
·Clinical Research· Current
Issue IF in JCR CiteScore ·Submission· In Press Recent Accepted PMC RSS
Citation: Li QJ, Zhao PX, Zhang XJ, Yi Y, Chen DY, Ma JM, Ma XM. Association of
the macrophage migration inhibitory factor promoter polymorphisms with benign
lymphoepithelial lesion of lacrimal gland. Int J Ophthalmol
2017;10(8):1229-1232
Association of the macrophage migration inhibitory
factor promoter polymorphisms with benign lymphoepithelial lesion of lacrimal
gland
Qin-Jian Li1, Peng-Xiang Zhao1, Xu-Juan Zhang1,
Yang Yi1, Dan-Ying
Cheng2, Jian-Min Ma3, Xue-Mei Ma1
1College of Life Science and Bio-engineering, Beijing University of
Technology, Beijing 100124, China
2Institute of Infectious Diseases, Beijing Ditan Hospital, Capital
Medical University, Beijing 100015, China
3Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital
Medical University, Beijing Ophthalmology &Vision Science Key Lab, Beijing
100730, China
Co-first
authors: Qin-Jian Li and Peng-Xiang Zhao
Correspondence
to: Peng-Xiang Zhao and Xue-Mei Ma. College of Life Science and
Bio-engineering, Beijing University of Technology, Beijing 100124, China.
zpx@bjut.edu.cn and xmma@bjut.edu.cn; Jian-Min Ma.
Beijing
Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University,
Beijing Ophthalmology &Vision Science Key Lab, Beijing 100730, China.
jmma@sina.com
Received:
2016-12-23
Accepted: 2017-05-16
Abstract
AIM: To
identify the association of the macrophage migration inhibitory factor (MIF)
gene polymorphism with the susceptibility of benign lymphoepithelial lesions
(BLEL) of the lacrimal gland.
METHODS: A
total of 40 BLEL of lacrimal gland cases were matched with 40 healthy subjects
(HS). Extraction the plasma and whole blood DNA of patients of lacrimal gland
BLEL and HS. Elisa and polymerase chain reaction was used to determine in plasma
contents of MIF and MIF gene SNP-173G>C and STR -794 CATT(5-8)
polymorphism, respectively.
RESULTS: The
MIF levels in plasma were significantly higher in patients with lacrimal gland
BLEL versus HS (P<0.001). The -173 G>C MIF polymorphism was
significantly associated with lacrimal gland BLEL, with a significantly higher
frequency of the C allele in lacrimal gland BLEL patients compared with HS
(OR=2.38, 95% CI=1.07-5.31, P=0.032), and the -173 C/x is more frequent
in patients than in HS, P=0.037. Besides, we found that the carriage
rate of the MIF -173C/x is associated with higher plasma levels of MIF in the
BLEL of lacrimal gland.
CONCLUSION: MIF
-173G/C variants play an insidious role in susceptibility of BLEL of lacrimal
gland. Otherwise, there is no statistically significant correlation exists
between MIF-794 CATT (5-8) and BLEL of lacrimal gland.
KEYWORDS:
benign lymphoepithelial lesion; lacrimal gland; macrophage
migration inhibitory factor; gene polymorphism
DOI:10.18240/ijo.2017.08.07
Citation: Li QJ, Zhao PX, Zhang XJ, Yi Y, Chen DY, Ma JM, Ma XM. Association of
the macrophage migration inhibitory factor promoter polymorphisms with benign
lymphoepithelial lesion of lacrimal gland. Int J Ophthalmol
2017;10(8):1229-1232
INTRODUCTION
Benign
lymphoepithelial lesion (BLEL)[1], also referred
to as Mikulicz disease[2], is a relatively rare
disease, with the major clinical manifestations being symmetrical and painless
enlargement of the bilateral lacrimal glands and/or the salivary glands[3-4]. The cause and pathogenesis of BLEL
remain unclear. Clinically, BLEL can be treated with glucocorticoid therapy,
but glucocorticoid resistance is a frequent occurrence[5].
Macrophage
migration inhibitory factor (MIF) is a pro-inflammatory cytokine first
identified in 1966 during studies of the delayed-type hypersensitivity reaction
and characterized as a soluble product of activated T lymphocytes that inhibits
macrophage migration in vitro[6-7].
MIF has been shown to act as a critical mediator of host defence with a role in
septic shock and chronic inflammatory and autoimmune diseases[8-9]. Furtherly, MIF has the unique ability to override the
inhibitory effects of glucocorticoid on the immune system[10-11].
MIF
gene is located in chromosome 22 (22q11.2) and contains two clinically relevant
polymorphisms within the promoter region that have been associated with
susceptibility to several diseases[12-16].
A short tandem repeat (STR) polymorphism is located at locus -794 (rs5844572),
which is a microsatellite repetition of cytosine-adenine-thymine-thymine
(CATT), and the repeat length (5 to 8 repetitions) which correlates with
increased gene expression and with circulating MIF levels[12].
Likewise, the single nucleotide polymorphism (SNP) -173 G>C MIF (rs755622)
has been found at location -173 of the MIF gene with a change from guanine (G)
to cytosine (C). Similar to the functions of STR polymorphism above, this
-173*C allele is also reported to associated with mRNA expression and circulating
MIF levels[17]. More fundamentally, some studies
indicated that MIF-173G/C gene polymorphism may increase the risk of
glucocorticoid resistance in a series of diseases including juvenile arthritis,
nephrotic syndrome and colitis[18-20].
In
this study, we evaluated the association of the -794 CATT(5-8) and
-173G>C MIF polymorphisms with glucocorticoid susceptibility to lacrimal
gland BLEL.
SUBJECTS AND METHODS
Participants
or Samples A total of
40 patients with lacrimal gland BLEL were registered and treated at the Beijing
Tongren Hospital between September 2013 and April 2016. The study population
comprised 11 males and 29 females, the ages ranged from 23 to 63y with a median
age of 47y. The enlargement of the lacrimal in patients with BLEL was found to
be uncongested and symmetric or unilateral as well as asymptomatic and
nontender to palpation. All BLEL diagnosis was confirmed by post-surgical
histological examinations. Forty subjects with healthy subjects (HS) were
recruited as a control group comprised of 20 male and 20 female subjects, aged
between 29 and 64y.
Peripheral
blood samples of 40 patients with lacrimal gland BLEL and 40 HS from Beijing
Tongren Hospital and University Hospital of Beijing University of Technology
are prepared for this trial. The study protocol was approved by the Ethics
Committee of Beijing University of Technology and adhered to the tenets of the
Declaration of Helsinki. Written informed consent was obtained from all
participants before their enrollment (Table 1).
Table
1 Clinical characteristics in the study population
|
Groups |
Age (a) |
Gender |
Affected
eyes |
|||
|
M |
F |
L |
R |
Bilateral |
||
|
BLEL
(n=40) |
47 (23-63)a |
11 |
29 |
6 |
10 |
24 |
|
HS
(n=40) |
49.2
(29-64)a |
20 |
20 |
|
|
|
aMinimum-maximum.
Pretreatments Whole blood
was centrifuged at 2000 rpm for 10min; the upper layer was then carefully
removed into a clean tube and stored at -20℃; whole blood DNA was at last
extracted from the left blood cells by using Genomic DNA Extraction from blood system
(TIANGEN® China).
Genotyping
of the SNP-173G>C and STR -794 CATT(5-8) Polymorphism To analyze
the SNP-173G>C MIF polymorphism, we amplified polymorphic fragments by
conventional polymerase chain reaction (PCR). Amplification of a 497 bp
fragment was completed using the primers as follows: forward primer 5’-CCCCGC
CCC ATC TCA AAC ACA-3’ and reverse primer 5’-CCGCCG CTG AGC TAC GTG CC-3’.
Cycling conditions were as follows: initial denaturing at 94℃ for 5min followed
by 30 cycles of 30s at 94℃, 30s at 58℃ , and 30s at 72℃ and then a final
extension of 5min at 72℃.
The
-794 CATT(5-8) MIF polymorphism was analyzed by conventional polymerase
chain reaction (PCR) and amplification of a 346 bp fragment was completed using
the primers as follows: forward primer 5’-TGCAGGAACCAATACCCATAG G-3’ and
reverse primer 5’-AATGGTAAACTCGGGGAC-3’. Of 30 cycles and an annealing
temperature of 58℃ were used.
Amplification
products were sequenced by Sangon Biotech (Shanghai, China), and the sequential
peaks showed genotyping results of the SNP-173G>C MIF and STR -794 CATT(5-8)
MIF polymorphism.
Enzyme-linked
Immunosorbent Assay for Macrophage Migration Inhibitory Factor The
determination of MIF plasma levels was performed by commercial ELISA Kits (RayBio®
USA) according to manufacturer’s instructions. The sensitivity of MIF detection
was 6 pg/mL.
Statistical
Analysis Data
analysis was performed using IBM SPSS Statistics ver.20, GraphPad Prism6
software and Revman 5.3. Student’s t-test for parametric variables (data
presented as mean±SD), and Mann-Whitney U test for nonparametric
variables (data presented as median and 5th to 95th
percentiles). Genotype and allele distribution in the study groups was
determined by direct counting and was expressed as frequencies with standard
errors (SE), and their association with the disease was studied using odds
ratios (OR) and 95% confidence intervals (95%CI). The genotype and allele
frequencies were calculated by the Chi-square test. P<0.05 was
considered statistically significant.
RESULTS
We
analyzed the association of the SNP-173 G>C MIF and STR -794 CATT(5-8)
MIF polymorphism with the susceptibility to BLEL of lacrimal gland. The -173
G>C MIF polymorphism was significantly associated with lacrimal gland BLEL,
with a significantly higher frequency of the C allele in lacrimal gland BLEL
patients (22/80; 27.50%) compared with HS (11/80; 13.75%) (OR=2.38,
95%CI=1.07-5.31, P=0.032). Furthermore, we found that the G/G genotype
was more frequent in HS (30/40, 75.00%) than in patients (21/40, 50.25%); thus,
the C/x
was
more frequent in patients (47.50%) than in HS (25.00%) (P=0.037).
Otherwise, there was no statistically significant correlation existed between
MIF-CATT(5-8) and the morbidity risk rate of lacrimal gland BLEL
(Table 2).
Table
2 Distribution of genotypes at -794 and -173 loci of MIF gene in patients and
HS
|
Parameters |
Patients, n=40 (%) |
HS, n=40 (%) |
OR (95%CI) |
P |
|
SNP-173 |
|
|
|
|
|
Genotype |
|
|
|
0.002 |
|
G/G |
21 |
30 |
- |
- |
|
G/C |
16 |
9 |
2.07
(0.78-5.51) |
NS |
|
C/C |
3 |
1 |
3.16
(0.31-31.78) |
NS |
|
Allele |
|
|
|
|
|
G |
58 |
69 |
- |
- |
|
C |
22 |
11 |
2.38
(1.07-5.31) |
0.032 |
|
Do |
|
|
|
|
|
GG |
21 |
30 |
- |
- |
|
CC+GC |
19 |
10 |
2.71
(1.05-7.00) |
0.037 |
|
STR-794 |
|
|
|
|
|
Genotype |
|
|
|
0.900 |
|
5/5 |
3 |
4 |
0.73
(015-3.49) |
NS |
|
5/6 |
20 |
22 |
0.82
(0.34-1.97) |
NS |
|
5/7 |
2 |
2 |
1.00
(0.13-7.47) |
NS |
|
6/6 |
11 |
8 |
- |
- |
|
6/7 |
3 |
4 |
0.73
(0.15-3.49) |
NS |
|
7/7 |
1 |
0 |
3.08
(0.12-77.80) |
NS |
|
Allele |
|
|
|
0.691 |
|
5 |
28 |
32 |
0.81
(0.43-1.53) |
NS |
|
6 |
45 |
42 |
- |
- |
|
7 |
7 |
6 |
1.18
(0.38-3.69) |
NS |
|
Do |
|
|
|
1.000 |
|
5/5+5/6+6/6 |
34 |
34 |
- |
- |
|
7/x |
6 |
6 |
1.00
(0.31-3.24) |
NS |
The
MIF level in plasma was significantly higher in patients of lacrimal gland BLEL
(mean 11.07 ng/mL, range 2.01-33.41 ng/mL) versus HS (mean 1.71 ng/mL, range
0.98-2.71 ng/mL) (P<0.001; Figure 1). As shown in Figure 2, a total
of 40 patients with lacrimal gland BLEL were genotyped for the -173
polymorphism of the MIF gene and evaluated for MIF levels in plasma. We found
that patients carrying the MIF-173*C allele had higher MIF levels of
serological MIF, which were significantly higher than those of patients with
the GG genotype (P=0.0041). Although the plasma level of MIF in patients
with MIF-794 CATT(7/x) was elevated, but no significant difference
was observed.
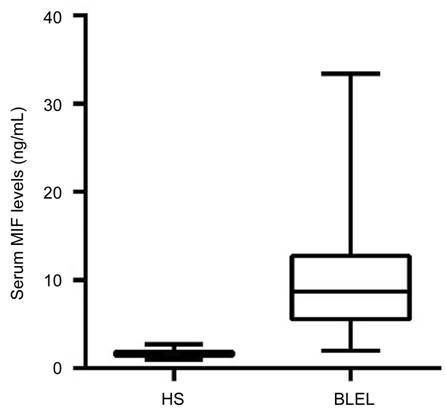
Figure
1 Plasma levels of macrophage MIF in healthy subjects and lacrimal gland
BLEL Note that
the lacrimal gland BLEL subjects had a significantly increase in MIF
concentration of plasma when compared with healthy subjects. Comparison among
groups was performed using Mann-Whitney U test; P<0.001.
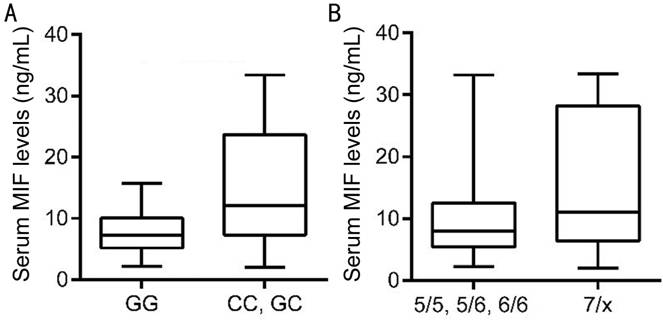
Figure
2 Plasma levels of macrophage MIF in patients with lacrimal gland BLEL A: According to the carriage of only the
MIF-173*G allele (GG) or to the carriage of the MIF-173*C allele (GC or CC), P=0.0041;
B:According to the allele frequencies of MIF-794 CATT(5/5, 5/6, 6/6)
or to the allele frequencies of MIF-794 CATT(7/x), P>0.05.
Comparison among groups was performed using Mann-Whitney U test.
DISCUSSION
Lacrimal
glands BLEL is characterized by unilateral or symmetric bilateral swelling of
the lacrimal glands, the etiology and pathogenesis of which, remain ill
defined, is relatively less studied over the last decade.
In
this study, we investigated the association of -173G>C MIF and -794 CATT(5-8)
MIF polymorphism with the risk of BLEL of lacrimal gland in Beijing population.
The patients with BLEL in this study were mainly middle-aged females with a
male-to-female ratio of 1:3. The median age was 47y (range 23-63y). The
enlargement of the lacrimal in patients with BLEL was found to be uncongested
as well as asymptomatic and nontender to palpation. We found that -173 G to C
mutations located in promoter region might be a potential risk factor. However,
we did not find a significant association between -794 CATT(5-8) MIF
polymorphism with the risk of BLEL of lacrimal gland.
MIF-173
G to C mutations are increasingly recognized causes of immune-system disorders,
including acute myeloid leukemia, erythema nodosum and psoriatic arthritis[21-23]. We found the similar situation
in BLEL of lacrimal gland. Besides, we found that the carriage rate of the MIF
-173C/x was related to higher plasma levels of MIF in the BLEL of lacrimal
gland. There were already massive evidences that elevated MIF overcomes the
inhibitory effects of glucocorticoids on TNF-alpha, IL-6 and IL-8 production,
restores IL-2 and IFN-gamma production, and antagonizes the glucocorticoid
inhibition of the production of several enzymes and cell surface molecules[24]. However, glucocorticoid therapy is the main method
of drug treatment in the BLEL of lacrimal gland. In line with this, higher
carried allele MIF -173C and higher plasma levels of MIF in patients with BLEL
of lacrimal gland were consistent with poorer response to glucocorticoid
treatment, with a higher risk of local recurrence. Thereby, the detection of
MIF -173G/C polymorphism could be a good index that can determine the curative
effect of glucocorticoid therapy in BLEL of lacrimal gland.
In
summary, we investigated for the first time the association between the
functional MIF polymorphisms and BLEL of lacrimal gland. Our results suggested
that MIF-173 G to C mutations played an insidious role in susceptibility of
BLEL of lacrimal gland, and plasma MIF expression. Further studies are still
needed to deeply reveal the mechanism of its mighty function.
ACKNOWLEDGEMENTS
Foundations: Supported
by the National Natural Science Foundation of China (No.81602408; No.81371052).
Conflicts
of Interest: Li QJ, None; Zhao PX, None; Zhang XJ, None; Yi
Y, None; Chen DY, None; Ma JM, None; Ma XM, None.
REFERENCES
1 Ferlito A, Cattai N. The so-called 'benign lymphoepithelial lesion'.
Part II. Clinical and pathological considerations with regard to evolution. J Laryngol Otol 1980;94(11):1283-1301. [CrossRef] [PubMed]
2 Stone JH, Zen Y, Deshpande V. IgG4-related disease. New Engl J Med 2012;366(6):539-551. [CrossRef] [PubMed]
3 Metwaly H, Cheng J, Ida-Yonemochi H, Ohshiro K, Jen KY, Liu AR, Saku
T. Vascular endothelial cell participation in formation of lymphoepithelial
lesions (epi-myoepithelial islands) in lymphoepithelial sialadenitis (benign
lymphoepithelial lesion). Virchows Arch
2003;443(1):17-27. [CrossRef] [PubMed]
4 Divatia M, Kim SA, Ro JY. IgG4-related sclerosing disease, an emerging
entity: a review of a multi-system disease. Yonsei
Med J 2012;53(1):15-34. [CrossRef] [PMC free article] [PubMed]
5 Tang DR, Shi XF, Sun FY, Zhao H, Jin YJ. Clinical features and therapy
of benign lymphoepithelial lesion. Zhonghua
Yan Ke Za Zhi 2009;45(5):441-445. [PubMed]
6 David JR. Delayed hypersensitivity in vitro: its mediation by
cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A
1966;56(1):72-77. [CrossRef]
7 Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with
delayed-type hypersensitivity. Science
1966;153(3731):80-82. [CrossRef] [PubMed]
8 Calandra T, Roger T. Macrophage migration inhibitory factor: a
regulator of innate immunity. Nat Rev
Immunol 2003;3(10):791-800. [CrossRef] [PubMed]
9 Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage
migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect 2002;4(4):449-460. [CrossRef]
10 Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T,
Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine
production. Nature
1995;377(6544):68-71. [CrossRef] [PubMed]
11 Santos L, Hall P, Metz C, Bucala R, Morand EF. Role of macrophage
migration inhibitory factor (MIF) in murine antigen-induced arthritis:
interaction with glucocorticoids. Clin
Exp Immunol 2001;123(2):309-314. [CrossRef]
12 De la Cruz-Mosso U, Bucala R, Palafox-Sánchez CA, Parra-Rojas I,
Padilla-Gutiérrez JR, Pereira-Suárez AL, Rangel-Villalobos H, Vázquez-Villamar
M, Angel-Chávez LI, Muñoz-Valle JF. Macrophage migration inhibitory factor:
association of -794 CATT5-8 and -173 G>C polymorphisms with TNF-α in systemic lupus erythematosus. Hum
Immunol 2014;75(5):433-439. [CrossRef] [PMC free article] [PubMed]
13 Martinez-Guzman MA, Alvarado-Navarro A, Pereira-Suarez AL, Muñoz-Valle
JF, Fafutis-Morris M. Association between STR -794 CATT5-8 and SNP -173 G/C
polymorphisms in the MIF gene and Lepromatous Leprosy in Mestizo patients of
western Mexico. Hum Immunol 2016;77(10):985-989.
[CrossRef] [PubMed]
14 Coban N, Onat A, Yildirim O, Can G, Erginel-Unaltuna N. Oxidative
stress-mediated (sex-specific) loss of protection against type-2 diabetes by
macrophage migration inhibitory factor (MIF)-173G/C polymorphism. Clin Chim Acta 2015;438:1-6. [CrossRef] [PubMed]
15 Das R, Koo MS, Kim BH, Jacob ST, Subbian S, Yao J, Leng L, Levy R,
Murchison C, Burman WJ, Moore CC, Scheld WM, David JR, Kaplan G, MacMicking JD,
Bucala R. Macrophage migration inhibitory factor (MIF) is a critical mediator
of the innate immune response to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A
2013;110(32):E2997-E3006. [CrossRef] [PMC free article] [PubMed]
16 Wang FF, Huang XF, Shen N, Leng L, Bucala R, Chen SL, Lu LJ. A
genetic role for macrophage migration inhibitory factor (MIF) in adult-onset
Still's disease. Arthritis Res Ther
2013;15(3):R65. [CrossRef] [PMC free article] [PubMed]
17 Radstake TR, Sweep FC, Welsing P, Franke B, Vermeulen SH,
Geurts-Moespot A, Calandra T, Donn R, van Riel PL. Correlation of rheumatoid
arthritis severity with the genetic functional variants and circulating levels
of macrophage migration inhibitory factor. Arthritis
Rheum 2005;52(10):3020-3029. [CrossRef] [PubMed]
18 Tong X, He J, Liu S, Peng S, Yan Z, Zhang Y, Fan H. Macrophage migration
inhibitory factor -173G/C gene polymorphism increases the risk of renal
disease: a meta-analysis. Nephrology
(Carlton) 2015;20(2):68-76. [CrossRef] [PubMed]
19 Vivarelli M, D'Urbano LE, Insalaco A, Lunt M, Jury F, Tozzi AE,
Ravelli A, Martini A, Donn R, De Benedetti F. Macrophage migration inhibitory
factor (MIF) and oligoarticular juvenile idiopathic arthritis (o-JIA):
association of MIF promoter polymorphisms with response to intra-articular
glucocorticoids. Clin Exp Rheumatol
2007;25(5):775-781. [PubMed]
20 Nohara H, Okayama N, Inoue N, Koike Y, Fujimura K, Suehiro Y,
Hamanaka Y, Higaki S, Yanai H, Yoshida T, Hibi T, Okita K, Hinoda Y.
Association of the -173 G/C polymorphism of the macrophage migration inhibitory
factor gene with ulcerative colitis. J
Gastroenterol 2004;39(3):242-246. [CrossRef] [PubMed]
21 Ramireddy L, Lin CY, Liu SC, Lo WY, Hu RM, Peng YC, Peng CT.
Association study between macrophage migration inhibitory factor-173
polymorphism and acute myeloid leukemia in Taiwan. Cell Biochem Biophys 2014;70(2):1159-1165. [CrossRef] [PubMed]
22 Karakaya B, van Moorsel CH, van der Helm-van Mil AH, Huizinga TW,
Ruven HJ, van der Vis JJ, Grutters JC. Macrophage migration inhibitory factor
(MIF) -173 polymorphism is associated with clinical erythema nodosum inLöfgren's
syndrome. Cytokine
2014;69(2):272-276. [CrossRef] [PubMed]
23 Morales-Zambrano R, Bautista-Herrera LA, De la Cruz-Mosso U,
Villanueva-Quintero GD, Padilla-Gutiérrez JR, Valle Y, Parra-Rojas I,
Rangel-Villalobos H, Gutiérrez-Ureña SR, Muñoz-Valle JF. Macrophage migration
inhibitory factor (MIF) promoter polymorphisms (-794 CATT5-8 and -173 G>C):
association with MIF and TNFαin psoriatic arthritis. Int J Clin Exp Med 2014;7(9):2605-2614.
[PMC free article] [PubMed]
24 Aeberli D, Leech M, Morand EF. Macrophage migration inhibitory factor
and glucocorticoid sensitivity. Rheumatology (Oxford) 2006;45(8): 937-943. [CrossRef] [PubMed]