/or incomplete membranous staining required at least
×200 magnification to confirm positive staining; 2+ for weak to moderate,
continuous membranous staining that was not readily apparent at low (×40)
magnification; 3+ for strong membranous staining that was readily apparent at
low magnification. Only membranous staining was scored. We estimated the
fraction of tumor cells in each case that had 0+, 1+, 2+, and 3+ intensity to
allow the calculation of an H score. The H score was calculated based on the following
formula: H score=(% Tumor 1+)+ 2×(% Tumor 2+)+ 3×(% Tumor 3+) The H score can,
therefore, range from 0 (cases with absent staining) to 300 (cases with 100% 3+
staining)[26].
Cell
Culture Human RB
cell lines Y79 and Weri-RB1 were obtained from the American Type Culture
Collection (ATCC, Manassas, VA, USA). The cells were cultured in RPMI 1640
medium, supplemented with 10% fetal bovine serum (FBS) (Gibco, Invitrogen,
India). The cells were grown under a humidified atmosphere of 5% CO2-95%
air at 37℃ and the media was changed every other day.
miRNA
Transfection The short
interfering mimics and inhibitor of miR-200c and negative control mRNA were
chemically synthesized by GenePharma (China). The miR-200c were transiently
transfected in Y79 and Weri-RB1 cell lines with Lipofectamine 2000 (Invitrogen)
as described in manufacture’s protocol respectively. Briefly, Lipofectamine®2000
(Invitrogen) (5 μL) combined with miRNA at a concentration of 30 nmol/L and
incubated in OPTI-MEM I reduced serum medium (Invitrogen, CA, USA) for 20min
prior to transfection. The combined medium was then added into a 6-well plate
with 5×104 cells per well. Cells were incubated at 37℃ for 6h
before replacement of medium. Then cells were harvested for 48h before further
analysis.
Western
Blot For the
analysis of protein expression in the Y79 and Weri RB cell lines, 1×106 cells
were washed with ice-cold PBS twice and lysed with cell lysis buffer at 4℃ for 30min.
Cell debris were removed by centrifugation at 15 000× g for 15min at 4℃. Equal
amounts of proteins were separated by 12% SDS-PAGE and transferred onto
nitrocellulose membrane. The membranes were first stained to confirm the
uniform transfer of all samples and then incubated in the blocking solution for
2h at room temperature. The membranes were first incubated with monoclonal
antibodies (rabbit Zeb1 antibody, SANTA, SC-25388n,1:500; Goat E-cadherin
antibody, SANTA, SC-31020, 1:500; Mouse Vimentin antibody, SANTA, SC-58901,
1:800; Rabbit N-cadherin antibody, Abcam, ab12221, 1:500; Mouse GAPDH antibody,
SANTA, SC-365062, 1:800) for 2h, after washed with PBS twice and tris buffered
saline tween (TBST) twice. The membranes were incubated with corresponding
horseradish peroxidase (HRP)-conjugated secondary antibodies (SANTA, USA) before
washing with TBST. GAPDH was used as the internal control. A BIO-RAD Western
blotting detection system was used to detect the immune-reactive proteins.
RNA
Extraction and Quantitative Polymerase Chain Reaction Analysis of MiR-200c TRIzol
reagent (Invitrogen) was used to extract the total RNAs from the fresh cells.
The miRNeasy Mini kit (Qiagen, USA) and miRNA Q-polymerase chain
reaction (PCR) Detection kit (GeneCopoeia, USA) were used in real-time PCR
assays. Quantitative PCR was performed using a ABI prism 7900HT REAL-Time PCR
System (Applied Biosystems). The procedure of PCR was performed as follows:
denaturation (95℃, 5min), amplification (40
cycles), denaturation (95℃, 10s), annealing (60℃, 20s),
elongation (72℃, 10s). Reactions were performed in a total volume of 20 μL in
triplicate. The miRNA levels were normalized to U6. The oligonucleotide primers
were as follows: has-miR-200c forward (5’-TAATACTGCCGGGTAATGATGGA-3') and
reverse (5’-TGGTGTCGTGGAGTCG-3'); U6RNA forward (5’-GCTTCGGCAGCACATATACTAAAAT-3')
and U6RNA reverse (5’-CGCTTCACGAATTTGCGTGTCAT-3'). The miRNA expression was
determined using the 2-ΔCt method.
Real-time
Reverse Transcription-polymerase Chain Reaction Total RNA
was prepared and reverse transcribed using RevertAid™ H Minus First Strand cDNA
Synthesis Kit (Fermentas) according to the manufacturer’s protocol. SYBR Green
real-time PCR was performed using the following primers (Invitrogen):
GAPDH-F:
5’-AAATTGAGCCCGCAGCCTCCC
GAPDH-R:
5’-GCGCCCAATACGACCAAATCCGT
Zeb1-F:
5’-TGACCTGCCAACAGACCAGACA
Zeb1-R:
5’-CCTTTCCTGTGTCATCCTCCCAGC
E-cadherin-F:
5’- TCCACGCCGAGCCCCAGTAT
E-cadherin-R:
5’- TCAGCCGCTTTAAGGCCCTCAT
Vimentin-F:
5’-TGGCCGCCTGCAGGATGAGAT
Vimentin-R:
5’-AGAGAAATCCTGCTCTCCTCGCCT
N-cadherin-F:
5’- CCCACCACGTACAAGGGTCAGGT
N-cadherin-R:
5’- ACGCTGGGGTATTGGGGGCA
Zeb1,
E-cadherin, Vimentin, N-cadherin and GAPDH-specific fragments were amplified by
40 cycles of PCR, respectively, each cycle comprising 20s at 94℃, 20s at 55℃, and 32s at
72℃. The relative mRNA levels were calculated using the 2-ΔCt method.
In Vitro Motility Assay In vitro motility
assay was performed with 6-well transwell chambers with 8-μm porous membrane
(Corning Incorporated, Corning, NY, USA)[28]. The upper chamber was
filled with serum-free media and the bottom chamber was filled with media
containing 10% FBS. Cells were washed three times with PBS and 10 000 cells
were added to the top chamber. Cells were incubated in a 5% CO2
humidified incubator for 22h at 37℃. After
removed the upper chamber , the migrated cells in the lower chamber were
counted.
Statistical
Analysis Linear
regression and Pearson correlation were calculated for association between
miR-200c and EMT markers (E-cadherin, Vimentin and N-cadherin) at RNA level.
The correlation coefficient was reported according to the R2
and P value. P value <0.05 was considered as statistical
significance.
RESULTS
Clinical
Features of Retinoblastoma Cases The study
included 15 boys (51.7%) and 14 girls (48.3%). The mean age at enucleation was
2.20±1.39y (ranged from 5mo to 8y). Bilateral tumor was detected in 4 children
(13.8%) and unilateral in 25 patients (86.2%). According to the ICRB[25,29],
20 patients were classified in stage D (69.0%), and 9 in stage E (31.0%). The
optic nerve was infiltrated in 9 patients (31.0%) and 20 patients were
non-infiltrated (69.0%). For differentiation degree, 15 samples (51.7%) were
differentiated, and 14 (48.3%) were undifferentiated. Five cases (17.2%) were
dead within one year after enucleation, 24 (82.8%) were survival after at least
3y follow-up. Of the 24 survival, 3 got relapse after treatment, and 21 were
relapse-free. Details of above information were listed in Table 1.
Table 1 Basic information of the 29 RB patients
|
Case
No. |
Gender |
OD/OS/OU |
Age |
Differentiation |
Optic
nerve infiltration |
Group of
ICRB |
Living
condition |
|
1 |
F |
OS |
2a |
Differentiated |
- |
D |
Survive |
|
2 |
F |
OS |
2a |
Differentiated |
- |
D |
Survive |
|
3 |
F |
OD |
1a |
Undifferentiated |
- |
D |
Survive |
|
4 |
F |
OU |
2a |
Differentiated |
- |
D |
Survive |
|
5 |
F |
OU |
4a |
Differentiated |
- |
D |
Survive |
|
6 |
M |
OS |
2a |
Differentiated |
- |
D |
Survive |
|
7 |
M |
OD |
2a |
Differentiated |
- |
D |
Survive |
|
8 |
F |
OD |
3a |
Undifferentiated |
+ |
E |
Survive |
|
9 |
F |
OD |
3a |
Differentiated |
- |
D |
Survive |
|
10 |
M |
OS |
3a |
Differentiated |
- |
D |
Survive |
|
11 |
F |
OD |
2a |
Differentiated |
- |
D |
Survive |
|
12 |
M |
OU |
5mo |
Differentiated |
- |
D |
Survive |
|
13 |
M |
OD |
2a |
Differentiated |
+ |
E |
Survive |
|
14 |
F |
OS |
6mo |
Undifferentiated |
- |
D |
Survive |
|
15 |
F |
OD |
2a |
Undifferentiated |
- |
D |
Survive |
|
16 |
M |
OS |
2a |
Undifferentiated |
- |
D |
Survive |
|
17 |
F |
OD |
1a |
Undifferentiated |
+ |
E |
Survive |
|
18 |
F |
OS |
2a |
Undifferentiated |
+ |
E |
Survive |
|
19 |
M |
OS |
3a |
Differentiated |
- |
D |
Survive |
|
20 |
F |
OD |
3a |
Differentiated |
- |
D |
Survive |
|
21 |
M |
OD |
2a |
Differentiated |
+ |
E |
Survive |
|
22 |
M |
OS |
2a |
Undifferentiated |
- |
D |
Recurrent,
survive |
|
23 |
M |
OU |
2a |
Undifferentiated |
- |
D |
Recurrent,
survive |
|
24 |
M |
OD |
1a |
Undifferentiated |
+ |
E |
Recurrent,
survive |
|
25 |
M |
OD |
1a |
Differentiated |
- |
D |
Die |
|
26 |
F |
OD |
2a |
Undifferentiated |
+ |
E |
Die |
|
27 |
M |
OS |
8a |
Undifferentiated |
+ |
E |
Die |
|
28 |
M |
OS |
3a |
Undifferentiated |
+ |
E |
Die |
|
29 |
M |
OD |
1a |
Undifferentiated |
- |
D |
Die |
Decreased
Expression of E-cadherin in Retinoblastoma Specimens with Bad Prognosis To evaluate
the association of EMT and clinical features in RB, expression of epithelial
marker E-cadherin, mesenchymal marker Vimentin and N-cadherin, stem cell marker
CD133 and drug-resistance protein ABCG2 in 29 specimens were analyzed by
immunohistochemistry. In the preliminary observation, we found the clue to
association with E-cadherin and outcomes, then H score was used to quantitate
the expression[26-27]. The student t-test analysis showed
that the expression of E-cadherin had no significant difference between the two
groups of male and female, of differentiated and undifferentiated type, of
optic nerve involved and uninvolved. However it is significantly lower expressed
in the cases with bad prognosis (Table 1). The expressions of E-cadherin in the
specimens of 5 dead patients were significantly lower than those 24 survivals (P<0.05).
E-cadherin was significant higher expressed in the 21 relapse-free tumors when
compared with 8 recurrent and metastatic tumors (including 5 death) (P<0.01)
(Table 2). However when we analyzed the expression of Vimentin, N-cadherin,
CD133 and ABCG2 with the above-mentioned clinical features, no significant
difference had been detected (data not shown). The immune-histochemical
pictures of two patients who had different expression of E-cadherin and
prognosis was showed in Figure 1.
Table 2 Association of E-cadherin expression
immunohistochemically with clinical features in RB cases
|
Clinical
features |
Case No. |
H-score of E-cadherin |
P |
H-score of N-cadherin |
P |
H-score of ABCG |
P |
H-score of CD133 |
P |
|||||
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
|||||||
|
Sex |
F |
14 |
106.07 |
35.00 |
0.346 |
149.29 |
24.01 |
0.368 |
139.29 |
21.74 |
0.198 |
150.71 |
23.03 |
0.479 |
|
M |
15 |
91.13 |
47.37 |
156.67 |
18.77 |
150.33 |
23.26 |
157.33 |
26.31 |
|||||
|
Differentiation |
Differentiated |
15 |
108.27 |
35.01 |
0.191 |
156.67 |
22.89 |
0.363 |
140.67 |
19.81 |
0.298 |
159.33 |
23.74 |
0.245 |
|
Undifferentiated |
14 |
87.71 |
47.02 |
149.29 |
19.79 |
149.64 |
25.61 |
148.57 |
25.07 |
|||||
|
Optic
nerve involvement |
Optic
nerve not involved |
20 |
99.40 |
38.07 |
0.884 |
145.56 |
16.67 |
0.208 |
144.25 |
21.72 |
0.797 |
155.50 |
22.82 |
0.664 |
|
Optic
nerve involved |
9 |
96.00 |
51.67 |
156.50 |
22.77 |
146.67 |
26.46 |
151.11 |
29.34 |
|||||
|
Survival
or not |
Survival |
24 |
107.00 |
35.24 |
0.012 |
151.30 |
22.01 |
0.385 |
146.04 |
23.45 |
0.600 |
152.50 |
22.31 |
0.442 |
|
Death |
5 |
56.80 |
49.92 |
160.00 |
18.97 |
140.00 |
21.21 |
162.00 |
35.64 |
|||||
|
Recurrence
and metastasis |
Relapse-free |
21 |
110.48 |
27.62 |
0.009 |
153.33 |
21.76 |
0.369 |
141.19 |
20.61 |
0.149 |
152.38 |
20.71 |
0.542 |
|
Relapse |
8 |
66.50 |
56.70 |
161.25 |
18.08 |
155.00 |
26.73 |
158.75 |
33.99 |
|||||
P<0.05:
Statistical significance in t-test.
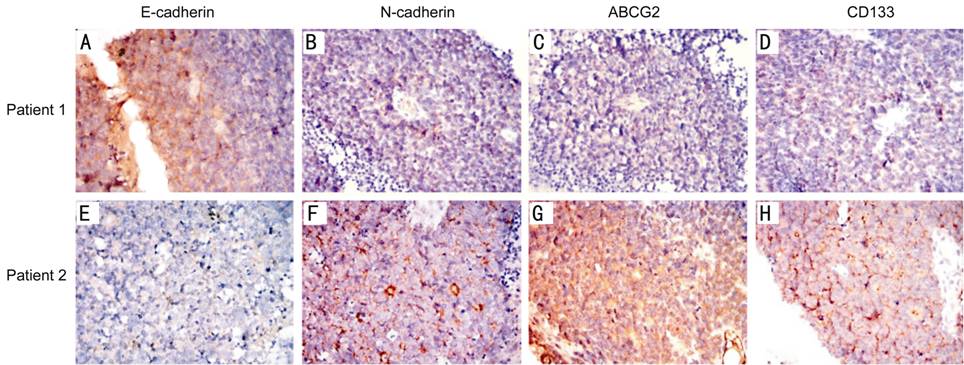
Figure
1 Expression of E-cadherin, N-cadherin, ABCG2 and CD133 in two patients with
completely opposite prognosis (100×) In the immunohistochemical test, positive
expression of the target antibody was displayed in brown. Patient 1 (case 13):
male, 2 years old of onset, E class in ICRB, survived more than 10y after
enucleation. The tumor sample showed strongly positive expression of E-cadherin
and nearly negative expression of N-cadherin, ABCG2 and CD133. Patient 2
(case 25): male, 1 year old of onset, D class in ICRB, died one year after
enucleation. E-cadherin was low expressed in the tumor, while the expression of
N-cadherin, ABCG2 and CD133 was strongly positive.
MiR-200c
Represses Zeb1 and Enhances the Expression of E-cadherin in Retinoblastoma
Cells To
investigate whether the miR-200c regulates the Zeb1 protein and EMT process in
RB cells, the Weri-RB1 and Y79 cells were transfected with miR-200c mimics,
inhibitors or control mRNA. The expression of Zeb1 was repressed by the
transfection of miR-200c mimics and enhanced by miR-200c inhibitors in both
mRNA level and protein level. The suppression of Zeb1 in miR-200c-mimic-transfected
cells was accompanied by a restoration of E-cadherin and decreased expression
of mesenchymal markers N-cadherin and Vimentin (mRNA and protein) (P<0.05).
On the contrary, transfection of miR-200c inhibitor caused a significant
reduction of E-cadherin and a concomitant induction of Vimentin and N-cadherin
(P<0.05) (Figure 2), which induced EMT. Immunofluorescence expression
of E-cadherin was shown in Figure 3. We use Pearson correlation and linear
regression to analyze the correlation between miR-200c and E-cadherin,
Vimentin, N-cadherin mRNA. It shows that E-cadherin mRNA correlated
significantly with miRNA-200c (P<0.01), while Vimentin and N-cadherin
mRNA negatively correlated with miRNA-200c (P<0.05). R2
and P values were shown in scatter plot graphs in Figure 4.
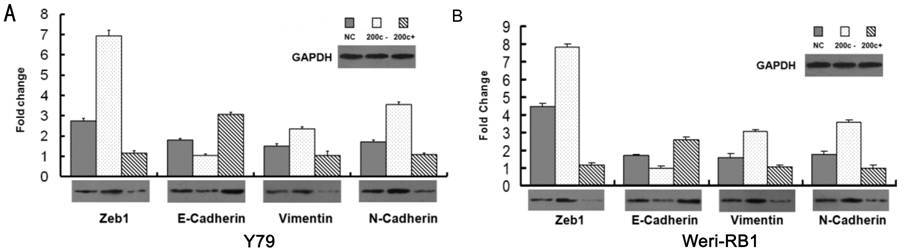
Figure
2 MiRNA-200c inhibits EMT in the Y79 and Weri-RB1 cells in both mRNA level and
protein level A: Y79
cells; B: Weri-RB1 cells. Cells were transfected with miR-200c inhibitor or
miR-200c mimics in vitro. Over-expression of miR-200c improves
E-cadherin expression and depresses Zeb1, N-cadherin and Vimentin, statistical
significance was detected in both protein and mRNA level (P<0.05).
Inhibition of miR-200c up-regulated the expression Zeb1, Vimentin and
N-cadherin, while down-regulated E-cadherin (P<0.05). The mRNA and
protein expression of Zeb1, E-cadherin, Vimentin and N-cadherin was quantified
by real-time RT-PCR (the bar charts) and Western blot (the panels)
respectively.
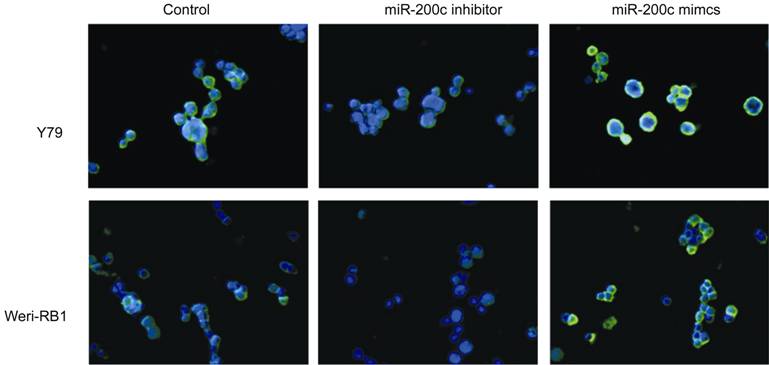
Figure
3 Immunofluorescence analysis of E-cadherin expression (green)
in Y79 and Weri-RB1 cells (400×) Cells
were counterstained with DAPI.
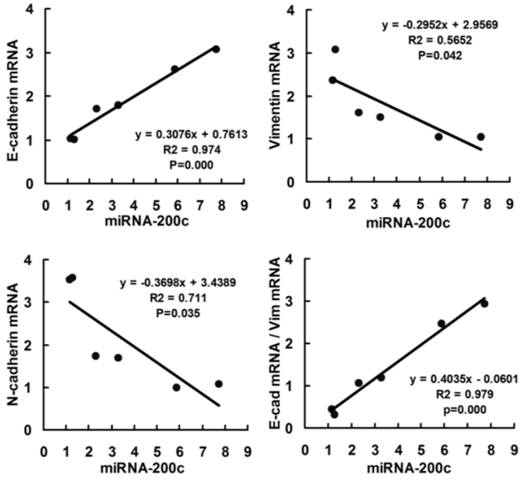
Figure
4 Scatter plot showing correlation between miR-200c and EMT markers
(E-cadherin, Vimentin and N-cadherin) in mRNA level in two RB cell lines Data of 6 groups (control, miR-200c+ and
miR-200c- in Y79 and Weri-RB1 cell lines) were plotted and linear regression
was applied for correlation analyzed.
MiR-200c
Inhibit the Migration of Retinoblastoma Cells It has been
previously demonstrated that the miR-200 family members can decrease the
migration and invasion in tumor cells[7,30-31]. We observed that the
migration of RB cells can be reversely regulated by the expression of miR-200c.
Reintroduction of miR-200c to Y79 and Weri-RB1 cells results in a 67.77% and a
54.74% decrease in migration respectively (Figure 5). On the contrary, when the
miR-200c was down-regulated with transfection of miR-200c inhibitors, the
migrating force increases 144% in Y79 and 164.25% in Weri-RB1 cells (Figure 5).
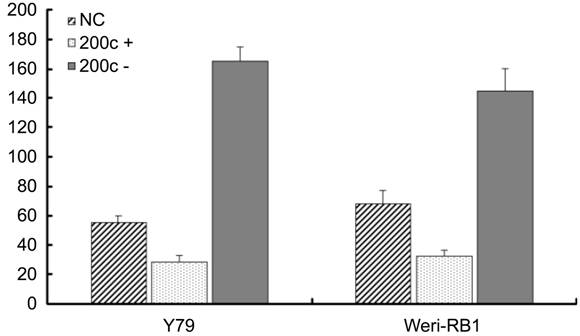
Figure
5 MiR-200c inhibited the in vitro migration of retinoblastoma cells in
motility assay After 24h
incubation with miR-200c, the cells migrated into under chambers were
quantified. The average cell number of 5 high magnification views was recorded
as the cell density. MiR-200c mimics transfected group (miR-200c+) showed
reduced cell migration, the difference is statistically significant (P<0.05),
however, the transfection of miRNA-200c inhibitors (miR-200c-) significantly
enhance the migration of the two RB cell lines (Y79 and Weri-RB1) (P<0.05).
DISCUSSION
In
the 29 samples of RB patients and the two RB cell-lines (Weri-RB1 and Y79),the co-expression of E-cadherin,
Vimentin and N-cadherin was detected in all the samples. It indicated the
middle status between the epithelial and mesenchymal phenotypes, like most of
cancer cells. Lower expression of E-cadherin protein was detected significantly
in the patients with recurrent and metastatic tumor. E-cadherin is a member of
Ca2+ dependent transmembrane glycoproteins that regulate cell-cell
adhesion[32-33]. Loss of E-cadherin reduces the cell-cell adhesion
and promotes the cellular mobility and invasion in tumors, so that leads to bad
prognosis[18,20,23]. Combining with the data in other
epithelial-derived tumors including gastrointestinal cancer, non-small cell
lung cancer, and cervical carcinoma[18,20,23], the prognosis of RB
may be somehow forecasted with the expression of E-cadherin. However, only 29 specimens
with complete clinical information were involved into our research. The sample
size, especially the sample with bad prognosis, is quite small, so the
statistical bias is inevitable. A larger sample with longer follow-up
information is required to further prove the correlation between E-cadherin and
prognosis.
In
vitro study of RB cell lines (Y79 and Weri-RB1), we proved that
over-expression of miR-200c repressed Zeb1 and improved the expression of
E-cadherin. Conversely, transfection of miR-200c inhibitor increased the
expression of Zeb1 and repressed E-cadherin. Other researches had showed that
miR-200c regulated E-cadherin and EMT by inhibition of Zeb1 in other cancers.
Zeb1 binds to the E-box of E-cadherin and inhibits its transcription[7,24].
The migration of RB cells could also be depressed by over-expression of
miR-200c. This might be ascribed to its positive effect to E-cadherin.
E-cadherin was involved into the cell-cell adhesion and has been proved
depressing migration and invasion[34]. Nevertheless, miR-200c itself
could also affect several genes related to migration and invasion like ARHGDIB,
NTRK2, EPHB1 and FN1[31]. Park et al[7]
transfected colorectal cancer cell HTC116 repeatedly every three days with
LNA-200 and detected that cells change from cobblestone to a spindle-like
morphology after 15d. However no significant morphological changes of RB cells
were observed in our research. The transient transfected RNA could not
integrate into the DNA of host cells and only affected the expression of the
function protein. Stable transfection of miR-200c and Zeb1 should be done to
further investigate its effect on EMT and MET in vitro.
Process of
EMT promotes the invasiveness and mobility of tumor cells, so that enables
tumor dissemination. However, the disseminated cells need self-renewal
capability to form metastatic tumor. Researches showed that EMT cells express
higher stem cell markers[5,35]. MiR-200c had been shown to inhibit
tumor-genesis by suppressing stem cell factor Bmi-1
in
several cancer cells[14,36-37]. Our research also found that
miR-200c can reduce the expression of cancer stemness gene Bmi-1 in Y79 and
Weri-RB1 cells (data not shown here). The tumorgenicity regulation of miR-200c
would be further investigated in vivo.
In
conclusion, E-cadherin is correlated with the prognosis of RB, lower E-cadherin
generally indicates bad prognosis. E-cadherin can be up-regulated by
over-expression of miR-200c in Y79 and Weri-RB1 cell-lines in vitro, so
that MET be activated and migration of tumor cells be inhibited.
ACKNOWLEDGEMENTS
Authors’ Contributions: Gao L was
responsible for the study design, statistical analysis and the interpretation
of the results. Shao XL and Chen Y were
responsible for data acquisition, Shao XL prepared the manuscript. All authors
critically reviewed the manuscript for important intellectual content and
approved the final manuscript.
Foundations: Supported by the
National Natural Science Foundation of China (No.81072221); National Science Foundation of Hunan
Province (No.14JJ2005).
Conflicts of Interest: Shao XL,
None; Chen Y, None; Gao L, None.
REFERENCES
1 Lamouille S, Xu J, Derynck R.
Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15(3):178-196. [CrossRef] [PMC free article] [PubMed]
2 Mallini P, Lennard T, Kirby J, Meeson
A. Epithelial-to-mesenchymal transition: what is the impact on breast cancer
stem cells and drug resistance. Cancer
Treat Rev 2014;40(3):341-348. [CrossRef] [PubMed]
3 Polyak K, Weinberg RA. Transitions
between epithelial and mesenchymal states: acquisition of malignant and stem
cell traits. Nat Rev Cancer 2009;9:265-273. [CrossRef] [PubMed]
4 Thiery JP, Acloque H, Huang RY, Nieto
MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139(5):871-890. [CrossRef] [PubMed]
5 Mani SA, Guo W, Liao MJ, Eaton EN,
Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL,
Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition
generates cells with properties of stem cells. Cell 2008;133(4):704-715. [CrossRef] [PMC free article] [PubMed]
6 Sreenivasan S, Thirumalai K, Danda R,
Krishnakumar S. Effect of curcumin on miRNA expression in human Y79
retinoblastoma cells. Curr Eye Res
2012;37(5):421-428. [CrossRef] [PubMed]
7 Park SM, Gaur AB, Lengyel E, Peter ME.
The miR-200 family determines the epithelial phenotypeof cancer cells by
targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 2008;22:894-907. [CrossRef] [PMC free article] [PubMed]
8 Jiang C, Chen X, Alattar M, Wei J, Liu
H. MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis of gastric
cancer. Cancer Gene Ther 2015;22(6):291-301. [CrossRef] [PubMed]
9 Pan Y, Meng M, Zhang G, Han H, Zhou Q.
Oncogenic microRNAs in the genesis of leukemia and lymphoma. Curr Pharm Des 2014;20(33): 5260-5267. [CrossRef]
10 Lee M, Kim EJ, Jeon MJ. MicroRNAs
125a and 125b inhibit ovarian cancer cells through post-transcriptional
inactivation of EIF4EBP1. Oncotarget
2016;7(8):8726-8742. [CrossRef] [PMC free article] [PubMed]
11 Zhou CX, Wang CL, Yu AL, Wang QY,
Zhan MN, Tang J, Gong XF, Yin QQ, He M, He JR, Chen GQ, Zhao Q. MiR-630
suppresses breast cancer progression by targeting metadherin. Oncotarget 2016;7(2): 1288-1299. [CrossRef] [PMC free article] [PubMed]
12 Chen CP, Sun ZL, Lu X, Wu WX, Guo WL,
Lu JJ, Han C, Huang JQ, Fang Y. miR-340 suppresses cell migration and invasion
by targeting MYO10 in breast cancer. Oncol
Rep 2016;35(2):709-716. [PubMed]
13 Tryndyak VP, Beland FA, Pogribny IP.
E-cadherin transcriptional down-regulation by epigenetic and microRNA-200
family alterations is related to mesenchymal and drug-resistant phenotypes in
human breast cancer cells. Int J Cancer
2010;126(11):2575-2583. [CrossRef]
14 Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen
A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt
U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes
tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol 2009;11(12):1487-1495. [CrossRef] [PubMed]
15 Villegas VM, Hess DJ, Wildner A, Gold
AS, Murray TG. Retinoblastoma. Curr Opin
Ophthalmol 2013;24(6):581-588.
[CrossRef] [PubMed]
16 Benavente CA, Dyer MA. Genetics and
epigenetics of human retinoblastoma. Annu
Rev Pathol 2015;10:547-562. [CrossRef] [PubMed]
17 Yu CL, Tucker MA, Abramson DH,
Furukawa K, Seddon JM, Stovall M, Fraumeni JF Jr, Kleinerman RA. Cause-specific
mortality in long-term survivors of retinoblastoma. J Nati Cancer Inst 2009;101(8):581-591. [CrossRef] [PMC free article] [PubMed]
18 Yan B, Zhang W, Jiang LY, Qin WX,
Wang X. Reduced E-cadherin expression is a prognostic biomarker of non-small
cell lung cancer: a meta-analysis based on 2395 subjects. Int J Clin Exp Med 2014;7(11): 4352-4356. [PMC free article] [PubMed]
19 Zhang Z, Liu X, Feng B, Liu N, Wu Q,
Han Y, Nie Y, Wu K, Shi Y, Fan D. STIM1, a direct target of microRNA-185,
promotes tumor metastasis and is associated with poor prognosis in colorectal
cancer. Oncogene 2015;34(37):4808-4820. [CrossRef] [PMC free article] [PubMed]
20 Huang X, Qian Y, Wu H, Xie X, Zhou Q,
Wang Y, Kuang W, Shen L, Li K, Su J, Shen L, Chen X. Aberrant expression of
osteopontin and e-cadherin indicates radiation resistance and poor prognosis
for patients with cervical carcinoma. J
Histochem Cytochem 2015;63(2):88-98. [CrossRef] [PMC free article] [PubMed]
21 Han MW, Lee JC, Kim YM, Cha HJ, Roh
JL, Choi SH, Nam SY, Cho KJ, Kim SW, Kim SY. Epithelial-mesenchymal transition:
clinical implications for nodal metastasis and prognosis of tongue cancer. Otolaryngol Head Neck Surg
2015;152(1):80-86. [CrossRef] [PubMed]
22 Aigner K, Dampier B, Descovich L,
Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P,
Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A. The
transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by
repressing master regulators of epithelial polarity. Oncogene 2007;26(49):6979-6988. [CrossRef] [PMC free article] [PubMed]
23 Bojmar L, Karlsson E, Ellegard S,
Olsson H, Björnsson B, Hallböök O, Larsson M, Stål O, Sandström P. The role of
microRNA-200 in progression of human colorectal and breast cancer. PLoS One 2013; 8(12):e84815. [CrossRef] [PMC free article] [PubMed]
24 Gregory PA, Bracken CP, Smith E, Bert
AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ,
Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ. An autocrine
TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance
of epithelial-mesenchymal transition. Mol
Biol Cell 2011;22(10):1686-1698. [CrossRef] [PMC free article] [PubMed]
25 Shields CL, Mashayekhi A, Au AK, Czyz
C, Leahey A, Meadows AT, Shields JA. The International Classification of
Retinoblastoma predicts chemoreduction success. Ophthalmology 2006;113(12):2276-2280. [CrossRef] [PubMed]
26 Radu OM, Foxwell T, Cieply K, Navina
S, Dacic S, Nason KS, Davison JM. HER2 amplification in gastroesophageal
adenocarcinoma: correlation of two antibodies using gastric cancer scoring
criteria, H score, and digital image analysis with fluorescence in situ
hybridization. Am J Clin Pathol
2012;137(4):583-594. [CrossRef] [PMC free article] [PubMed]
27 Detre S, Saclani Jotti G, Dowsett M.
A "quickscore" method for immunohistochemical semiquantitation:
validation for oestrogen receptor in breast carcinomas. J Clin Pathol 1995;48(9):876-878. [CrossRef]
28 Niu K, Shen W, Zhang Y, Zhao Y, Lu Y.
MiR-205 promotes motility of ovarian cancer cells via targeting ZEB1. Gene 2015;574(2):330-336. [CrossRef] [PubMed]
29 Kaliki S, Shields CL, Rojanaporn D,
Al-Dahmash S, McLaughlin JP, Shields JA, Eagle RC Jr. High-risk retinoblastoma
based on international classification of retinoblastoma: analysis of 519
enucleated eyes. Ophthalmology 2013;120(5):997-1003. [CrossRef] [PubMed]
30 Korpal M, Lee ES, Hu G, Kang Y. The
miR-200 family inhibits epithelial-mesenchymal transition and cancer cell
migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and
ZEB2. J Biol Chem
2008;283(22):14910-14914. [CrossRef] [PMC free article] [PubMed]
31 Cochrane DR, Spoelstra NS, Howe EN,
Nordeen SK, Richer JK. MicroRNA-200c mitigates invasiveness and restores
sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther 2009;8(5):1055-1066. [CrossRef] [PMC free article] [PubMed]
32 van Roy F, Berx G. The cell-cell
adhesion molecule E-cadherin. Cell Mol
Life Scie 2008;65(23):3756-3788. [CrossRef] [PubMed]
33 Liang Z, Sun XY, Xu LC, Fu RZ.
Abnormal expression of serum soluble E-Cadherin is correlated with
clinicopathological features and prognosis of breast cancer. Med Sci Monit 2014;20:2776-2782. [PMC free article] [PubMed]
34 Molina-Ortiz I, Bartolome RA,
Hernandez-Varas P, Colo GP, Teixido J. Overexpression of E-cadherin on melanoma
cells inhibits chemokine-promoted invasion involving p190RhoGAP/p120ctn-dependent
inactivation of RhoA. J Biol Chem
2009;284(22):15147-15157. [CrossRef] [PMC free article] [PubMed]
35 Giannoni E, Bianchini F, Masieri L,
Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate
cancer cells and cancer-associated fibroblasts stimulates
epithelial-mesenchymal transition and cancer stemness. Cancer Res 2010;70(17):6945-6956. [CrossRef] [PubMed]
36 Liu S, Tetzlaff MT, Cui R, Xu X.
miR-200c inhibits melanoma progression and drug resistance through
down-regulation of BMI-1. Am J Pathol
2012;181(15):1823-1835. [CrossRef] [PMC free article] [PubMed]
37 Liu L, Qiu M, Tan G, Liang Z, Qin Y,
Chen L, Chen H, Liu J. miR-200c inhibits invasion, migration and proliferation
of bladder cancer cells through down-regulation of BMI-1 and E2F3. J Transl Med 2014;12:305. [CrossRef] [PMC free article] [PubMed]