
·Basic Research· Current
Issue IF
in JCR CiteScore
Submission In
Press Recent
Accepted PMC RSS
Citation:
Wang W, Liu GX, Li YH, Li XD, He Y. Inhibitory effect of tenomodulin versus
ranibizumab on in vitro angiogenesis. Int J Ophthalmol 2017;10(8):1212-1216
Inhibitory effect of tenomodulin
versus ranibizumab on in vitro angiogenesis
Wei Wang1,
Guang-Xu Liu2, Yue-Hua Li1, Xue-Dong Li1, Yan He2
1Department
of Ophthalmology, Beijing Chao-Yang Hospital, Capital Medical University,
Beijing 100020, China
2Department
of Epidemiology and Health Statistics, School of Public Health, Capital Medical
University, Beijing 100069, China
Correspondence
to: Wei Wang. Department of Ophthalmology, Beijing Chao-Yang Hospital, Capital
Medical University, Beijing 100020, China. wendy_wen81@hotmail.com
Received:
2017-02-01
Accepted: 2017-05-02
Abstract
AIM: To
evaluate anti-angiogenic effect of tenomodulin (TNMD) and ranibizumab on cell
proliferation and capillary-like morphogenesis of vascular endothelial cells
under the stimulation of vascular endothelial growth factor (VEGF) in vitro.
METHODS: The
effects of TNMD and ranibizumab on VEGF-induced proliferation of human
umbilical vein endothelial cells (HUVECs) were evaluated by MTT assay, and the
effects of TNMD and ranibizumab on capillary-like structures formed by HUVECs
under the stimulation of VEGF were examined in culture. Capillary-like
morphogenesis of HUVECs was quantitatively evaluated, and total lengths of
tube-like structures per field were measured in a masked way.
RESULTS: HUVECs
with both ranibizumab and TNMD protein showed MTT reduction in VEGF-stimulated
cell proliferation as expected, while MTT absorbance in the HUVECs with TNMD
was significantly declined than that with ranibizumab (P<0.01). The
capillary-like structures formed by HUVECs were markedly impaired by the
presence of both TNMD and ranibizumab in the culture medium. The total length
of the capillary-like structures per field was significantly shorter in the
medium with TNMD than that of ranibizumab (P<0.01). The inhibitory effect of
TNMD on tube formation in vitro angiogenesis was significantly stronger than
that of ranibizumab.
CONCLUSION: TNMD
may have stronger inhibitory effect than ranibizumab on in vitro angiogenesis.
KEYWORDS: tenomodulin;
ranibizumab; inhibitory effect; proliferation; angiogenesis
DOI:10.18240/ijo.2017.08.04
Citation:
Wang W, Liu GX, Li YH, Li XD, He Y. Inhibitory effect of tenomodulin versus
ranibizumab on in vitro angiogenesis. Int J Ophthalmol 2017;10(8):1212-1216
INTRODUCTION
Neovascular
eye diseases such as diabetic retinopathy, central retinal vein occlusion, and
wet age-related macular degeneration (AMD) are characteristic of ocular
neovascularization, the pathological vascular proliferation that impairs
eyesight[1-2]. Neovascular age-related
macular degeneration (NVAMD) is a primary cause of blindness in elderly
populations among those diseases[3]. The disease is
characterized by the abnormal growth of arteries and veins (neovascularisation)
in the macula, the leakage of these blood vessels leads to swelling and damage
to the macula, resulting in a fibrous scar that cause uncorrectable vision
loss[4]. Therapies against NVAMD target new blood vessels. Ranibizumab
is one of the most frequently used anti-vascular endothelial growth factor
(VEGF) agents injected intravitreally to treat NVAMD[3].
Ranibizumab (also referred to as lucentis) is a humanized recombinant
monoclonal antibody fragment (Fab), targeting the inhibition of human VEGF-A.
It is combined with the VEGF-A subtype (i.e. VEGF110, VEGF121 and VEGF165) with
a high affinity, which inhibits the binding of VEGF-A to its receptor VEGFR-1
and VEGFR-2. VEGFA binding to its receptor, leading to the formation of
vascular endothelial cell proliferation and angiogenesis, and increased
vascular leakage, all of which are thought to be associated with NVAMD
progress[5]. Lucentis was shown to be effective in
AMD-associated choroidal neovascularization (CNV) compared with photodynamic
therapy or no treatment[6-7]. But for the
duration and efficiency of treatment, repeated injections intravitreally are
inevitable which may result in further safety risks and increased costs of
patients. Due to the lack of a long-term convincing body of evidence regarding
safety, the systemic safety of intravitreal lucentis repeatedly is still need
to be assessed[4]. Previous studies have reported the adverse
events including hypertension, stroke and myocardial infarction etc[4]. Thus, doctors have been searching for more effective anti
angiogenic drugs to prevent intraocular neovascular disorders.
Tenomodulin
(TNMD) is a new member of the tumor necrosis factor family[8],
which has been identified as a transmembrane angiogenesis inhibitor[9]. Few studies have confirmed TNMD as an angiogenesis
inhibitor, which inhibits vascular endothelial cell proliferation and tube
morphology in vitro, and suppresses tumorigenesis in vivo[10-13]. In our earlier article, we explored the role of TNMD in
retinal neovascularization in vivo, and concluded that TNMD inhibits pathologic
vascular proliferation in the mouse model of oxygen-induced retinopathy[14].
In this
study, we would like to recommend TNMD, a more potent anti-VEGF agent by
analyzing the inhibitory effect of TNMD versus ranibizumab in vitro
angiogenesis.
MATERIALS AND METHODS
Materials TNMD (1 mg/mL) was purchased from Abcam
(LA, USA). Kept at -20℃, in sterile PH 7.4, 0.01 mol/L phosphate buffered saline (PBS)
once reconstituted. TNMD protein is stable at 2℃-4℃ for at least six weeks. The
antibody has a strong hydrophobic, high concentrations lead to precipitation,
and freeze-thaw cycles can be repeated 2-3 times. Ranibizumab (lucentis injections,
10 mg/mL) was obtained from Novartis, China, stored at 2℃-8℃ and cannot be frozen.
Methods
Cell culture Human umbilical vein endothelial cells
(HUVECs, KG110, KeyGen BioTECH, China) were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) (Hyclone, USA) including 10% fetal bovine serum (FBS)
(Gibco, USA) in 5% CO2 at 37℃, media were changed in each 2 to 3d. Cells were used for the
experiments between passages 3 and 6, within these passages, HUVECs kept their
endothelial characteristics, such as the cobblestone-like morphogenesis.
Human
umbilical vein endothelial cell proliferation assay Cellular proliferation was determined
using MTT assay, which was described previously[10].
Briefly, HUVECs at passages 3-6 were harvested with trypsin (KeyGen BioTECH,
China) and suspended in DMEM at a density of 50 000 cells/mL. The cells were
seeded into 96-well (Corning, USA) microplates (100 μL per well) and grown for
24h. The cells were then starved in FBS free culture medium for 6h and
stimulated with VEGF (Sino Biological Inc., China) or VEGF with lucentis (0.25,
0.5, 1, 2 μg/mL) or VEGF with TNMD (0.25, 0.5, 1, 2 μg/mL) respectively at the
indicated concentrations for 12h. After the stimulation, 10 μL of MTT at 5
mg/mL (Amresco, USA) was put into each well, and the cells were then incubated
for another 4h. After 150 μL of dimethyl sulfoxide (DMSO) (Applichem, Germany)
was added and mixed thoroughly for 10min, optical density was then measured by
a microplate reader (Themo Multiscan MK3, USA) at 490 nm.
Matrigel
tube formation assay The 24-well
tissue culture plates (Corning, USA) were coated with Matrigel Matrix (400 μL
per well, BD, USA) and incubated at 37℃ for 30min. HUVECs starved in 1% FBS containing culture medium for
4h were harvested with trypsin and were seeded at a density of 60 000 cells per
well on polymerized Matrigel in the existence of VEGF (100 ng/mL) or VEGF with
lucentis and VEGF with TNMD at the indicated concentrations. Cells were also
seeded in 10% FBS containing culture medium as a positive control. The plate
was incubated at 37℃ for 6h and
then photographed (Olympus IX81, Japan). To quantitatively assess the
capillary-like morphogenesis of HUVECs, total lengths of capillary-like
structures per field were measured in a masked way, using image processing and
analysis software (Image J software, National Institutes of Health, USA, NIH
Image J Version 1.61, acquired from the public domain
http://rsb.info.nih.gov/nih-image via the National Institute of Health,
Bethesda, MD, USA). Each experiment was performed at least 3 times.
Statistical
Analysis Each experiment was done
at least thrice, and the data were statistically analyzed by using SPSS 13.0,
one-way ANOVA, followed by Scheffe’s multicomparison test. P value of <0.05
was considered statistically significant.
RESULTS
Vascular
Endothelial Growth Factor Screened for Optimum Concentration of Cell
Proliferation HUVECs were seeded
into 96-well microplates (100 μL per well) and grown for 24h. The cells were
then starved in FBS free culture medium (0.5% FBS) for 6h and stimulated with
VEGF of different concentration (25, 50, 100, 200 ng/mL) for another 24h.
VEGF-induced endothelial proliferation was evaluated by measurement of MTT
assay. HUVECs were significantly stimulated by VEGF at the indicated
concentration, up to 100 ng/mL (P<0.01) (Figure 1).
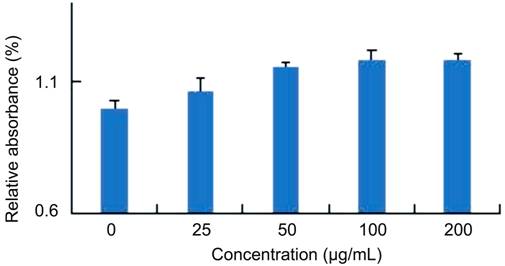
Figure 1
VEGF screened for optimum concentration of cell proliferation.
Comparing
Suppressive Effect of Tenomodolin/Lucentis on Vascular Endothelial Growth
Factor-induced Endothelial Proliferation
The suppressive effect of TNMD and lucentis on VEGF-induced endothelial
proliferation was assessed by measurement of MTT assay. HUVECs were
significantly stimulated by VEGF at the indicated concentration, up to 100
ng/mL (Figure 1). HUVECs with both TNMD and lucentis protein showed MTT
reduction in VEGF-stimulated cell proliferation as expected, in contrast, MTT
absorbance in the HUVECs with TNMD significantly declined than that with
lucentis (P<0.01) (Figure 2).
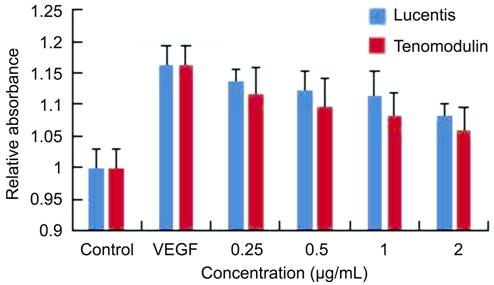
Figure 2
Inhibitory effect of TNMD and lucentis on VEGF-induced endothelial
proliferation The suppressive
effect of TNMD and lucentis on VEGF-induced endothelial proliferation was
evaluated by measurement of MTT assay.
Comparison
Inhibitory Effect of Lucentis/Tenimodulin on Vascular Endothelial Growth
Factor-mediated Human Umbilical Vein Endothelial Cell Tube Formation in Vitro
Angiogenesis To compare the
suppressive effects of lucentis and TNMD in vitro angiogenesis, capillary like
morphogenesis of HUVECs was evaluated by culturing in various conditioned
media. HUVECs were flated on the matrix in the existence of 100 ng/mL VEGF. The
capillary-like structures formed by HUVECs were markedly impaired by the
existence of both TNMD and lucentis in the culture medium. The total length of
the capillary-like structures in each field was significantly shorter in the
medium with TNMD than that of lucentis (Figure 3). The inhibitory effect of
TNMD on tube formation in vitro angiogenesis was significantly stronger than
that of lucentis (P<0.01).
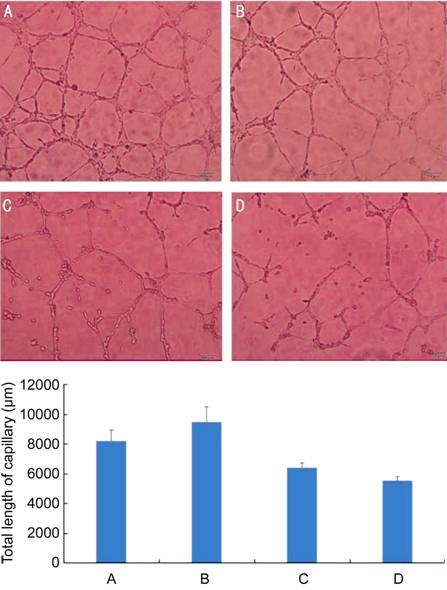
Figure 3 Suppressive
effect of TNMD/lucentis on VEGF-induced endothelial proliferation and on in
vitro angiogenesis (×100) The
tube morphogenesis of HUVECs in different conditioned culture medium. A: 1% FBS
containing medium as positive control; B: Medium including 1% FBS and 100 ng/mL
VEGF; C: Medium including 1% FBS, 100 ng/mL VEGF and lucentis 1 μg/mL; D:
Medium including 1% FBS, 100 ng/mL VEGF and TNMD 10 μg/mL. Bar chart: quantitative
assessment of the length of the capillary structures by image computer
analysis. Data are means±SD of three independent experiments. The total length
of HUVECs incubated in the medium containing TNMD had significant differences
with that of in the other three different conditioned culture medium (P<0.01).
DISCUSSION
Neovascular
eye disease is a main cause of severe vision loss at present worldwide. The
treatment of intraocular neovascular disease is being innovated by intravitreal
therapies targeting VEGF[15]. Intravitreal injection
anti-VEGF agents, aim to prevent the growth of abnormal blood vessels in the
eye to stop vision loss and, in some cases, improve vision. Although
ranibizumab as an anti-VEGF agent is one of the most frequently used anti-VEGF
drugs injected intravitreally to treat wet AMD[3], and has
been proved to be effective with respect to preserving or improving visual
acuity, the major eye adverse events detected in clinical tests such as a low
frequency of ocular inflammation, a slightly elevated risk of monocular
hemorrhage, stroke[15] and so on keep exist. High cost is
also a problem need to be concerned in developing countries.
Recently,
many research labs have been trying to better understand the molecular
mechanisms of the occurrence of neovascularization and possibilities for
recovery from retinopathy or maculopathy. Currently, a lot of new protein class
molecules with important regulatory functions have been discovered and
identified[16].
TNMD, a more
potent anti-VEGF agent, primarily expressed in dense hypovascular connective
tissues such as tendon, ligament, and sclera, vitreous body of eye[17-19]. Three-fold higher TNMD gene
expression levels have been observed in adipocytes and adipose tissue as
compared to other human tissues[20]. Jelinsky et al[21] reported that the TNMD expression was four times higher in
tendons than in the adipose tissue, moderate TNMD expression is demonstrated in
cartilages and bones. TNMD has various biological functions. Tolppanen et al[22] summarized that TNMD could have genetic associations with
the central obesity, inflammations, serum level of system immune mediators,
AMD, Alzheimer disease, type 2 diabetes, glucose and lipid metabolism. TNMD is
one of the most downregulated genes in patients with metabolic syndrome
symptoms, impaired fasting glycaemia and weight reduction intervention[23]. TNMD also plays a crucial role in cardiac valve tissues
degeneration by control of the angiogenesis and the matrix metalloproteinase
synthesis[24]. It has been reported that TNMD inhibits
proliferation and tube morphogenesis of vascular endothelial cells in vitro and
has a potent anti-tumor effect in vivo. Clinical and laboratory studies have
also reported strong evidence indicating that tumor angiogenesis is inhibited
by administrating anti-angiogenic inhibitory factors. Our earlier study has
reported that it is effective in preventing ischemic-induced retinopathy and
pathologic angiogenesis[14] when TNMD be injected in the
vitreous body of C57BL/6 mice with an oxygen-induced retinopathy.
In this
study, we analyzed the inhibitory effect of ranibizumab versus TNMD in vitro
angiogenesis by comparing the anti-angiogenic effect of TNMD and lucentis
protein on cell proliferation and capillary-like morphogenesis of vascular
endothelial cells under the stimulation of VEGF in vitro. HUVECs with both
lucentis and TNMD protein showed MTT reduction in VEGF-stimulated cell
proliferation as expected, in contrast, MTT absorbance in the HUVECs with TNMD
significantly declined than that with lucentis. The capillary-like structures
formed by HUVECs were markedly impaired with the culture medium containing both
TNMD and lucentis. The total length of the capillary-like structures in each
field was significantly shorter in the medium with TNMD than that of lucentis
(Figure 2). The inhibitory effect of TNMD on tube formation in vitro
angiogenesis was significantly stronger than that of lucentis.
In
conclusion, these results indicate that TNMD may have stronger inhibitory
effect than ranibizumab on in vitro angiogenesis. This is an interesting
finding and also a relatively shallow study that further research and
confirmation on TNMD such as toxicity, safety check, duration of action, etc.
is necessary. The observations may provide us with a more effective and better
role in the treatment of pathologic neovascular conditions in the near future.
ACKNOWLEDGEMENTS
Foundation: Supported
by the Cooperation Fund for Clinic and Scientific Research of Capital Medical
University (No.13JL13).
Conflicts of
Interest: Wang W, None; Liu GX, None; Li YH, None; Li XD, None; He Y, None.
REFERENCES
1 Berg K, Pedersen TR, Sandvik L, Bragadottir R. Comparison of
ranibizumab and bevacizumab for neovascular age-related macular degeneration
according to the LUCAS treat-and-extend protocol. Ophthalmology
2015;122(1):146-152. [CrossRef] [PubMed]
2 Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS.
Anti-vascular endothelial growth factor for neovascular age-related macular
degeneration. Cochrane Database Syst Rev 2014;8:CD005139. [CrossRef]
3 Solomon SD, Lindsley KB, Krzystolik MG, Vedula SS, Hawkins BS.
Intravitreal bevacizumab versus ranibizumab for treatment of neovascular
age-related macular degeneration. Ophthalmology 2016;123(1):70-77.e1. [CrossRef] [PMC
free article]
[PubMed]
4 Moja L, Lucenteforte E, Kwag KH, et al. Systemic safety of
bevacizumab versus ranibizumab for neovascular age-related macular
degeneration. Cochrane Database Syst Rev 2014;9:CD011230. [CrossRef]
8 Zhai Y, Ni J, Jiang GW, Lu J, Xing L, Lincoln C, Carter KC,
Janat F, Kozak D, Xu S, Rojas L, Aggarwal BB, Ruben S, Li LY, Gentz R, Yu GL.
VEGI, a novel cytokine of the tunor necrosis factor family, is an angiogenesis
inhibitor that suppresses the growth of colon carcinomas in vivo. FASEB J
1999;13(1):181-189. [PubMed]
9 Oshima Y, Shukunami C, Honda J, Nishida K, Tashiro F, Miyazaki
J, Hiraki Y, Tano Y. Expression and localization of tenomodulin, a
transmembrane type chondromodulin-I-related angiogenesis inhibitor, in mouse
eyes. Invest Ophthalmol Vis Sci 2003;44(5):1814-1823. [CrossRef]
10 Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is
necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol
2005;25(2):699-705. [CrossRef] [PMC
free article]
[PubMed]
11 Oshima Y, Sato K, Tashiro F, Miyazaki JI, Nishida, K, Hiraki Y,
Tano Y, Shukunami C. Anti-angiogenic action of the C-terminal domain of
tenomodulin that shares homology with chondromodulin-I. J Cell Sci 2004;117(Pt
13):2731-2744. [CrossRef] [PubMed]
12 Shukunami C, Oshima Y, Hiraki Y. Chondromodulin-I and
tenomodulin: a new class of tissue-specific angiogenesis inhibitors found in
hypovascular connective tissues. Biochem Biophys Res Commun 2005;
333(2):299-307. [CrossRef] [PubMed]
13 Funaki H, Sawagucbi S, Yaoeda K, Koyama Y, Yaoita E, Funaki S,
Shirakashi M, Oshima Y, Shukunami C, Hiraki Y, Abe H, Yamamoto T. Expression
and localization of angiogenic inhibitory factor, chondromodulin-I, in adult
rat eye. Invest Ophthalmol Vis Sci 2001;42(6): 1193-1200. [PubMed]
14 Wang W, Li ZQ, Sato T, Oshima Y. Tenomodulin inhibits retinal
neovascularization in a mouse model of oxygen-induced retinopathy. Int J Mol
Sci 2012;13(11):15373-15386. [CrossRef] [PMC
free article]
[PubMed]
15 Tolentino M. Systemic and ocular safety of intravitreal
anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol 2011;56(2):
95-113. [CrossRef] [PubMed]
16 Alexandrov VP, Naimov SI. A prospectus of Tenomodulin. Folia
Med 2016;58(1):19-27. [CrossRef]
17 Brandau O, Meindl A, Fassler R, Aszodi A. A novel gene, tendin,
is strongly expressed in tendons and ligaments and shows high homology with
chondromodulin-I. Dev Dyn 2001;221(1):72-80. [CrossRef] [PubMed]
18 Shukunami C, Oshima Y, Hiraki Y. Molecular cloning of
tenomodulin, a novel chondromodulin-I related gene. Biochem Biophys Res Commun
2001;280(5):1323-1327. [CrossRef] [PubMed]
19 Yamana K, Wada H, Takahashi Y, Sato H, Kasahara Y, Kiyoki M.
Molecular cloning and characterization of CHM1L; a novel membrane molecule
similar to chondromodulin-I. Biochem Biophys Res Commun 2001;280(4):1101-1106.
[CrossRef] [PubMed]
20 Saiki A, Olsson M, Jernas M, et al. Tenomodulin is highly
expressed in adipose tissue, increases in obesity, and down-regulated during
diet-induced weught loss. J Clin Endocrinol Metab 2009;94(10):3987-3994. [CrossRef] [PubMed]
21 Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective
genes identified from rat and human musculoskeletal tissues. J Orthop Res 2010;28(3):289-297.
[PubMed]
22 Tolppanen AM, Kolehmainen M, Pulkkinen L, Uusitupa M.
Tenomodulin gene and obesity-related phenotypes. Ann Med 2010;42(4): 265-275. [CrossRef] [PubMed]
23 Kolehmainen M, Salopuro T, Schwab US, Kekäläinen J, Kallio P,
Laaksonen DE, Pulkkinen L, Lindi VI, Sivenius K, Mager U, Siitonen N, Niskanen
L, Gylling H, Rauramaa R, Uusitupa M. Weight reduction modulates expression of
genes involved in extracellular matrix and cell death: the GENOBIN study. Int J
Obes (Lond) 2008;32(2): 292-303. [CrossRef] [PubMed]
24 Kusumoto D, Fukuda K. The role of angiogenetic factors in the
pathogenesis and the progression of cardiac valve disease. Clin Calcium
2013;23(4):481-488. [PubMed]