
·Basic Research· Current
Issue IF in JCR CiteScore Rank ·Online Submission· Articles in Press PMC RSS
Citation: Kim SJ, Kim MJ, Choi MY, Kim YS, Yoo JM, Hong EK,
Ju S, Choi WS. Aralia elata inhibits neurodegeneration by downregulating
O-GlcNAcylation of NF-κB
in diabetic mice. Int J Ophthalmol 2017;10(8):1203-1211
Aralia elata inhibits neurodegeneration by downregulating
O-GlcNAcylation of NF-κB
in diabetic mice
Seong-Jae Kim1, Min-Jun Kim2,
Mee-Young Choi2,3, Yoon-Sook Kim2,3, Ji-Myong Yoo1,3,
Eun-Kyung Hong4, Sunmi Ju5, Wan-Sung Choi2,3
1Department of Ophthalmology, School of Medicine, Gyeongsang National
University, Jinju 52727, Korea
2Department of Anatomy and Convergence Medical Science, School of
Medicine, Gyeongsang National University, Jinju 52727, Korea
3Institute of Health Science, Gyeongsang National University, Jinju
52727, Korea
4Medvill Co., Ltd., Seoul 08511, Korea
5Division of Pulmonology and Allergy, Department of Internal Medicine,
School of Medicine, Gyeongsang National University, Jinju 52727, Korea
Correspondence to: Wan-Sung Choi. Department of Anatomy and Neurobiology, School of
Medicine, Institute of Health Science, Gyeongsang National University, Jinju
52727, Korea. choiws@gnu.ac.kr
Received:
2017-03-21
Accepted: 2017-06-12
Abstract
AIM:
To investigate the role of O-GlcNAcylation of nuclear factor-kappa B (NF-κB) in
retinal ganglion cell (RGC) death and analysedthe effect of Aralia elata (AE)
on neurodegeneration in diabetic mice.
METHODS: C57BL/6mice
with streptozotocin-induced diabetes were fed daily with AE extract or control
(CTL) diet at the onset of diabetes mellitus (DM). Two months after injection
of streptozotocin or saline, the degree of cell death and the expression of
O-GlcNAc transferase (OGT), N-acetyl-b-D-glucosaminidase (OGA), O-GlcNAcylated
proteins, and O-GlcNAcylation of NF-κB were examined.
RESULTS: AE
did not affect the metabolic status of diabetic mice. The decrease in the inner
retinal thickness (P<0.001 vs CTL, P<0.01 vs
DM) and increases in RGCs with terminal deoxynucleotidyl transferase-mediated
dUTP nick end labelling (P<0.001 vs CTL, P<0.0001 vs
DM), glial activation, and active caspase-3 (P<0.0001 vs CTL,
P<0.0001 vs DM) were blocked in diabetic retinas of AE
extract-fed mice. Expression levels of protein O-GlcNAcylation and OGT were
increased in diabetic retinas (P<0.0001 vs CTL), and the level
of O-GlcNAcylation of the NF-κB p65 subunit was higher in diabetic retinas than
in controls (P<0.0001 vs CTL). AE extract downregulated O-GlcNAcylation
of NF-κB and prevented neurodegeneration induced by hyperglycemia (P<0.0001
vs DM).
CONCLUSION: O-GlcNAcylation
of NF-κB is concerned in neuronal degeneration and that AE prevents
diabetes-induced RGC apoptosis via downregulation of NF-κB O-GlcNAcylation.
Hence, O-GlcNAcylation may be a new object for the treatment of DR, and AE may
have therapeutic possibility to prevent diabetes-induced neurodegeneration.
KEYWORDS: Aralia elata; diabetic
retinopathy; neurodegeneration; nuclear factor-kappa B; O-GlcNAc; O-GlcNAc
transferase; mice
DOI:10.18240/ijo.2017.08.03
Citation: Kim SJ, Kim MJ, Choi MY, Kim YS, Yoo JM, Hong EK,
Ju S, Choi WS. Aralia elata inhibits neurodegeneration by downregulating
O-GlcNAcylation of NF-κB
in diabetic mice. Int J Ophthalmol 2017;10(8):1203-1211
INTRODUCTION
Diabetic retinopathy (DR), a disorder affecting the
microvascular structure of the retina, remains a major sight-threatening
disease for working adults and a common complication of diabetes[1-2]. In the early stages of the
diseases, retinal ganglion cells (RGCs), the major neuronal cells of the
retina, and glial cells are compromised[3].
Moreover, various studies report neuronal apoptosis in diabetic retinas as well
as activation of glial cells, which is another feature of retinal
neurodegeneration[4-6].
Nowadays, it is evident that considerable damage to retinal neurons is already
present at early stages of DR, before any abnormal microcirculatory changes can
be detected by fundusexamination[7-8].
Nonetheless, the key regulators of neurodegeneration in DR remain uncertain,
and further studies are required to identify new therapeutic agents for
neuroprotection in the early stages of DR.
O-GlcNAcylation is ansignificant protein
post-translational modification that participates in addition of a single
O-linked b-N-acetylglucosamine (O-GlcNAc) to the hydroxyl groups of serine
and/or threonine protein[9-10].
O-GlcNAcylation is concerned in regulating various nuclear or cytoplasmic
proteins in a manner similar to protein phosphorylation. But unlike
phosphorylation, only two enzymes, O-GlcNAc transferase (OGT) and N-acetyl-b-D-glucosaminidase
(OGA) are responsible for the addition of O-GlcNAc to serine/threonine
residuesand O-GlcNAcelimination[9]. Accumulating
evidence reveals the important roles that O-GlcNAcylation participates in
several cellular processes, including transcription, degradation of protein,
regulation of signal transduction and cell cycle, stress responses[10-11]. Hyperglycaemia increases O-GlcNAcylated
proteins in cells, which may play important roles in the pathogenesis of
diabetes[12]. Furthermore, the changes of
O-GlcNAcsignaling have been involved in the pathogenesis of diabetic
complications, including diabetic cardiomyopathy, diabetic nephropathy, and DR[13-16]. In the animal model of DR,
O-GlcNAcylated proteins were increased with higher glucose levels in retinal
endothelial cells and pericytes, and the OGT-positive cells were located in the
ganglion cell layer (GCL), inner nuclear layer (INL), and inner plexiform layer
(IPL). Additionally, the number of terminal deoxynucleotidyl
transferase-mediated dUTP nick end labelling (TUNEL)-positive RGCs that
colocalized with OGT was notably higher in diabetic mice than in control[15,17]. Also, previous studies report
that increased expression and O-GlcNAcylation of NF-kB participate in several
human diseases, including DR and cancer[17-18].
Aralia elata (AE) generally distributes in several provinces in East Asia, such as
China, Japan, and South Korea. The number of patients with diabetes and DR in
East Asia is increasing, especially in China. Currently, China currently has
10.6% of its population with diagnosed diabetes and this number has more than
doubled from 4.5% in the past 6y, and is catching up with developed
counterparts like the USA[19]. The prevalence of
DR in diabetic subjects was 9.7% in China[20],
15.9% in Korea[21], and 23.2% in Japan[22]. Furthermore, these rates are also increasing, and so
the economic burden of treatment of DR is high in these countries[19]. The water extract of AE has been traditionally used
to treat diabetes in Korea, China, and Japan. Moreover, in a previous study,
authors report that AE prevents hyperglycaemia-induced RGC apoptosis and
downregulates tonicity response element binding protein in DR[23]. Therefore, it would be meaningful for these
countries to study whether AE really has the effect of inhibiting the
progression of DR.
The current study investigates the role of
O-GlcNAcylation in promoting neurodegeneration. We also examined whether
increases in O-GlcNAcylation of the nuclear factor-kappa B (NF-kB) contributes
to increased apoptosis of RGCs in DR. Finally, we analysed the effect of
extracts of AE on neurodegeneration in DR.
MATERIALS AND METHODS
Animals C57BL/6 mice were provided from the KOATEC (Pyeongtaek, Korea). All
animal procedures for this study were in a adherence to the ARVO statement for
the Use of Animals in Ophthalmic and Vision Research, and were kept in
accordance with the Institutional Animal Care and Use Committee of Gyeongsang
National University (Jinju, Korea). To induce diabetes, mice were
intraperitoneally injected with streptozotocin (55 mg/kg, STZ; Sigma, St.
Louis, MO, USA) dissolved in sodium citrate once a day for 5d. Control mice
injected phosphate-buffered saline (PBS). Blood glucose levels were checked
every 2wk using a glucometer (Abbott, Alameda, CA, USA) after 8h of fasting.
Diabetes was established by blood glucose levels >250 mg/dL at 1wk after the
final injection of STZ. Mice were killed 2mo after the last injection of STZ or
saline.
Preparation and Administration of Aralia elata Extract A freeze-dried powder of AE was obtained from Medvill Co., Ltd. (Seoul,
Korea) and prepared as described previously[24].
The AE powder was dissolved and diluted in 0.9% saline and administered to mice
at doses of 20 or 100 mg/kg body weight with an oral feeding tube once a day
for 7wk after diabetes induction. Finally, the mice were divided into four
groups as follows: 1) saline-treated control group; 2) saline-treated diabetic
group; 3) 20 mg/kg AE-treated diabetic group; 4) 100 mg/kg AE-treated diabetic
group. Each group included 10 mice and a total of 40 mice were used in this
study.
Assessment of Changes in Inner Retinal Thickness Collected retinas were immersed in 4% paraformaldehyde for 6h, and then
washed several times in PBS, cryoprotected in 30% sucrose overnight at 4℃,
frozen in liquid nitrogen with O.C.T. compound (Sakura, Tokyo, Japan), and
finally cryosectioned on a cryostat (Leica 8400E; Leica, Tokyo, Japan). Samples
were stained with haematoxylin and eosin (H&E), and the length (µm) from
the GCL to the tip of the INL was measured as inner retinal thickness. The
comparison of the inner retinal thickness between diabetic and control groups
was performed in four different retinas per group at a distance of about
0.8-1.0 mm from the optic nerve head.
Antibodies and Cell Death Assay Kit Anti-NF-kB (p65 subunit), anti-glial fibrillary acidic protein (GFAP),
and OGA antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX,
USA). A rabbit polyclonal anti-caspase-3 antibody was purchased from Cell
Signaling Technology (Danvers, MA, USA). Antibodies against O-GlcNAc were
acquired from Thermo Fisher Scientific (Waltham, MA, USA), and antibodies
against OGT were purchased from Sigma (St. Louis, MO, USA). Anti-β-actin
antibody was purchased from Abcam (Cambridge, UK). Anti-NeuN antibody was
purchased from Chemicon (Nuernberg, Germany).
A TUNEL kit (In Situ Cell Death Detection Kit;
Roche, Grenzach, Germany) was used to detect apoptosis according to the
manufacturer’s guidance. Total numbers of TUNEL-positive RGCs were counted in
the GCL (approximately 100 µm) in three random fields per retina from 5-6
retinas per group.
Western Blot Analysis Proteins were extracted from four retinas of each group, and 30 mg of
protein were subjected to 10% sodium dodecyl sulphate polyacrylamide gel
electrophoresis and transferred to a nitrocellulose membrane. The membranes
were incubated with antibodies against O-GlcNAc, OGT, OGA, anti-caspase-3, and
NF-kB (p65 subunit) followed by a horseradish peroxidase-conjugated anti-rabbit
secondary IgG (Thermo Fisher Scientific). Blots were reprobed with an anti-b-actin
antibody to control. All Western blot data are representative of four
independent experiments.
Immunoprecipitation Protein from retinas were mixed with agarose beads (Santa Cruz
Biotechnology), incubated for 1h at 4℃, and centrifuged. The supernatants were
incubated with 2 mg of antibody overnight at 4℃. And, samples were incubated
with agarose beads for 2h at 4℃. The negative control was prepared with only
agarose beads without an antibody. The protein-bead complex was washed and then
collected by centrifuge. The complex were boiled in loading buffer to separate
the agarose beads and electrophoresed on 10% gels. Proteins were transferred to
membranes and then probed with antibodies.
Immunohistochemistry and Image Capture Retinal sections (10-mm thick) were prepared as described previously[23,25-26]. The
sections were incubated with primary antibodies against O-GlcNAc, OGT, OGA,
GFAP, and NF-kB (p65 subunit) and followed by an Alexa 488-conjugated goat
anti-rabbit secondary IgG (Molecular Probes, Carlsbad, CA, USA). The sections
were counterstained with 4,6-diamidino-2-phenylindole (DAPI) and mounted in
ProLong Gold Antifade Reagent (Invitrogen, Carlsbad, CA, USA). All retinal
images were captured at a distance of about 0.8-1.0 mm from the optic nerve
head using a JP IX2-DSU disk scanning confocal microscope (Olympus, Hamburg,
Germany). Quantitative analyses were performed with ImageJ analysis software
(Molecular Devices, Sunnyvale, CA, USA).
Statistical Analysis Comparisons among experimental groups were operated by one-way ANOVA
using Prism 5 (GraphPad Software, San Diego, CA, USA). All data are
representatives of four independent experiments, and are presented as
mean±standard error. P values <0.05 were considered statistically
significant.
RESULTS
The Effect of Aralia elata Administration on
Body Weight and Blood Glucose Concentration After Induction of Diabetes Body weights and blood glucose levels are shown in Table 1. Control mice
showed a steady increase in body weight during the experiment, whereas diabetic
mice exhibited a decrease in body weight. The blood glucose concentrations of
diabetic mice significantly and steadily increased, whereas control mice
maintained normal glucose concentrations throughout the experiment. AE extract
did not significantly affect the body weight of any of the mice. Blood glucose
levels of 20 mg/kg AE-treated diabetic mice were maintained at slightly lower
concentrations compared with saline-treated diabetic mice except for the 4wk
after induction of diabetes. In the group administered with 40 mg/kg AE, blood
glucose levels were slightly lower than in the saline-treated diabetic group,
especially the difference was statistically significant in the 2wk after
induction of diabetes (Table 1).
Table 1 Effect of AE administration on body weight and
blood glucose concentrations
|
Time |
Body
weight (g) |
Blood
glucose (mg/dL) |
||||||
|
Control |
DM |
DM-AE20 |
DM-AE100 |
Control |
DM |
DM-AE20 |
DM-AE100 |
|
|
Week
0 |
21.7±1.8 |
21.5±0.5 |
22.4±0.97 |
22.2±1.6 |
133±23.2 |
141±19.7 |
142±17.8 |
136±27.1 |
|
Week
2 |
22.2±0.7 |
19.4±2.0a |
20.6±1.5 |
20.4±1.3 |
178±19.7 |
443±23.5a |
437±57.2 |
398±42.3c |
|
Week
4 |
23.4±1.5 |
20.2±1.3a |
19.8±1.8 |
19.5±1.7 |
152±22.1 |
497±65.8a |
426±55.0 |
402±33.9 |
|
Week
7 |
24.9±2.2 |
20.1±1.7a |
20.3±1.8 |
19.3±2.1 |
157±24.7 |
445±61.7a |
449±78.6 |
428±20.7 |
AE: Aralia elata; AE20: 20 mg/kg AE; AE100: 100
mg/kg AE; DM: Diabetes mellitus; aP<0.05 vs CTL; cP<0.05 vs DM.
Diabetic Retinal Neurodegeneration and Neuroprotective
Effects of Aralia Elata We examined RGC apoptosis, glial activation, changes in inner retinal
thickness, and activation of caspase-3. Next, we investigated whether treatment
with AE extract protected against these changes. TUNEL-positive cells were
mainly located in the GCL of diabetic mice, with some in the INL and OPL
(Figure 1A). The numbers of TUNEL-positive cells were increased in the GCL of
the diabetic group compared with the control (P<0.0001) (Figure 1B).
Treatment with AE extract decreased the number of TUNEL-positive apoptotic
cells in the diabetic retinas (Figure 1A, 1B). Moreover, glial activation was
noted in diabetic retinas, which was prevented by treatment with AE extract
(Figure 1C).
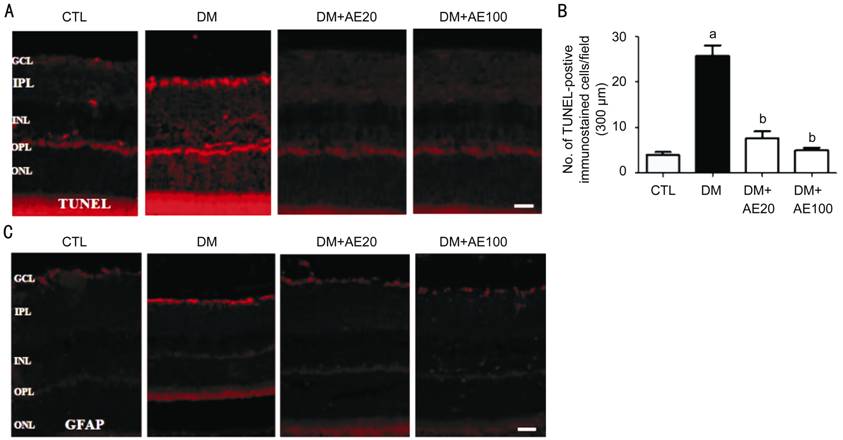
Figure 1 Effect of AE on retinal cell death and glial
activation in the GCL with diabetic DR Representative
immunofluorescence images of TUNEL (A) and GFAP (C) in retinas of control or
diabetic mice with or without AE. Quantification of TUNEL-positive cells in the
GCL (B). aP<0.0001 vs CTL; bP<0.0001
vs DM. Scale bar, 50 mm. AE: Aralia elata; AE20: 20 mg/kg AE;
AE100: 100 mg/kg AE; CTL: Control; DM: Diabetes mellitus; GCL: Ganglion cell
layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer
plexiform layer; ONL: Outer nuclear layer.
Inner retinal thickness was thinner in diabetic
retinas than in control retinas (Figure 2). AE treatment increased the inner
retinal thickness compared with the untreated diabetic group (Figure 2).
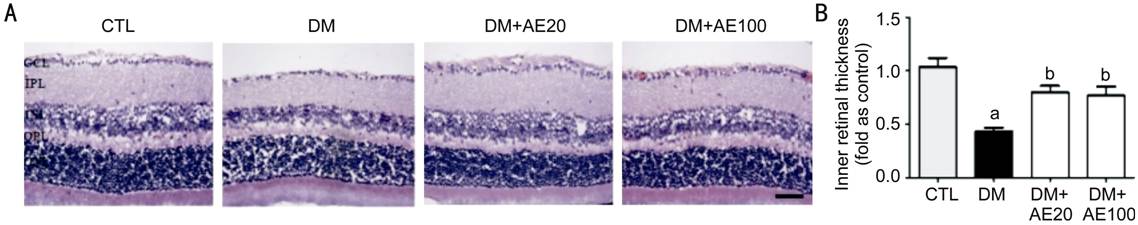
Figure 2 Effect of AE on changes in inner retinal
thickness
A: Representative H&E images of retinas from control or diabetic
mice with or without AE treatment; B: Inner retinal thickness was measuredand
presented as normalized to CTL. aP<0.001 vs CTL, bP<0.01
vs DM. Scale bar, 50 mm. AE: Aralia elata; AE20: 20 mg/kg AE;
AE100: 100 mg/kg AE; CTL: Control; DM: Diabetes mellitus; GCL: Ganglion cell
layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer
plexiform layer; ONL: Outer nuclear layer.
The level of active caspase-3 was significantly increased
in diabetic retinas compared with that in controls. This increase was blocked
by treatment with AE extract (P<0.001 vs diabetic group)
(Figure 3).
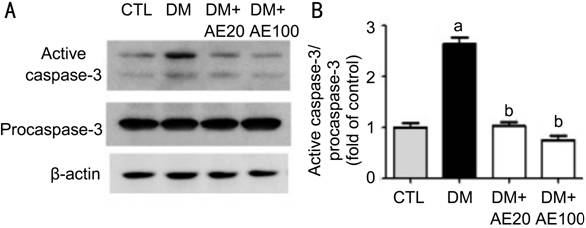
Figure 3 Effect of AE treatment on activation of
caspase-3 Representative Western blot (A) and quantification (B) of active
caspase-3 and procaspase-3 in the retinas of control and diabetic mice with or
without AE treatment. Band intensity was normalized to b-actin. aP<0.0001
vs CTL; bP<0.0001 vs DM. AE: Aralia elata;
AE20: 20 mg/kg AE; AE100: 100 mg/kg AE; CTL: Control; DM: Diabetes mellitus.
Changes in Protein O-GlcNAcylation, O-GlcNAc
Transferase, N-acetyl-β-D-glucosaminidase and Effects of Aralia Elata O-GlcNAcylation of retinal proteins was notably increased in the Western
blot analysis (Figure 4A). Quantification showed that the levels of OGT were
higher in diabetic retinas than in controls (Figure 4B). However, there were no
significant changes in OGA expression between control and diabetic groups
(Figure 4C). In the AE-treated diabetic groups, O-GlcNAcylation of proteins and
OGT were decreased comp ared to the saline-treated diabetic group (Figure 4A,
4B). On the other hand, treatment with AE extract exerted no apparent effect on
the protein levels of OGA (Figure 4C).
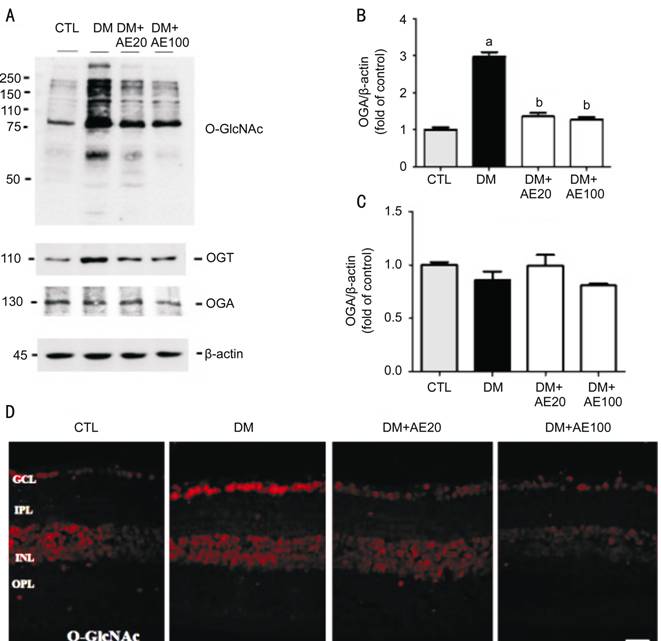
Figure 4 Effect of AE on changes of protein
O-GlcNAcylation, OGT, and OGA in DR Representative Western
blots of protein O-GlcNAcylation, OGT, and OGA (A) and quantification of OGT
and OGA (B, C) in the retinas of control or diabetic mice with or without AE
treatment. Band intensity was normalized to b-actin. (D) Representative
immunofluorescence images for protein O-GlcNAcylation in retinas.Scale bar, 50 mm.
aP<0.0001 vs CTL; bP<0.0001 vs
DM. AE: Aralia elata; AE20: 20 mg/kg AE; AE100: 100 mg/kg AE; CTL: Control; DM:
Diabetes mellitus; GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL:
Inner nuclear layer; OGA: b-D-N-acetylglucosaminidase; OGT: O-GlcNAc
transferase; OPL Outer plexiform layer; ONL: Outer nuclear layer.
Immunohistochemical studies showed a high
concentration of proteins with O-GlcNAcylation localized in the GCL (and INL)
of diabetic mice (Figure 4D). Treatment with AE extract inhibited these
changes.
Relationship Between Retinal Ganglion Cell Death and
O-GlcNAc Transferase Expression and Effects of Aralia Elata To determine whether OGT affects RGC death in DR, triple
immunofluorescence staining was performed for OGT, NeuN, and TUNEL.
Immunoreactivity for OGT was markedly increased in the GCL of diabetic retinas
compared to control retinas, whereas treatment with AE extract attenuated these
changes (Figure 5A). Interestingly, most OGT-positive cells colocalized with
TUNEL and NeuN staining in the retinal GCL of both diabetic and control mice
(white arrows, Figure 5A). Furthermore, the total number of OGT- and
TUNEL-positive RGCs was greater in diabetic retinas compared with controls, but
AE treatment reduced RGC death in the diabetic retinas (P<0.0001 vsuntreated
diabetic group) (Figure 5B).
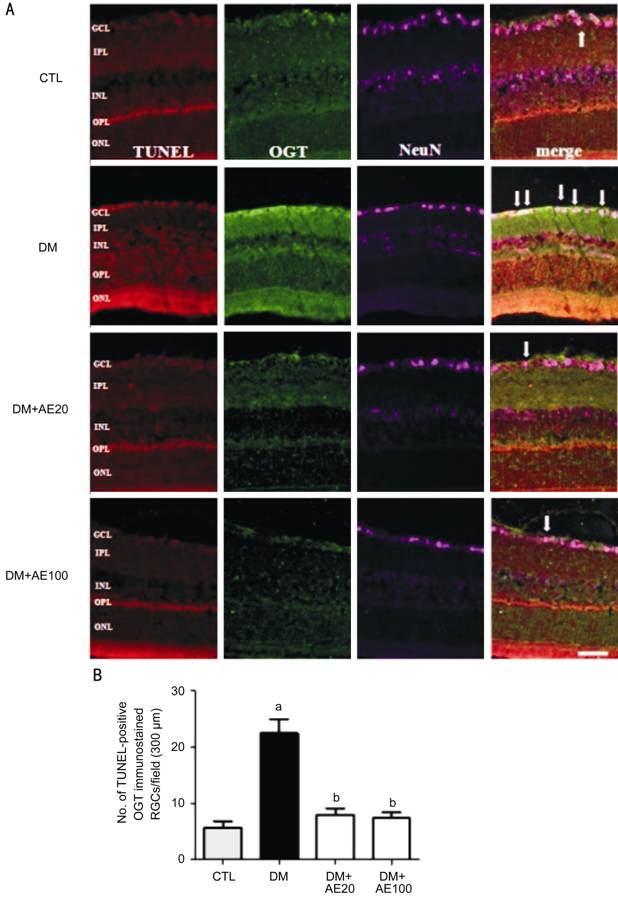
Figure 5 Effect of AE on OGT and RGC death in diabetic
mice A: Representative triple staining for OGT, TUNEL, and NeuN in the
retinas of control and diabetic mice with or without AE. The white arrows
indicate TUNEL-positive cells that were stained for OGT and NeuN in the retinas
of control and diabetic mice. Scale bar, 50 µm. Quantification of
TUNEL-positive RGCs that double-labelled for OGT in the retinas of control or
diabetic mice with or without AE (B). aP<0.0001 vs
CTL; bP<0.0001 vs DM. AE: Aralia elata;
AE20: 20 mg/kg AE; AE100: 100 mg/kg AE; CTL: Control; DM: Diabetes mellitus;
GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer;
OGT: O-GlcNAc transferase; OPL: Outer plexiform layer; ON: Outer nuclear layer;
RGC: Retinal ganglion cell.
Aralia Elata Suppresses NF-κB Expression and Decreases Levels of O-GlcNAc-modified
NF-κB in DR The levels of NF-kB (p65 subunit) were increased in diabetic retinas
compared with controls in Western blot analysis (P<0.0001) (Figure
6A, 6B). However, AE extract treatment reduced levels of NF-kB in DR (P<0.0001).
Furthermore, we found that NF-kB immunoreactivity was colocalized with TUNEL,
and nuclear translocation of NF-kB was significantly increased in the GCL of DR
(Figure 6C, boxed area). Interestingly, NF-kB colocalization with TUNEL was
notably reduced in the GCL of diabetic mice treated with AE compared with
untreated mice (Figure 6C).
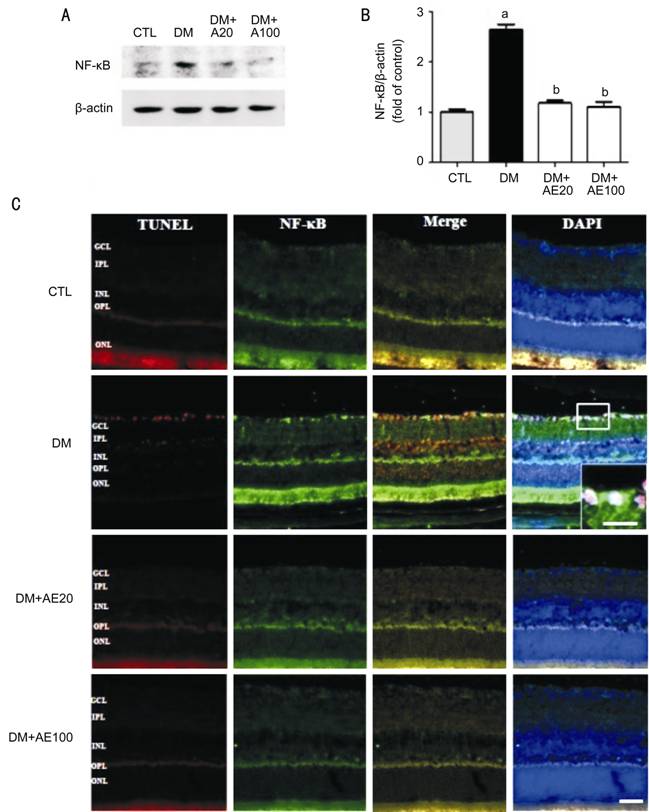
Figure 6 Effect of AE administration on levels of NF-kB Representative
Western blot and quantification of NF-kB (A, B), and immunofluorescent studies
for TUNEL, NF-kB, and DAPI (nuclear counterstain) in the retinas of control or
diabetic mice with or without AE treatment (C). The boxed area shows
colocalization of NF-kB with TUNEL and nuclear translocation of the NF-κB p65
subunit in the GCL of diabetic retinas. Band intensity was normalized to b-actin.
aP<0.0001 vs CTL; bP<0.0001 vs
DM. AE: Aralia elata; AE20: 20 mg/kg AE; AE100: 100 mg/kg AE; CTL:
Control; DM: Diabetes mellitus, Scale bar: 50 mm.
Finally, we assessed O-GlcNAcylation of NF-kB in DR
using co-immunoprecipitation assays (Figure 7A). As expected, O-GlcNAcylation
of the p65 subunitof NF-kB was greater in the diabetic retinas than in control
retinas (Figure 7A). However, AE treatment reduced the amount of NF-kB
O-GlcNAcylation in diabetic retinas (P<0.0001 vs untreated
diabetic group) (Figure 7).
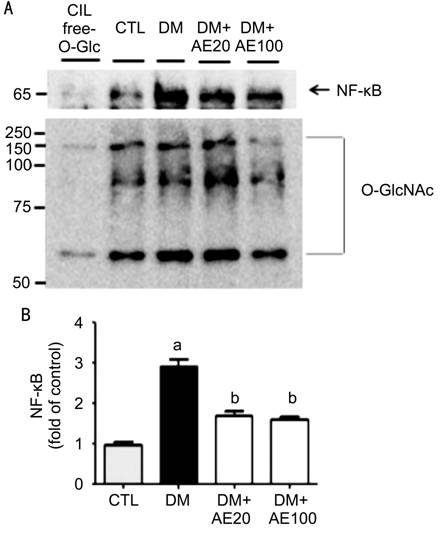
Figure 7 Effect of AE treatment on NF-kB O-GlcNAcylation in diabetic retinas Representative Western blots (A) and
quantification (B) of the levels of NF-kB (p65 subunit) that
co-immunoprecipitated with anti-O-GlcNAc antibodies in lysates from retinas of
control or diabetic mice with or without AE treatment. Densitometry of
co-immunoprecipitated NF-kB to O-GlcNAc was normalized to IgG. aP<0.0001
vs CTL; bP<0.0001 vs DM. AE: Aralia elata;
AE20: 20 mg/kg AE; AE100: 100 mg/kg AE; CTL: Control.
DISCUSSION
In the present study, we suggest that O-GlcNAcylation
of NF-kB is involved in RGC death and that AE treatment prevents
diabetes-induced RGC apoptosis via downregulation of NF-kB
O-GlcNAcylation in DR. One of the earliersigns in DR is the abnormalities of
capillary circulation with leakage of retinal vessels in the inner retina[27]. Until recently, considerable attention was given to
protection of retinal circulations, with less understanding given to
neuroprotection in DR[3]. Nonetheless, various
studies showed that retinal neuronal changes occur before clinically detectable
microvascular abnormalities[28].
Several factors have been involved in in the
pathogenesis of DR, including increases of vascular endothelial growth factor[29], tumour necrosis factor (TNF)-a[30],
advanced glycation end products[31], inflammation[32], and several polyol pathways[33].
Among them, NF-kB plays crucial roles in the induction of vascular
permeability, angiogenesis, and neurodegeneration in DR[3,34]. Numerous studies showed that NF-kB is activated
through multiple pathways in DR. First, hyperglycemia-induced oxidative stress
leads to the activation of NF-kB[35]. Next, TNF-a-mediated
NF-kB activation is concerned in diabetes-related leukostasis, inflammation,
and apoptosis[36]. Specifically, TNF-a mediates
phosphorylation of the p65 subunit of NF-kB at Ser536, which was shown to be
reciprocally applied by O-GlcNAc[37]. Altered
O-GlcNAcylation of NF-kB leads to an increased nuclear translocation of RelA
and increases NF-kB transcriptional activity[38].
Previous study also reports that O-GlcNAcylation of NF-kB is important for its
nuclear translocation and acceleration of cancer metastasis[18],
and hyperglycemia-induced activation and RGC death in DR[17].
Consistent with these studies, our data show that OGT protein expression was
increased and related to RGC death in diabetic retinas (especially, in the
GCL). Moreover, we suggested that NF-kB underwent O-GlcNAcylation, and that
increased O-GlcNAcylation and translocation of the p65 subunit contributed to
RGC death.
Neurodegeneration of retina is a crucial component of
DR and is typically accomplished by a decreased number of RGCs, a thinning
inner retina, and an increased numbers of apoptotic cells[28].
Our current study revealed a notable decrease in inner retinal thickness, a
marked increase in the number of TUNEL-positive cells, and increased glial
activation in the diabetic retinas, consistent with the findings of earlier
investigations. Importantly, AE treatment reversed these changes in the
diabetic mice. Additionally, we found that AE reduced levels of
NF-κBO-GlcNAcylation, which is known to play aimportant role in RGC apoptosis
in DR[17]. Consistent with this, some studies
show that inhibiting O-GlcNAcylation in retinal vascular endothelial cells
protects the vascular integrity and reduces the expression of vascular
endothelial growth factor in vitro[39],
and O-GlcNAcylation and nuclear translocation of p65 subunit of NF-kB increases
its transcriptional activities[18].
Unfortunately, more specific OGT inhibitors generally do not work well in most
living cells or animals and also affect O-GlcNAcylation of many proteins[40]. Therefore, inhibition of NF-kB O-GlcNAcylation by AE
administration represents a promising target for successful neuroprotection. In
addition, it is well known that AE exhibits numerous biological activities,
including cytoprotective, anti-inflammatory, antioxidative, antiviral, and
antidiabetic properties[24,41].
Indeed, the AE extract used in this study contained phenolic compounds [i.e.
3, 4-dihydroxybenzoic acid (DHBA), chlorogenic acid, and caffeic acid] as
revealed by high-performance liquid chromatography analysis[42].
Ban et al[43] reported that DHBA
safeguards against amyloid beta protein-induced neuronal cell death, and some
reports show that chlorgenic acid and caffeic acid have neuroprotective actions[44]. Our previous study showed that AE prevents
hyperglycemia-induced RGC apoptosis and downregulates tonicity response element
binding protein in DR[23]. Consequently, AE may
have therapeutic potential to regulate O-GlcNAcylation of proteins and prevent
diabetes-induced retinal neurodegeneration in DR.
Taken together, our findings indicate that
O-GlcNAcylation of NF-kB contributes to neuronal degeneration andthatAE
treatment prevents diabetes-induced RGC apoptosis via downregulation of
NF-kB O-GlcNAcylation. Thus, O-GlcNAcylation may be a new target for treatment
of DR, and AE may have therapeutic abilities to prevent diabetes and
neurodegeneration in DR. However, much more work is needed to understand the
mechanisms of O-GlcNAcylation and its relationship with other pathogenesis in
DR.
ACKNOWLEDGEMENTS
Foundations: Supported by the Basic Science Research Program Through the National
Research Foundation (NRF) of Korea Funded by the Ministry of Science, ICT, and
Future Planning 2014049413, NRF-2015R1A5A2008833 and NRF-2015R1C1A1A02037702.
Conflicts
of Interest: Kim SJ, None; Kim MJ, None; Choi MY,
None; Kim YS, None; Yoo JM, None; Hong EK, None; Ju S, None; Choi WS, None.
REFERENCES
1 Kempen JH, O'Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE,
Taylor HR, Hamman RF. The prevalence of diabetic retinopathy among adults in
the United States. Arch Ophthalmol 2004;122(4):552-563. [CrossRef] [PubMed]
2 Zimmet P, Alberti KG, Shaw J. Global and societal implications of
the diabetes epidemic. Nature
2001;414(6865):782-787. [CrossRef] [PubMed]
3 Ola MS, Nawaz MI, Khan HA, Alhomida AS. Neurodegeneration and
neuroprotection in diabetic retinopathy. Int
J Mol Sci 2013;14(6865): 2559-2572. [CrossRef] [PMC free article] [PubMed]
4 Barber AJ. A new view of diabetic retinopathy: a neurodegenerative
disease of the eye. Prog Neuropsychopharmacol
Biol Psychiatry 2003; 27(2):283-290. [CrossRef]
5 Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL.
Neuronal and glial cell abnormality as predictors of progression of diabetic
retinopathy. Curr Pharm Des
2007;13(26):2699-2712. [CrossRef]
6 Qian H, Ripps H. Neurovascular interaction and the pathophysiology
of diabetic retinopathy. Exp Diabetes Res
2011;2011:693426. [CrossRef] [PMC free article] [PubMed]
7 Satoh S, Iijima H, Imai M, Abe K, Shibuya T. Photopic
electroretinogram implicit time in diabetic retinopathy. Jpn J Ophthalmol 1994;38(2):178-184. [PubMed]
8 Jindal V. Neurodegeneration as a primary change and role of neuroprotection
in diabetic retinopathy. Mol Neurobiol 2015;51(3):878-884. [CrossRef] [PubMed]
9 Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk
between O-GlcNAcylation and phosphorylation: roles in signaling, transcription,
and chronic disease. Annu Rev Biochem 2011;80:825-858. [CrossRef] [PMC free article] [PubMed]
10 Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic
link between diabetes and cancer? Trends
Biochem Sci 2010;35(10):547-555. [CrossRef] [PMC free article] [PubMed]
11 Bond MR, Hanover JA. O-GlcNAc cycling: a link between metabolism
and chronic disease. Annu Rev Nutr 2013;33:205-229. [CrossRef] [PubMed]
12 Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow
JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling
links O-GlcNAc transferase to insulin resistance. Nature 2008;451(7181):964-969. [CrossRef] [PubMed]
13 McLarty JL, Marsh SA, Chatham JC. Post-translational protein
modification by O-linked N-acetyl-glucosamine: its role in mediating the adverse
effects of diabetes on the heart. Life
Sci 2013;92(11):621-627. [CrossRef] [PMC free article] [PubMed]
14 Park MJ, Kim DI, Lim SK, Choi JH, Han HJ, Yoon KC, Park SH. High
glucose-induced O-GlcNAcylated carbohydrate response element-biding protein
(ChREBP) mediates mesangial cell lipogenesis and fibrosis: the possible role in
the development of diabetic nephropathy. J
Biol Chem 2014;289(19):13519-13530. [CrossRef] [PMC free article] [PubMed]
15 Gurel Z, Sieg KM, Shallow KD, Sorenson CM, Sheibani N. Retinal
O-linked N-acetylglucosamine protein modifications: implications for postnatal
retinal vascularization and the pathogenesis of diabetic retinopathy. Mol Vis 2013;19:1047-1059. [PMC free article] [PubMed]
16 Semba RD, Huang H, Lutty GA, Van Eyk JE, Hart GW. The role of
O-GlcNAc signaling in the pathogenesis of diabetic retinopathy. Proteomics Clin Appl
2014;8(3-4):218-231. [CrossRef] [PMC free article] [PubMed]
17 Kim SJ, Yoo Ws, Choi MY, Chung IY, Yoo JM, Choi WS. Increased
O-GlcNAcylation of NF-κB enhances retinal ganglion cell death in streptozotocin-induced
diabetic retinopathy. Curr Eye Res
2016;41(2):249-257. [CrossRef] [PubMed]
18 Phoomak C, Vaeteewoottacharn K, Sawanyawisuth K, Seubwai W,
Wongkham C, Silsirivanit A, Wongkham S. Mechanistic insights of O-GlcNAcylation
that promote progression of cholangiocarcinoma cells via nucear translocation
of NF-κB. Sci Rep 2016;13(1):27853. [CrossRef] [PMC free article] [PubMed]
20 Pang C, Jia L, Jiang S, Liu W, Hou X, Zuo Y, Gu H, Bao Y, Wu Q,
Xiang K, Gao X, Jia W. Determination of diabetic retinopathy prevalence and
associated risk factors in Chinese diabetic and pre-diabetic subjects: Shanghai
diabetic complications study. Diabetes
Metab Res Rev 2012;28(3):276-283. [CrossRef] [PubMed]
21 Song SJ, Han K, Choi KS, Rhee EJ, Park CY, Park JY, Ko KS, Lee KU,
Ko KS; Task Force Team for Diabetes Fact Sheet of the Korean Diabetes
Association. Trends in diabetic retinopathy and related medical practices among
type 2 diabetes patients: Results from the National Insurance Service Survey
2006-2013. J Diabetes Investig 2017.
[CrossRef] [PubMed]
22 Funakoshi M, Azami Y, Matsumoto H, Ikota A, Ito K, Okimoto H,
Shimizu N, Tsujimura F, Fukuda H, Miyagi C, Osawa R, Miura J. Socioeconomic
status and type 2 diabetes complications among young adult patients in Japan. PLoS One 2017;12(4):e0176087. [CrossRef] [PMC free article] [PubMed]
23 Kim SJ, Yoo WS, Kim HJ, Kwon JE, Hong EK, Choi M, Han Y, Chung I,
Seo S, Park J, Yoo JM, Choi WS. Aralia elata prevents neuronal death by
downregulating tonicity response element binding protein in diabetic
retinopathy. Ophthalmic Res 2015;54(2):85-95. [CrossRef] [PubMed]
24 Kim YH, Kim YS, Kang SS, Cho GJ, Choi WS. Resveratol inhibits
neuronal apoptosis and elevated Ca2+/calmodulin-dependent protein kinase II
activity in diabetic mouse retina. Diabetes
2010;59(7):1825-1835. [CrossRef] [PMC free article] [PubMed]
25 Kim YH, Kim YS, Roh GS, Choi WS, Cho GJ. Resveratol blocks
diabetes-induced early vascular lesions and vascular endothelial growth factor
induction in mouse retinas. Acta
Ophthalmol 2012;90(1):e31-37. [CrossRef] [PubMed]
26 Lee JH, Ha YW, Jeong CS, Kim YS, Park Y. Isolation and tandem mass
fragmentations of an anti-inflammatory compound from Aralia elata. Arch Pharm Res 2009;32(6):831-840. [CrossRef] [PubMed]
27 Cunha-Vaz J, Faria de Abreu JR, Campos AJ. Early breakdown of the
blood-retinal barrier in diabetes. Br J
Ophthalmol 1975;59(11):649-656.
[CrossRef]
28 Villarroel M, Ciudin A, Hernandez C, Simo R. Neurodegeneration: an
early event of diabetic retinopathy. World
J Diabetes 2010;1(2):57-64. [CrossRef] [PMC free article] [PubMed]
29 Eichler W, Kuhrt H, Hoffmann S, Wiedemann P, Reichenbach A. VEGF
release by retinal glia depends on both exygen and glucose supply. Neuroreport 2000;11(16):3533-3537. [CrossRef]
30 Flyvbjerg A. Diabetic angiopathy, the complement system and the
tumor necrosis factor superfamily. Nat
Rev Endocrinol 2010;6(2):94-101. [CrossRef] [PubMed]
31 Chen M, Curtis TM, Stitt AW. Advanced glycation end products and
diabetic retinopathy. Amino Acids
2013;20(26): 3234-3240. [CrossRef]
32 Frey T, Antonetti DA. Alterations to the blood-retinal barrier in
diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal 2011;15(5):1271-1484. [CrossRef] [PubMed]
33 Obrosova IG, Kador PF. Aldose reductase/polyol inhibitors for
diabetic retinopathy. Curr Pharm
Biotechnol 2011;12(3):373-385.
[CrossRef] [PubMed]
34 Kern TS. Contributions of inflammatory processes to the
development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007;2007: 95103. [CrossRef]
35 Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM. Recent
advances in understanding the biochemical and molecular mechanism of diabetic
retinopathy. J Diabetes Complicat
2012;26(1):56-64. [CrossRef] [PubMed]
36 Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA.
TNFα is required for late BRB breakdown in diabetic retinopathy, and its
inhibition prevents leukostasis and protects vessels and neurons from
apoptosis. Invest Ophthalmol Vis Sci
2011;52(3):1336-1344. [CrossRef] [PMC free article] [PubMed]
37 Xing D, Gong K, Feng W, Nozell SE, Chen YF, Chatham JC, Oparil S.
O-GlcNAc modification of NF-κB p65 inhibits TNF-α-induced inflammatory mediator
expression in rat aortic smooth muscle cells. PLoS One 2011;6(8):e24021. [CrossRef] [PMC free article] [PubMed]
38 Yang WH, Park SY, Nam HW, Kim DH, Kang JG, Kang ES, Kim YS, Lee
HC, Kim KS, Cho JW. NFkappaB activation is associated with its O-GlcNAcylation
state under hyperglycemic conditions. Proc
Natl Acad Sci U S A 2008;105(45):17345-17350. [CrossRef] [PMC free article] [PubMed]
39 Xu C, Liu G, Liu X, Wang F. O-GlcNAcylation under hypoxic conditions
and its effect of the blood-retinal barrier in diabetic retinopathy. Int J Mol Med 2014;33(3):624-632. [PubMed]
40 Gloster TM, Zandberg WF, Heinonen JE, Shen DL, Deng L, Vocadlo DJ.
Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within
cells. Nat Chem Biol
2011;7(3):174-181. [CrossRef] [PMC free article] [PubMed]
41 Yoshikawa M, Matsuda H, Harada E, Murakami T, Wariishi N, Yamahara
J, Murakami N. Elatoside E, a new hypoglycemic principle from the root cortex
of Aralia elata seem: structure-related hypoglycemic activity of oleanolic acid
glycosides. Chem Pharm Bull
1994;42(6): 1354-1356. [CrossRef]
42 Chung YS, Choi YH, Lee SJ, Choi SA, Lee JH, Kim H, Hong EK. Water
extract of Aralia elata prevents cataractogenesis in vitro and in vivo. J Ethnopharmacol 2005;101(1-3):49-54. [CrossRef] [PubMed]
43 Ban JY, Cho SO, Jeon SY, Bae K, Song KS, Seong YH. 3,
4-Dihydroxybenzoic acid from Smilacis chinae rhizome protects amyloid-β protein
(25-35)-induced neurotoxicity in cultured rat cortical neurons. Neurosci Lett 2007;420(2):184-188. [CrossRef] [PubMed]
44 Tremblay F, Waterhouse J, Nason J, Kalt W. Prophylactic
neuroprotection by blueberry-enriched diet in a rat model of light-induced
retinopathy. J Nutr Biochem
2013;24(4):647-655. [CrossRef] [PubMed]