
·Clinical Research· Current
Issue IF in JCR CiteScore ·Submission· In Press Recent Accepted PMC RSS
Citation: Lee W, Bae HW, Kim CY, Seong GJ. The change of
anterior segment parameters after cataract surgery in normal-tension glaucoma. Int
J Ophthalmol 2017;10(8):1239-1245
The change of anterior segment parameters after
cataract surgery in normal-tension glaucoma
Wonseok Lee, Hyoung Won Bae, Chan Yun Kim, Gong Je
Seong
Institute of Vision Research, Department of Ophthalmology, Yonsei
University College of Medicine, Seoul 03722, Korea
Correspondence to: Gong Je Seong.
Institute of Vision Research, Department of Ophthalmology, Yonsei University
College of Medicine, #211 Eonjuro, Gangnam-gu, Seoul 03722, Korea.
gjseong@yuhs.ac
Received:
2016-11-08
Accepted: 2017-03-06
Abstract
AIM: To
investigate the change of anterior chamber angle morphology and intraocular
pressure (IOP) reduction after cataract surgery in patients with normal-tension
glaucoma (NTG) using swept-source optical coherence tomography (SS-OCT).
METHODS: This
prospective, comparative, observational study recruited patients into two
groups. Group 1 was the control group including normal subjects except those
with cataracts (cataract group, n=67 eyes of 67 patients), and group 2
was NTG group including patients who were diagnosed with NTG and cataracts (n=43
eyes of 43 patients), which were treated with phacoemulsification and
intraocular lens implantation. Before surgery, and at postoperative 1 and 6mo,
anterior chamber angles were evaluated by SS-OCT under dark conditions using
three-dimensional angle analysis scan protocol. Angle opening distance (AOD),
angle recess area (ARA), and trabecular-iris surface area (TISA) at four
quadrants (temporal, nasal, superior, and inferior) were calculated
automatically by SS-OCT, after the observer marked scleral spurs.
RESULTS: A
total of 106 patients (54 males and 52 females) were enrolled in the study.
Angle parameters, AOD, ARA, and TISA were increased after surgery in both
groups. However, changes of angle parameters were only significant in group 2.
In group 2, preoperative IOP was 13.2±2.9 mm Hg, and postoperative IOP at 1 and
6mo were 10.5±3.0 and 10.7±2.8 mm Hg, respectively. In group 1, preoperative
IOP was 12.4±2.8 mm Hg, and postoperative IOP at 1 and 6mo were 11.6±2.5 and
12.0±2.8 mm Hg, respectively. After cataract surgery, angle parameters changed
significantly while IOP significantly reduced and was maintained in group 2 (P<0.001).
The changes in angle parameters (ΔAOD500, ΔTISA500 at temporal; ΔAOD500,
ΔARA500 at nasal) were linearly correlated with postoperative IOP changes.
CONCLUSION: Cataract
surgery may have improved anterior chamber angle parameters and decreased IOP
in NTG patients.
KEYWORDS: normal-tension glaucoma; cataract
surgery; intraocular pressure reduction; swept-source optical coherence
tomography; angle parameters
DOI:10.18240/ijo.2017.08.09
Citation: Lee W, Bae HW, Kim CY, Seong GJ. The change of anterior segment
parameters after cataract surgery in normal-tension glaucoma. Int J
Ophthalmol 2017;10(8):1239-1245
INTRODUCTION
Cataract and glaucoma are both very common eye diseases in older
patients, and their prevalence significantly increases with increasing age.
Thus, large numbers of glaucoma patients also have cataracts, which can
decrease visual acuity, contrast sensitivity, and examination accuracy. For
these reasons, many glaucoma patients undergo cataract surgery. Cataract and
glaucoma are major causes of blindness in the world[1].
Cataract is the leading cause of blindness, and it accounts for 50% of
blindness worldwide. Glaucoma is the leading cause of irreversible blindness,
with an estimated prevalence of about 2.4% in the world[2-3]. According to previous reports, in Asia, the prevalence
of normal-tension glaucoma (NTG) refers to glaucoma with an intraocular
pressure (IOP) below 21 mm Hg, is higher than other regions around the world[4]. It is widely known that glaucoma progression is
associated with IOP and hemodynamic status, and controlling IOP is the only
existing treatment to prevent NTG progression[3].
The structures and functions of angles are important in aqueous humour
outflow. It is well-known that open angle status, rather than closed-angle
status, is a more favorable structure in aqueous humour drainage. Lens
extraction using cataract surgery creates more space in posterior chamber as
well as in the angle, especially in older patients. With aging, lens becomes
thicker and anterior chamber narrows. Oxidative stress markers hinder
trabecular meshwork functioning, and increasing levels of superoxide dismutase
(SOD) and catalase (CAT) activities in anterior chamber have been correlated
with the severity of cataracts[5-6].
Cataract surgery is a positive factor that benefits the prevention of
closed-angle glaucoma, and multiple studies have reported that cataract surgery
can lower IOP in glaucoma patients.Lens extraction using cataract surgery is helpful
for closed-angle glaucoma with severely narrowed angles. After cataract
surgery, configuration of angle was widened, and it lowered IOP[7-9]. However, many controversies remain
for open angle glaucoma patients, which led us to investigate the effects of
cataract surgery in open angle glaucoma.
Recently, anterior segment swept-source optical coherence tomography
(AS-SS-OCT) has been used for evaluation of anterior segment (AS)
configurations, although previous evaluations only used gonioscopy. Ultrasound
biomicroscopy (UBM) assessment of the angle is more objective and reproducible,
but its contact may be uncomfortable to the patient[10].
Anterior segment optical coherence tomography (AS-OCT) is a non-contact method
that can perform both quantitative and objective evaluations[10-11]. When only scleral spur is marked, the built-in
software can automatically calculate many AS parameters. Compared to
time-domain optical coherence tomography (TD-OCT), the currently used SS-OCT
has more horizontal and depth resolution, so that high speed, high resolution,
and three-dimensional imaging of the angle, anterior lens surface, and full
thickness morphology of the cornea can be obtained. Furthermore, analytical
properties of AS-SS-OCT are being continuously refined[11-12].
We investigated angle configuration change and IOP reduction after
cataract surgery in NTG patients. Before surgery, not a lot of differences
could be found between glaucoma and non-glaucoma patients in terms of angle
configuration and function. However, we
hypothesized that the changes in angle structures might be different between
two groups after cataract surgery. According to the changes in angle
configuration, greater IOP reduction might be obtained in NTG. We can expect better IOP control by cataract surgery in NTG patients.
SUBJECTS AND METHODS
Ethics Statement The study protocol followed tenets of the Declaration of Helsinki, and
was approved by the Institutional Review Board of Gangnam Severance Hospital,
Yonsei University College of Medicine. Informed consent was obtained from all
subjects.
Study Patients Between January and December 2015, patients from Gangnam Severance
Hospital Eye Center (Seoul, Korea) were recruited. This prospective, comparative,
observational study divided patients into two groups. Group 1 was cataract
group, including normal patients except for those with cataracts. Group 2 was
NTG group, consisting of NTG patients with cataracts; this group was further
defined as either having or not having glaucomatous optic nerve changes and
visual field (VF) defects. NTG was defined by a glaucoma specialist based on
the following factors: 1) glaucomatous VF defect confirmed by two reliable VF
tests; 2) typical appearance of glaucomatous optic nerve head (ONH) that
includes cup/disc (C/D) ratio >0.7 and C/D ratio asymmetry >0.2, with
diffuse or focal neuroretinal rim thinning, disc haemorrhage, or vertical
elongation of the optic cup; 3) maximum untreated IOP at <21 mm Hg, as determined
by three repeated measurements taken at different times on separate visits
during follow-up; 4) normal anterior chamber with open angle
status on slit-lamp and gonioscopic examinations.
Inclusion and Exclusion Criteria
All patients were Shaffer grade ≥3, with
open angle status. Gonioscopy was performed on the first visit (day 1) using
four-mirror goniolens (G-4 High Mag, Volk Optical, Inc., OH, USA) in
non-dilated status. Under topical anaesthesia (Proparacaine Hydrochloride,
Alcaine®; Alcon, Fort Worth, TX, USA), quadrants were studied with
the four-mirror lens. When scleral spur was visible and angle opened wide
(about 30°-45°) in all quadrants, which was graded by Shaffer grade ≥3.
Patients did not have any other ocular disease affecting the aqueous outflow or
angle morphology, except for cataract and glaucoma. Clinical exclusion criteria
included closed-angle glaucoma, neovascular glaucoma, age-related macular
degeneration, and proliferative diabetic retinopathy. Patients with prior
corneal surgery, trabeculoplasty, cycloablation, or any incisional glaucoma
procedure (such as trabeculectomy, tube shunt, or deep sclerectomy) were also
excluded. If cataract surgery was performed on both eyes, the most affected
eyes (worse visual acuity in group 1 and worse glaucoma field in group 2) were
enrolled.
Study Examinations All patients completed an assessment of visual acuity, Goldmann
applanation tonometry, gonioscopy, axial length measurement, and indirect
ophthalmoscopy. IOP measurement was performed at the clinic in Gangnam
Severance Hospital, using Goldmann applanation tonometry (AT900®;
Haag-Streit, Koeniz, Switzerland) with topical anaesthesia (proparacaine HCl,
Alcaine®; Alcon, Fort Worth, TX, USA). A single ophthalmologist
(Choi W) performed IOP measurement three times, and the average value was used
for analysis. Patients’ data or study enrolment statuses were masked. IOP
measurement was performed at the latest procedure (OCT was performed ahead of
IOP measurements) during clinical operation time (from 9:00 a.m. to noon), with
well-calibrated Goldmann applanation tonometry. Axial length was determined by
non-contact type laser biometry (IOL-Master500®; Carl Zeiss Meditec,
Dublin, CA, USA). Central corneal thickness was measured by a contact-type
ultrasound pachymeter (US-500 Echoscan, Nidek Co., Ltd., Gamagori, Japan).
AS-SS-OCT was performed before surgery and on postoperative 1 and 6mo.
Preoperative assessments (visual acuity, tonometry, gonioscopy, and AS-SS-OCT)
were performed before surgery.
Surgical Procedures Patients were prescribed pupil dilating medication [5 mg phenylephrine
HCl, 5 mg tropicamide (Mydrin-P®; Taejoon Pharmaceutical, Seoul,
Korea)] before surgery. One surgeon (Seong GJ) performed all cataract
operations under topical anaesthesia (proparacaine HCl, Alcaine®;
Alcon, Fort Worth, TX, USA). A 2.75 mm clear corneal incision was made at
temporal side of the cornea, and anterior chamber was filled with an ophthalmic
viscoelastic device (Healon®; Abbott Laboratories, Chicago, IL,
USA). An approximate 5.5-6.0 mm of continuous curvilinear capsulorrhexis was
performed. Lens extraction was done by phacoemulsification (INFINITI®;
Alcon), and foldable intraocular lens (Hoya iSert®, Hoya, Tokyo,
Japan) was inserted into the capsular bag. Corneal wound was sutured with one
knot at the temporal incision site, and suture knot was removed at
postoperative 2wk. Patients were then treated with gatifloxacin eye drops
(Handok, Seoul, Korea) four times per day for 2wk. Prednisolone acetate eye
drops (Allergan, Irvine, CA, USA) were used four times per day for 4wk. There
were no complications during or after the surgery.
Anterior Segment Parameters Before
starting the main study, we checked the repeatability and reproducibility of
SS-OCT with scleral spur marking. In our anterior OCT instrument (Casia SS-1000; Tomey, Nagoya, Japan), there was built-in software for
automatic calculation of AS parameters that was initiated as soon as scleral
spur was marked. Therefore, scleral spur marking was considered very important
for reliability of AS parameter determinations in this study. A total of 30 randomly
selected patients, involving 30 eyes, were checked by AS-SS-OCT. Two
investigators marked the scleral spur site at separate spaces at different
times, and this procedure was repeated after 1wk. Intrapersonal and
interpersonal correlation coefficients were obtained for these determinations.
AS parameters were obtained by AS-SS-OCT (Casia SS-1000). One operator
obtained all angle images in the undilated state under dark, identical room
conditions. To obtain the entire angle images, upper eyelids were gently raised
by the examiner using a long cotton tip. If eyelids were tightened and the eye
was not exposed in one image, the examiner separately obtained images of the
four quadrants. For example, the examiner asked the patient to look forward and
raised the upper eyelid for the superior quadrant for imaging, and images of
the other quadrants were obtained in a second imaging. Using the angle analysis
mode of Casia SS-1000, images were obtained of the nasal, temporal, superior,
and inferior angle quadrants by moving the arrow bar. The examiner was blinded
to the diagnosis of the patient. Images were also analysed by two other
investigators who were blinded to the diagnosis of the patient. The best images
were selected after analyses using the automatic calculating software in
AS-SS-OCT, in order to obtain several AS parameters. Angle opening distance
(AOD) at 500 µm (AOD500), 750 µm (AOD750) from the scleral spur, trabecular-iris
surface area at 500 µm (TISA500), 750 µm (TISA750), angle recess area at 500 µm
(ARA500), 750 µm (ARA750) were obtained automatically, after marking the
scleral spur (Figures 1, 2)[12].
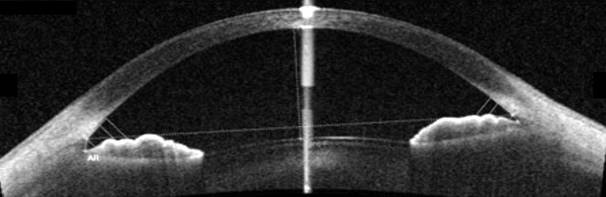
Figure 1 Angle evaluation with SS-OCT and automatically obtained angle
parameters
AOD, ARA, TISA and TIA attemporal
side were 0.522, 0.223, 0.189, 38.0 (at 500 µm) and 0.763, 0.392, 0.357, 40.5 (at 750 µm). At nasal side, angle parameters were 0.344, 0.145, 0.127, 26.4 (at 500 µm) and 0.598, 0.273, 0.256, 32.6 (at 750 µm).
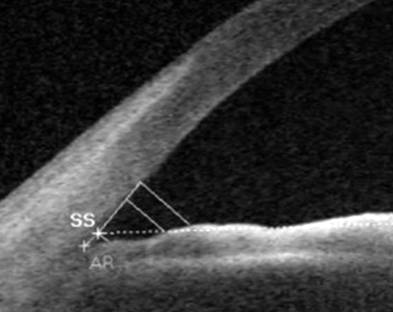
Figure 2
Scleral spur (SS), angle recess (AR) and 500/750 µm points from scleral spur were indicated in analyzed program.
Statistical Analysis The repeated longitudinal data were analysed by SPSS, version 20 software
for windows (IBM, Chicago, IL, USA) based on longitudinal, parametric, paired t-test,
Chi-square test, and multiple regression test. For intraclass and interclass
correlation coefficients, two-way mixed effects model was used. All patients
were included and all results were considered significant at P<0.05.
RESULTS
Characteristics of the Study Patients A total of 110 patients were enrolled. In the end, 4 patients (3 from
group 1, 1 from group 2) were excluded due to loss of follow-up. Group 1
included 64 eyes of 64 patients, comprised of 30 males and 34 females. Group 2
included 42 eyes of 42 patients, comprised of 24 males and 18 females. The mean
age was 68.87±8.68y in group 1, and 68.00±10.66y in group 2 (Table 1).
Table 1
Patients characteristics mean±SD
|
Parameters |
Group 1 |
Group 2 |
P |
|
Age (a) |
68.87±8.68 |
68.00±10.66 |
0.944 |
|
Gender
(M:F) |
30:34 |
24:18 |
0.453a |
|
Laterality
(R:L) |
33:31 |
24:18 |
0.806a |
|
Axial
length (mm) |
24.01±1.16 |
24.26±1.53 |
0.362 |
|
CCT (μm) |
554 ±64 |
556±28 |
0.959 |
|
Refractive
error (SE) |
-0.41±0.85 |
-0.50±0.85 |
0.602 |
|
MD (dB) |
NA |
-4.78±5.16 |
NA |
|
Untreated
IOP (mm Hg) |
NA |
16.5±3.1 |
NA |
|
Preop. VA
(logMAR) |
0.26±0.15 |
0.21±0.03 |
0.854 |
|
Postop. VA
(logMAR) |
0.03±0.01 |
0.02±0.01 |
0.886 |
NTG:
Normal-tension glaucoma; CCT: Central corneal thickness; SE: Spherical
equivalent; VA: Visual acuity; Untreated IOP: Intraocular pressure before
anti-glaucoma medications. aP values by Chi-square test.
Repeatability and Reproducibility
AS parameters, AOD500, AOD750, ARA500,
ARA750, TISA500, and TISA750 had good repeatability and reproducibility.
Intraclass correlation coefficients of one investigator at an interval of 1wk
were 0.896-0.984, and interclass correlation coefficients for two investigators
were 0.906-0.980. Therefore, both repeatability and reproducibility were
acceptable for this study.
Intraocular Pressure Reduction After Cataract Surgery Group 2 (NTG group) had significant changes in IOP. In group 2, preoperative
IOP was 13.2±2.9 mm Hg, and postoperative IOP at 1
and 6mo were 10.5±3.0 and 10.7±2.8 mm Hg,
respectively. After cataract surgery, significant IOP
reduction was observed in group 2 (P<0.001) (Table 2). About 19% IOP
reduction was obtained after cataract surgery in NTG patients. Before cataract
surgery, the mean IOPs were not significantly different between two groups,
recording at 12.4±2.8 mm Hg in group 1 and
13.2±2.9 mm Hg in group 2 (P=0.078). However, significant reduction of
IOP was shown only in group 2. In addition, NTG patients used fewer
anti-glaucoma eye drops after cataract surgery than before surgery (P=0.005).
Before surgery, NTG patients used a mean of 1.53±0.61 species of anti-glaucoma
eye drops. Six months after cataract surgery, they used a mean of 0.71±0.83
species of anti-glaucoma eye drops. No patients required IOP-lowering
medications after cataract surgery in group 1 (cataract group). Among
patients with open angle glaucoma, 33 used prostaglandin analogues, eight used
selective beta blockers, 11 used fixed combination of timolol and dorzolamide,
and six used alpha agonist (brimonidine).
Twenty-one subjects used two species of anti-glaucoma eye drops, and three patients used three species. No
significant correlation was found between the use of prostaglandin analogue or
alpha agonist and angle parameters.
Table 2
Comparison of IOP
mm Hg; mean±SD
|
Groups |
Preop. |
Postop. 1mo |
Postop. 6mo |
P |
|
Group 1 |
12.4±2.8 |
11.6±2.5 |
12.0±2.8 |
0.065a; 0.082b |
|
Group 2 |
13.2±2.9 |
10.5±3.0 |
10.7±2.8 |
<0.001a; <0.001b |
aP values
between preop. and postop. 1mo; bP values between preop. and
postop. 6mo.
Anterior Chamber Parameters In both groups, anterior chamber depth (ACD) and anterior chamber volume
(ACV), which were measured by three-dimensional reconstruction program of the
AS-OCT after cataract surgery, significantly increased compared to before
surgery (Table 3). However, there was no significant difference between
two groups in regards to ACD and ACV (P=0.576, 0.164, respectively). The decrease of IOP in group 2
might not be due to changes of ACD and ACV, but could be due to increase in
angle parameters.
Table 3
Comparison of ACD and ACV
|
Groups |
ACD (mm) |
ACV (mm3) |
||||
|
Preop. |
Postop. |
P |
Preop. |
Postop. |
P |
|
|
Group 1 |
2.92±0.48 |
3.45±0.20 |
<0.001a |
159.96±30.66 |
175.22±21.42 |
<0.001a |
|
Group 2 |
3.07±0.45 |
3.45±0.50 |
<0.001a |
165.30±42.59 |
174.05±36.25 |
<0.001a |
|
P |
0.073b |
0.576b |
NA |
0.452b |
0.164b |
NA |
ACD:
Anterior chamber depth; ACV: Anterior chamber volume. aP
value comparison between preop. and postop.; bP value
comparison between groups 1 and 2.
Changes of Angle Configurations
Before cataract extraction in group 1, AS
parameters at the temporal side were as follows: AOD500, 0.52±0.29 mm; AOD750,
0.70±0.37 mm; ARA500, 0.25±0.13 mm2; ARA750, 0.41±0.21 mm2;
TISA500, 0.20±0.11 mm2; and TISA750, 0.35±0.19 mm2. At
postoperative 1 and 6mo at the temporal side, AOD500, AOD750, ARA500, ARA750,
TISA500, and TISA750 significantly increased. The values at nasal, superior,
and inferior sides are also increased. AS-OCT parameters significantly changed
when comparing preoperative and postoperative values.
In group 2, the changes of AS parameters were significant. Before
cataract extraction, the following parameters were determined at the temporal
side: AOD500, 0.52±0.24 mm; AOD750, 0.74±0.38 mm; ARA500, 0.26±0.16 mm2;
ARA750, 0.42±0.24 mm2; TISA500, 0.19±0.10 mm2; and
TISA750, 0.35±0.18 mm2. Anterior angle parameters were significantly
increased at postoperative 1 and 6mo at the temporal side. Data for nasal,
superior, and inferior side parameters are also increased. Changes in all
parameters before and 1 or 6mo after cataract surgery in NTG patients were all
statistically significant in all four quadrants. The variations of angle
parameters in group 2 (Figure 3) were greater than those of group 1. In
particular, nasal quadrant angle parameters (ARA500, ARA750, and TISA500) were
significantly increased compared to those of group 1. There was a 66.66%
increase in ARA500 in group 1, and a 138.09% increase in group 2 (P=0.031);
a 65.78% increase in ARA750 in group 1, and a 121.32% increase in group 2 (P=0.038);
and a 61.11% increase of TISA500 in group 1, and 106.25% increase in group 2 (P=0.045).
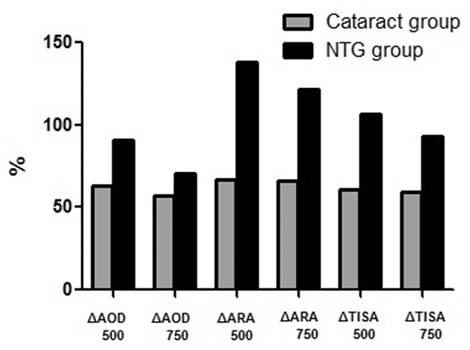
Figure 3 Comparisons of angle parameters variations (ΔAOD500/750, ΔARA500/750, ΔTISA500/750) at nasal quadrant in
control and NTG group.
Multiple regression analyses of angle parameter changes and IOPs showed
that the changes (Δ) of AOD500 at temporal and nasal sides, TISA500 at temporal
side, and ARA500 at nasal side were linearly correlated with postoperative IOP
(β=-14.686, -11.831, -23.671, and -14.263; P=0.022, 0.050, 0.050, and
0.047, respectively) (Table 4).
Table 4 Multiple
regressions of angle parameter changes and postop. IOP changes
|
Angle
parameters |
β |
t |
P |
R2 |
|
Temporal |
|
|
|
0.474 |
|
ΔAOD
500 |
-14.686 |
-2.417 |
0.022 |
|
|
ΔTISA
500 |
-23.671 |
-1.964 |
0.050 |
|
|
Nasal |
|
|
|
|
|
ΔAOD
500 |
-11.831 |
-2.037 |
0.050 |
|
|
ΔARA
500 |
-14.263 |
-2.070 |
0.047 |
|
DISCUSSION
AS-SS-OCT showed good repeatability and reproducibility in our study, as
well as in other previous studies[13-14].
The present prospective study is the first to evaluate the relationship between
angle configuration and IOP in NTG patients before and after cataract surgery.
IOP control is the most important factor in glaucoma patients, and lowering IOP
will minimize damage to the retinal nerve fiber layer. Taken together, the
present study is important in optimizing glaucoma treatment. After cataract
surgery, ACD, ACV were significantly increased. However, after surgery, no
difference was found between two groups in terms of ACD or ACV. The significant
decrease of IOP in group 2 might not be due to changes of ACD and ACV, but
could be due to a more significant increase in angle parameters.
However, the effect of IOP reduction in cataract surgery remains
controversial. In our study, group 1 (cataract group) also had slightly
decreased IOP at 6mo after surgery. According to angle widening, non-glaucoma
patients also might have lowered IOP, although the value may not be highly
significant (P=0.082). The average IOP of non-glaucomatous eye was
13.4±2.9 mm Hg, according to Namil study (Namil-myon,
a rural agricultural area in central Korea). The average IOP in this study was slight lower than that of Namil
study; however, preoperative IOP of two groups (groups 1 and 2) were not
significantly different. Besides, more IOP reduction was obtained in group 2 at
postoperative status. Meanwhile, Prata et al[15]
reported that cataract surgery could reduce IOP during the first few days after
surgery. However, IOP was not meaningfully reduced for longer time periods
(average 5y). Among many studies, the mean decrease of IOP values varied
between 1.1 and 5.3 mm Hg. According to Melancia et
al[16], 68 patients with open angle glaucoma who were controlled with medication
had clinically meaningful IOP (Hayashi group). However, other groups
(Shingleton and Mathalone groups) reported that the changes of IOP after
cataract surgery were not meaningful in primary open angle glaucoma (POAG).
Phacoemulsification results in angle widening and a decrease of IOP in
closed-angle glaucoma. After phacoemulsification, ACD and angle parameters
change significantly[7-10,15-19]. In elderly patients, lens
thickness from anterior to posterior surface is increased. The thick lens can
influence aqueous humour dynamics, due to shallower anterior chamber and narrower
angle. In older open angle glaucoma patients, including NTG, there are frequent
senile cataracts. IOP elevation is due to aqueous outflow resistance resulting
from trabecular meshwork alterations or collapse of Schlemm’s canal, which are
major causes of open angle glaucoma[20-21].
However, it is well-known that senile cataracts can disrupt aqueous humour
outflow. In these cases after cataract extraction, an increase of aqueous
outflow has been reported[22]. Cataract
extraction alone may at least partially correct the anatomical and
physiological problems in aqueous humour dynamics[9].
Senile POAG patients have both conventional pathway dysfunction (trabecular
meshwork disruption) and aqueous humour outflow barriers due to increasing lens
thickness, which cannot be adequately clinically evaluated by gonioscopy[23]. However, AS-OCT can detect open angles, with
narrowed status, more accurately than gonioscopic examination.
Aqueous humour is secreted from ciliary bodies and drained in a balanced
pattern at ocular AS, which includes the lens and the trabecular meshwork, to
achieve the following: provide nutrients, scavenge metabolic wastes, and
maintain the correct ocular pressure. Humans, like other living organisms, are
continuously exposed to reactive oxygen species (ROS) as a consequence of
biochemical reactions, as well as external pollutants. ROS are causes of many
degenerative diseases, including glaucoma, cataract, and macular degeneration.
Production of free radicals can result in degeneration of mammalian cells[2]. The cells of trabecular meshwork and Schlemm’s canal
can therefore be damaged by oxidative stress, which is considered to be the
major pathogenic mechanism of POAG. Significant correlations have been found
between oxidative damage of human trabecular meshwork DNA, VF defects, and IOP[2]. ROS have also been linked to POAG by increased flow
resistance in anterior chamber, due to high levels of hydrogen peroxide[2-3,24]. According to
the severity of nuclear cataract, SOD and CAT levels increase in aqueous humour[5]. Increasing ROS in anterior chamber activates
scavenging system of ROS in the lens. Imbalance of ROS scavenging system and
ROS production are the causes of cataract formation[24-25]. Whether ROS increases in anterior chamber precede
cataract formation, or cataract formation precedes the formation of ROS is not
known. However, many types of ROS can cause cataracts in the lens, so it is
possible that cataracts could be a major cause of ROS. Therefore,
it is possible that cataract extraction is an effective
treatment for decreasing ROS in anterior chamber. As the levels of ROS
decrease, trabecular meshwork in anterior chamber could be protected to prevent
open angle glaucoma.
During cataract surgery, spontaneous infusion and aspiration were
performed with balanced salt solution (BSS). Angles can be enlarged and cleaned
by viscoelastic material injection during surgery. One main cause of POAG is
alteration of trabecular meshwork due to plaque materials. During cataract
surgery, the use of BSS in anterior chamber can remove plaque materials from
the angle and trabecular meshwork. In pseudoexfoliation (PEX) syndrome, PEX
materials are removed during cataract surgery. Water-jet infusion of BSS
removed PEX materials from the angles, and no PEX material recurrence was seen
during a mean follow-up of 3y. The mean IOP also decreased significantly after
surgery[26]. In a similar manner, anterior
chamber irrigation during cataract surgery is helpful in removing plaque
materials, which can influence aqueous humour drainage function of trabecular
meshwork and Schlemm’s canal. In this study, all surgeries were performed through a temporal side clear corneal incision
followed by phacoemulsification. The direction of phaco-tip was roughly toward
the nasal quadrant; therefore, water-jet effect could be stronger in the nasal
quadrant. Water-jet might alter the angle structure; more precisely, the angle
geometry in the nasal quadrant could be significantly increased, as shown in
this study. Connective tissue structures in glaucoma
patients are more elastic to IOP increases than non-glaucoma patients[27]. Prostaglandin analogues, widely used in NTG
patients, modulate iris and uvea connective tissues via matrix
metalloproteinase (MMPs) in the anterior chamber[28].
Lens extraction using phacoemulsification and IOP implantation create more
space in posterior chamber. More loosening of connective tissues in the angle
can be directed toward posterior-inferior direction. Angle widenings could then
occur during these processes.
According to Koc et al[29], angle
width in all quadrants were significantly lower in older group (>41y) than
younger groups (<20 or 21-40y) in open angle, healthy subjects. Even with
open angle status, older patients have slightly narrowed angle width compared
to younger subjects. This might be the reason why ΔAOD 500 at nasal and
temporal quadrants was correlated with postoperative IOP. We thought
that differences in the composition of connective studies might greatly widen
the angle structures in glaucoma group after undergoing the same procedures
(cataract surgery), compared to non-glaucoma group
(cataract group). Wider angle structure in glaucoma eyes could lead to greater
IOP reduction, as compared to non-glaucoma eyes. No significant changes were
found in AVD or ACV; however, differences were significant for the angle
structures.
This study has some limitations. First, the duration of this study was
6mo. To evaluate long-term effects of cataract extraction for glaucoma
patients, longer follow-up periods are needed. Second, this study included a
relatively small number of participants (106 eyes of 106 patients). However,
data of this prospective study had a normal distribution, and the effects of
cataract surgery were statistically significant in NTG patients and normal
subjects. Third, we could not evaluate the diurnal variation of IOP
(especially at night), and the anti-glaucoma drugs used by all subjects were
not identical. However, we made an effort to diminish the bias of IOP
variations, and checked IOP at the same time (at 9:00 a.m. to noon). Fourth,
subjects enrolled this study were in relatively early stages of glaucoma
(-4.78±5.16 dB). We need to study IOP reduction in moderate or severe NTG
patients.
During the processes of phacoemulsification and acrylic monofocal
intraocular lens insertion, anterior angle can be widened in both elderly
glaucoma and normal patients. Especially in NTG patients, nasal quadrant angle
parameters can be significantly increased, and IOP can be reduced more than
non-glaucoma patients after cataract extraction. Therefore, our results suggest
that phacoemulsification and intraocular lens implantation can be simple and
convenient adjunctive treatments for glaucoma.
ACKNOWLEDGEMENTS
My special
thanks must go to Dr. Choi W, who had participated in IOP measurement in
outpatient clinic with double blind maneuver (patients’ data or study enrolment
statuses were masked). With his work, we could perform this study.
Conflicts of
Interest: Lee W, None; Bae
HW, None; Kim CY, None; Seong GJ, None.
REFERENCES
1 Chan W, Garcia JA, Newland HS, Muecke J, McGovern S, Selva D, Aung T,
Casson RJ. Killing two birds with one stone: the potential effect of cataract
surgery on the incidence of primary angle-closure glaucoma in a high-risk
population. Clin Exp Ophthalmol 2012;40(4):e128-134.
[CrossRef] [PubMed]
2 Babizhayev MA. Biomarkers and special features of oxidative stress in
the anterior segment of the eye linked to lens cataract and the trabecular
meshwork injury in primary open-angle glaucoma: challenges of dual combination
therapy with N-acetylcarnosine lubricant eye drops and oral formulation of
nonhydrolyzed carnosine. Fundam Clin
Pharmacol 2012;26(1):86-117. [CrossRef] [PubMed]
3 Zanon-Moreno V, Marco-Ventura P, Lleo-Perez A, Pons-Vazquez S,
Garcia-Medina JJ, Vinuesa-Silva I, Moreno-Nadal MA, Pinazo-Duran MD. Oxidative
stress in primary open-angle glaucoma. J
Glaucoma 2008;17(4):263-268. [CrossRef] [PubMed]
4 Kim CS, Seong GJ, Lee NH, Song KC; Namil Study Group, Korean Glaucoma
Society. Prevalence of primary open-angle glaucoma in central South Korea the
Namil study. Ophthalmology 2011;118(6):1024-1030.
[CrossRef] [PubMed]
5 Sawada H, Fukuchi T, Abe H. Oxidative stress markers in aqueous humor
of patients with senile cataracts. Curr
Eye Res 2009;34(1):36-41. [CrossRef] [PubMed]
6 Goyal A, Srivastava A, Sihota R, Kaur J. Evaluation of oxidative
stress markers in aqueous humor of primary open angle glaucoma and primary
angle closure glaucoma patients. Curr Eye
Res 2014;39(8):823-829. [CrossRef] [PubMed]
7 Roberts TV, Francis IC, Lertusumitkul S, Kappaqoda MB, Coroneo MT.
Primary phacoemulsification for uncontrolled angle-closure glaucoma. J Cataract Refract Surg
2000;26(7):1012-1016. [CrossRef]
8 Brown RH, Zhong L, Whitman AL, Lynch MG, Kilgo PD, Hovis KL. Reduced
intraocular pressure after cataract surgery in patients with narrow angles and
chronic angle-closure glaucoma. J
Cataract Refract Surg 2014;40(10):1610-1614. [CrossRef] [PubMed]
9 Lai JS, Tham CC, Chan JC. The clinical outcomes of cataract extraction
by phacoemulsification in eyes with primary angle-closure glaucoma (PACG) and
co-existing cataract: a prospective case series. J Glaucoma 2006;15(1):47-52. [CrossRef] [PubMed]
10 Zhou AW, Giroux J, Mao AJ, Hutnik CM. Can preoperative anterior
chamber angle width predict magnitude of intraocular pressure change after
cataract surgery? Can J Ophthalmol
2010;45(2):149-153. [CrossRef] [PubMed]
11 Fukuda R, Usui T, Tomidokoro A, Mishima K, Matagi N, Miyai T, Amano S,
Araie M. Noninvasive observations of peripheral angle in eyes after penetrating
keratoplasty using anterior segment fourier-domain optical coherence
tomography. Cornea
2012;31(3):259-263. [CrossRef] [PubMed]
12 Nolan WP, See JL, Aung T, Friedman DS, Chan YH, Smith SD, Zheng C,
Huang D, Foster PJ, Chew PT. Changes in angle configuration after
phacoemulsification measured by anterior segment optical coherence tomography. J Glaucoma 2008;17(6):455-459. [CrossRef] [PubMed]
13 Aptel F, Chiquet C, Gimbert A, Romanet JP, Thuret G, Gain P, Campolmi
N. Anterior segment biometry using spectral-domain optical coherence tomography. J Refract Surg 2014;30(5):354-360. [CrossRef] [PubMed]
14 Szalai E, Berta A, Hassan Z, Modis L Jr. Reliability and
repeatability of swept-source Fourier-domain optical coherence tomography and
Scheimpflug imaging in keratoconus. J
Cataract Refract Surg 2012;38(3):485-494. [CrossRef] [PubMed]
15 Prata TS, Ushida M, Dorairaj S. Cataract surgery alone cannot be
considered an IOP-lowering procedure for open-angle glaucoma patients. Arq Bras Oftalmol 2015;78(5):V-VI. [CrossRef] [PubMed]
16 Melancia D, Abeqao Pinto L, Marques-Neves C. Cataract surgery and
intraocular pressure. Ophthalmic Res
2015;53(3):141-148. [CrossRef] [PubMed]
17 Jamil AZ, Iqbal K, Ur Rahman F, Mirza KA. Effect of phacoemulsification
on intraocular pressure. J Coll
Physicians Surg Pak 2011;21(6):347-350. [PubMed]
18 Eid TM. Primary lens extraction for glaucoma management: a review
article. Saudi J Ophthalmol
2011;25(4):337-345. [CrossRef] [PMC free article] [PubMed]
19 Latifi G, Moghimi S, Eslami Y, Fakhraie G, Zarei R, Lin S. Effect of
phacoemulsification on drainage angle status in angle closure eyes with or
without extensive peripheral anterior synechiae. Eur J Ophthalmol 2013;
23(1):70-79. [PubMed]
20 Agarwal P, Daher AM, Agarwal R. Aqueous humor TGF-beta2 levels in
patients with open-angle glaucoma: a meta-analysis. Mol Vis 2015;21: 612-620. [PMC free article] [PubMed]
21 Diestelhorst M, Krieglstein GK. Effect of cataract extraction on
aqueous humor dynamics in patients with senile cataract. A prospective
fluorophotometric study. Fortschr
Ophthalmol 1991;88(2):128-131. [PubMed]
22 Mansouri M, Ramezani F, Moghimi S, Tabatabaie A, Abdi F, He M, Lin
SC. Anterior segment optical coherence tomography parameters in phacomorphic angle
closure and mature cataracts. Invest
Ophthalmol Vis Sci 2014;55(11):7403-7409. [CrossRef] [PubMed]
23 Pinazo-Duran MD, Gallego-Pinazo R, Garcia-Medina JJ, Zanon-Moreno V,
Nucci C, Dolz-Marco R, Martinez-Castillo S, Galbis-Estrada C, Marco-Ramirez C,
Lopez-Galvez MI, Galarreta DJ, Diaz-Llopis M. Oxidative stress and its
downstream signaling in aging eyes. Clin
Interv Aging 2014;9:637-652. [CrossRef] [PMC free article] [PubMed]
24 Samanta A, Kumar P, Machhua S, Rao GN, Pal A. Incidence of cystoid
macular oedema in diabetic patients after phacoemulsification and free radicals
link to its pathogenesis. Br J Ophthalmol
2014;98(9):1266-1272. [CrossRef] [PubMed]
25 Zoric L, Elek-Vlajic S, Jovanovic M, Kisic B, Djokic O, Canadanovic
V, Cosic V, Jaksic V. Oxidative stress intensity in lens and aqueous depending
on age-related cataract type and brunescense.
Eur J Ophthalmol 2008;18(5): 669-674. [PubMed]
26 Tran VT. Washout of pseudoexfoliation material combined with cataract
surgery: a new surgical approach to lower intraocular pressure in
pseudoexfoliationsyndrome. Int Ophthalmol
2015;35(2):209-214. [CrossRef] [PMC free article] [PubMed]
27 Steinhart MR, Cone-Kimball E, Nguyen C, Nguyen TD, Pease ME,
Chakravarti S, Oqlesby EN, Quigley HA. Susceptibility to glaucoma damage
related to age and connective tissue mutations in mice. Exp Eye Res 2014;119:54-60. [CrossRef] [PMC free article] [PubMed]
28 Weinreb RN, Kashiwagi K, Kashiwagi F, Tsukahara S, Lindsey JD.
Prostaglandins increase matrix metalloproteinase release from human ciliary
smooth muscle cells. Invest Ophthalmol
Vis Sci 1997;38(13): 2772-2780. [PubMed]
29 Koc M, Ozulken K, Ayar O, Karakurt A. Measurement of the anterior
chamber angle according to quadrants and age groups using Pentacam Scheimpflug
camera. J Glaucoma
2013;22(3):226-229. [CrossRef] [PubMed]