
·Meta-Analysis· Current
Issue IF in JCR CiteScore ·Submission· In Press Recent Accepted PMC RSS
Citation: Han LH, Yuan LF, Liang X, Jia X, Zhang ML.
Combined therapy versus anti-vascular endothelial growth factor monotherapy for
polypoidal choroidal vasculopathy: a Meta-analysis. Int J Ophthalmol
2017;10(8):1280-1289
Combined therapy versus anti-vascular endothelial
growth factor monotherapy for polypoidal choroidal vasculopathy: a
Meta-analysis
Long-Hui Han1, Li-Fei Yuan1,
Xu Liang2, Xin Jia1, Ming-Lian Zhang1
1Hebei Provincial Eye Institute, Hebei Provincial Eye Hospital, Xingtai
054001, Hebei Province, China
2Tianjin Eye Hospital, Tianjin 300020, China
Co-first authors: Long-Hui Han and Li-Fei Yuan
Correspondence to: Long-Hui Han. Hebei Provincial Eye
Institute, Hebei Provincial Eye Hospital, #399 East Quanbei Street, Xingtai
054001, Hebei Province, China. han-longhui@ 163.com
Received:
2016-11-08
Accepted: 2017-01-22
Abstract
AIM: To
evaluate the efficacy and safety of anti-vascular endothelial growth factor
(VEGF) combined with photodynamic therapy (PDT) versus anti-VEGF monotherapy
for polypoidal choroidal vasculopathy (PCV).
METHODS: We
conducted a Meta-analysis of 9 studies to compare the efficacy and safety
between combined therapy and anti-VEGF monotherapy for PCV. The programs of RevMan
5.3 and Stata 12.0 were used to analyze data.
RESULTS: The
best corrected visual acuity (BCVA) in combined therapy group were
significantly better than those of anti-VEGF monotherapy group at 6, 24 and
36mo, with pooled weighted means differences (WMDs) of 0.12 (0.06, 0.18), 0.25
(0.12, 0.38) and 0.28 (0.13, 0.43), respectively. The central retinal thickness
(CRT) reductions in combined therapy group were higher than that in anti-VEGF
monotherapy group at 1, 3, 6 and 9mo, with pooled WMDs of 63.90 (20.41,
107.38), 33.47 (4.69, 62.24), 30.57 (0.12, 60.01) and 28.00 (2.51, 53.49),
respectively. The regression rate of polyps in combined therapy group was much
higher than that in anti-VEGF monotherapy group [RD: 0.47 (0.26, 0.68); P<0.0001].
The adverse event retinal hemorrhage did not differ significantly between the
two groups.
CONCLUSION:
Our findings clearly document that anti-VEGF combined with PDT is a more
effective therapy for PCV compared with anti-VEGF monotherapy. Furthermore,
combined therapy does not increase the incidence of retinal hemorrhage.
KEYWORDS:
vascular endothelial growth factor; photodynamic therapy;
polypoidal choroidal vasculopathy
DOI:10.18240/ijo.2017.08.16
Citation: Han LH, Yuan LF, Liang X, Jia X, Zhang ML. Combined therapy versus
anti-vascular endothelial growth factor monotherapy for polypoidal choroidal
vasculopathy: a Meta-analysis. Int J Ophthalmol 2017;10(8):1280-1289
INTRODUCTION
Polypoidal
choroidal vasculopathy (PCV) is one of the common sight-threatening eye
diseases characterized by polypoidal and aneurysmal dilatations at the
terminals of the branching network in the inner choroid[1-3]. It results in severe visual loss in some patients
secondary to recurrent serosanguinous detachment of retinal pigment epithelium
or occasional massive submacular hemorrhage[4].
Although several treatment modalities for PCV are available currently, more
reliable evidences are still needed for ophthalmologists to make the best
choice.
Anti-vascular
endothelial growth factor (VEGF) therapy is a treatment modality that is being
investigated in PCV. The increased expression of VEGF in the eyes with PCV
provides a biologic rationale for the treatment with anti-VEGF agents[5-6]. Relevant studies demonstrated a
rapid resolution of exudative fluid from polypoidal lesions and subsequent
rapid visual recovery after anti-VEGF therapy[7-9]. Due to its rapid effects, simple operation and low
risk, anti-VEGF monotherapy is easy to achieve the patient’s satisfaction, so
it’s wildly used by many clinicians in the treatment of PCV. However, despite
the visual improvement, anti-VEGF monotherapy showed a limited effect on polyp
regression[10].
Photodynamic
therapy (PDT) has been widely used in the treatment of PCV, as various studies
have shown that it can result in regression of polyps and visual improvements[11-13]. However, evidence suggests
that PDT is only an efficient treatment in a short term[2,12-14]. Moreover, the visual
threatening hemorrhagic complications after PDT have been reported in up to 30%
of eyes, and repeated PDT induced choroidal ischemia, which can lead to the
increase of VEGF expression[5-6,12-16].
Therefore,
combining anti-VEGF with its anti-angiogenic and anti-permeability effects and
PDT with its angio-occlusive effects may lead to synergistic effects in PCV
treatment. To date, several studies comparing combined therapy (anti-VEGF
combined with PDT) with anti-VEGF monotherapy have been conducted[15,17-24]. However,
they only included a small sample size and no definitive conclusions have been
reached yet. Therefore, we performed a Meta-analysis of the available published
literature to compare the outcomes of combined therapy and anti-VEGF
monotherapy.
MATERIALS AND METHODS
This
Meta-analysis was reported in accordance with Cochrane Handbook for Systematic
Reviews of Interventions and the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) statement[25].
All stages of literature search, study selection, data extraction, and quality
assessment were performed independently by two reviewers (Han LH and Yuan LF).
And all disagreements were resolved by discussion until a consensus was
reached.
Literature
Search A systematic
search of the Cochrane Library, PubMed and Embase via Ovid database
system was performed to identify relevant studies. The following terms, adapted
for Ovid database, were used for the searches “polypoidal choroidal
vasculopathy” OR “PCV” AND “endothelial growth factor” OR “VEGF” OR
“angiogenesis inhibitor” OR “Lucentis” OR “Ranibizumab” OR “Bevacizumab” OR
“Avastin” OR “Pegaptanib” OR “Macugen” OR “Conbercept” OR “Aflibercept” OR
“Eylea” AND “photodynamic therapy” OR “PDT”. The “Include Related Terms”
function in Ovid database was also used to broaden the search, and the websites
of professional associations and Google Scholar were also searched for
additional information. The computer search was supplemented with manual
searches of the reference lists of all relevant studies, review articles and conference
abstracts. The final search was carried out in May 2016 and was updated on
January 6, 2017, without restrictions regarding publication year, language, or
methodological filter.
Inclusion
and Exclusion Criteria All
available randomized controlled trials (RCTs) and non-randomized comparative
studies (NRSs) that compared combined therapy (anti-VEGF combined with PDT)
with anti-VEGF monotherapy, and that had at least one of the quantitative
outcomes mentioned in the next section of this paper, were included. Reviews,
case reports, comments, editorials, letters, and registered protocols were
excluded.
Data
Extraction The
following information was extracted from each study: first author; year of
publication; study design; inclusion and exclusion criteria; location of the
trial; follow up; number of patients in each group; baseline patient
characteristics; and outcomes of interest. The numbers of withdrawal and
patients reporting adverse events were also recorded.
Outcome
Measures The
following outcomes were used to compare combined therapy with anti-VEGF
monotherapy: 1) visual outcomes: mean best corrected visual acuity (BCVA)
change at months 1, 3, 6, 9, 12, 24 and 36; 2) anatomical outcomes: mean change
in central retinal thickness (CRT) at months 1, 3, 6, 9, 12 and 24; regression
rate of polyps at month 3; 3) adverse events: incidence of retinal hemorrhage.
Quality
Assessment The
methodological quality of studies was assessed using a previously reported
quality assessment system for both randomized and non-randomized studies[26]. The system includes 27 items distributed to five
subscales: reporting (10 items), external validity (3 items), internal
validity-bias (7 items), internal validity-confounding (selection bias) (6
items), and power (1 item). And the total score for each study was presented as
a percentage of the maximum achievable score. The scores not lower than 50% are
considered to be of high quality.
Statistical
Analysis Data from
this Meta-analysis are presented in accordance with PRISMA guidelines. All
Meta-analyses and sensitivity analyses were performed using RevMan (version
5.3), and publication bias analyses were performed using Stata (version 12.0;
StataCorp, College Station, TX, USA). Weighted mean difference (WMD) and risk
difference (RD) were used to compare continuous and dichotomous variables,
respectively. And the outcomes were reported with 95% confidence interval (CI).
The
heterogeneity among the studies was accessed using a chi-square test with the
significance set at P<0.10. The percentage of heterogeneity was
evaluated using the I2 statistic, ranging from 0 to 100%. If
there was a statistical heterogeneity between studies (P<0.10, I2>50%),
a random-effect model was used to combine data. Otherwise, a fixed-effect model
was used (P>0.10, I2<50%).
Subgroup
analysis was performed according to type of study design (RCT or NRS).
Sensitivity analysis was performed by iteratively excluding each study and
recalculating the combined estimate based on the remaining studies, and only
outcomes that were reported in no less than four studies were included in
sensitivity analysis[2]. The potential publication
bias was evaluated with Begg’s and Egger’s tests using Stata software.
The
data are presented as mean±standard deviation (SD) or mean±95% CI. The
unavailable SD values were estimated according to Cochrane Handbook 5.3.5
(chapter 16.1.2). A P<0.05 was considered to be statistically
significant, except where otherwise specified.
RESULTS
Characteristics
of Included Studies Nine studies
including two RCTs[17-18] and
seven NRSs[15,19-24]
were included in the final analysis (Figure 1). The characteristics of the
included studies are shown in Table 1. A total of 317 cases (153 cases of
combined therapy and 164 cases of anti-VEGF monotherapy) were enrolled. PCV was
confirmed by indocyan-nine green angiograph (ICGA). ICGA and OCT were used in
the same way in all included studies. Characteristics of lesions and treatment
exposures included in the Meta-analysis are shown in Table 2. The quality assessment
is summarized in Table 3. All of the studies scored over 50% and were
considered to be of high quality.
Figure
1 Flow diagram of included studies for this Meta-analysis.
Table
1 Characteristics of studies included in this Meta-analysis
|
Studies (first
author, year) |
Design |
Center |
Location |
Follow-up (mo) |
No. of
eyes combined/anti- VEGF |
Mean age
(a) combined/anti- VEGF |
Sex (M/F)
combined /anti-VEGF |
|
Koh A,
2012 |
RCT |
7 |
Hong Kong,
Singapore, Korea, Taiwan, Thailand |
6 |
19/21 |
63.8±8.30/69.3±8.3 |
(11/8)/(15/6) |
|
Lim JY,
2012 |
RCT |
1 |
Korea |
12 |
5 / 5 |
57.8±7.9/68.6±7.2 |
(3/2)/(5/0) |
|
Sakurai M,
2014 |
NRS |
1 |
Japan |
12 |
17 / 30 |
74.8±5.8/73.9±8.1 |
(13/4)/(20/10) |
|
Lai TY,
2011 |
NRS |
1 |
Hong Kong |
12 |
16 / 7 |
71.3±9.8/64.6±7.9 |
(8/8)/(4/3) |
|
Kang HM,
2014 |
NRS |
1 |
Korea |
24 |
20 / 23 |
70.0±7.6/68.1±8.1 |
(NA)/(NA) |
|
Song MH,
2011 |
NRS |
1 |
Korea |
12 |
9 / 15 |
56.9±12.1/60.6±10.7 |
(0/9)/(6/9) |
|
Rouvas AA,
2011 |
NRS |
2 |
Greece |
12 |
9 / 10 |
64.67±NA/66.5±NA |
(4/5)/(4/6) |
|
Kikushima
W, 2016 |
NRS |
1 |
Japan |
12 |
33 / 33 |
73.4±8.3/72.7±8.5 |
(22/11)/(25/8) |
|
Sakai T,
2016 |
NRS |
1 |
Japan |
36 |
25 / 20 |
72.6±6.2/75.3±8.1 |
(21/4)/(13/7) |
RCT:
Randomized controlled trial; NRS: Non-randomized comparative study; PDT:
Photodynamic therapy; RF-PDT: Reduced-fluence photodynamic therapy; M/F:
Male/female; NA: Not available. Combined group: Eyes treated with intravitreal
anti-VEGF agents combined with PDT or RF-PDT; Anti-VEGF group: Eyes treated
with intravitreal anti-VEGF agents only. The data are shown as mean±standard
deviation (SD) or mean.
Table
2 Characteristics of lesions and treatment exposures included in this
Meta-analysis
|
Studies
(first author, year) |
Lesion GLD
(mm) |
Interventions |
No. of
treatments |
|||
|
Combined |
Anti-VEGF |
Combined |
Anti-VEGF |
Combined |
Anti-VEGF |
|
|
Koh A,
2012 |
NA |
NA |
PDT+IVR
0.5 mg (1-24h
after PDT) |
IVR 0.5
mg+ sham PDT |
1.4±0.5
PDT, 5.0±2.6
IVR |
7.4±2.4
IVR |
|
Lim JY,
2012 |
NA |
NA |
IVB 1.25
mg+PDT within 7d
before or after IVB) |
IVB 1.25
mg |
3.6±0.89
IVB, 1 PDT |
3.0±0 IVB |
|
Sakurai M,
2014 |
2576±1002 |
1474±909 |
IVR 0.5
mg+RF-PDT (1-24h
after IVR) |
IVR 0.5 mg |
3.4 IVR, 1
RF-PDT |
4.3 IVR |
|
Lai TY,
2011 |
3490±1170 |
3610±2240 |
PDT+IVR
0.5 mg (30min
after PDT) |
IVR 0.5 mg |
1.2 PDT,
3.4 IVR |
0.6 PDT,
4.0 IVR |
|
Kang HM,
2014 |
2815±910 |
2790±872 |
PDT+IVB
0.5 mg (the same
day as the PDT) |
IVR 0.5 mg
or IVB 1.25
mg |
1.33±0.17
PDT, 11.00±1.46 IVB |
10.12±1.46
IVR/IVB |
|
Song MH,
2011 |
NA |
NA |
PDT+IVR
0.5 mg (within 3d
after PDT) |
IVR 0.5 mg |
1 PDT,
4.33±2.78 IVR |
4.47±2.10
IVR |
|
Rouvas AA,
2011 |
NA |
NA |
IVR 0.5
mg+PDT (7±2d
after IVR) |
IVR 0.5 mg |
1.67 PDT,
5.0 IVR |
6.9 IVR |
|
Kikushima
W, 2016 |
1692±747 |
2041±1273 |
IVA 2
mg+PDT (15min
after the start of the injection ) |
IVA 2 mg |
3.42±0.94
IVA, 1 PDT |
4.6±1.6
IVA |
|
Sakai T,
2016 |
2800±823 |
2937±1040 |
IVR 0.5
mg+PDT (1 or 2d
after IVR) |
IVR 0.5 mg |
5.08±2.45
IVR, 1.32 PDT |
7.65±2.74
IVR, 0.3 PDT |
GLD:
Greatest linear dimension; PDT: Photodynamic therapy (6 mg/m2, 50
J/cm2, 600 mW/cm2, 83s); RF-PDT: Reduced-fluence
photodynamic therapy (6 mg/m2, 50 J/cm2, 42s); IVR:
Intravitreal ranibizumab; IVB: Intravitreal bevacizumab; IVA: Intravitreal
aflibercept; NA: Not available. Combined group: Eyes treated with intravitreal
anti-VEGF agents combined with PDT or RF-PDT; Anti-VEGF group: Eyes treated
with intravitreal anti-VEGF agents only. The data are shown as mean±standard
deviation (SD) or mean.
Table
3 Quality assessment for studies included in this Meta-analysis
|
Studies
(first author, year) |
Quality
score components |
Scores |
|||||
|
I |
II |
III |
IV |
V |
Total |
Percentage |
|
|
Koh A,
2012 |
11 |
3 |
6 |
3 |
0 |
23 |
71.88% |
|
Lim JY,
2012 |
11 |
1 |
5 |
4 |
0 |
21 |
65.63% |
|
Sakurai M,
2014 |
10 |
1 |
5 |
2 |
1 |
19 |
59.38% |
|
Lai TY,
2011 |
10 |
1 |
5 |
2 |
0 |
18 |
56.25% |
|
Kang HM,
2014 |
9 |
1 |
5 |
2 |
1 |
18 |
56.25% |
|
Song MH,
2011 |
10 |
1 |
5 |
2 |
0 |
18 |
56.25% |
|
Rouvas AA,
2011 |
9 |
1 |
5 |
2 |
0 |
17 |
53.13% |
|
Kikushima
W, 2016 |
9 |
1 |
5 |
2 |
1 |
18 |
56.25% |
|
Sakai T,
2016 |
10 |
1 |
5 |
2 |
1 |
19 |
59.38% |
I:
Reporting; II: External validity; III: Internal validity-bias; IV: Internal
validity-confounding (selection bias); V: Power.
Visual
Outcomes BCVA was one
of the most important criterion for evaluating efficacy. The pooled WMDs (with
95% CIs) of logMAR BCVA improvements from the baseline and the comparisons
between the two groups (combined therapy group vs anti-VEGF monotherapy
group) by Meta-analysis are presented in Table 4 and Figure 2.
Table
4 Comparisons of logMAR BCVA by Meta-analysis
|
Outcomes
of interest |
No. of
studies |
WMD (95%
CI) |
Heterogeneity |
Z |
P |
||
|
Chi2 |
P |
I2 |
|||||
|
Mean
logMAR improvement in combined therapy group (follow-up vs baseline) |
|||||||
|
Month 1 |
4 |
0.07
(-0.04, 0.18) |
3.26 |
0.35 |
8% |
1.32 |
0.19 |
|
Month 3 |
7 |
0.19
(0.12, 0.26) |
6.92 |
0.33 |
13% |
5.62 |
<0.00001 |
|
Month 6 |
7 |
0.23
(0.17, 0.29) |
4.66 |
0.59 |
0 |
7.05 |
<0.00001 |
|
Month 9 |
4 |
0.24
(0.16, 0.33) |
3.39 |
0.34 |
11% |
5.55 |
<0.00001 |
|
Month 12 |
8 |
0.24
(0.17, 0.30) |
5.49 |
0.60 |
0 |
6.79 |
<0.00001 |
|
Month 24 |
2 |
0.22
(0.09, 0.34) |
0.13 |
0.72 |
0 |
3.32 |
0.0009 |
|
Month 36 |
1 |
0.21
(0.06, 0.36) |
NA |
NA |
NA |
2.82 |
0.005 |
|
Mean
logMAR improvement in anti-VEGF monotherapy group (follow-up vs
baseline) |
|||||||
|
Month 1 |
4 |
0.05
(-0.04, 0.14) |
2.22 |
0.53 |
0 |
1.12 |
0.26 |
|
Month 3 |
7 |
0.11
(0.03, 0.19) |
2.32 |
0.77 |
0 |
2.79 |
0.005 |
|
Month 6 |
7 |
0.10
(0.02, 0.19) |
3.16 |
0.79 |
0 |
2.51 |
0.01 |
|
Month 9 |
4 |
0.13
(0.03, 0.23) |
2.03 |
0.57 |
0 |
2.52 |
0.01 |
|
Month 12 |
8 |
0.10
(0.02, 0.18) |
8.86 |
0.26 |
21% |
2.34 |
0.02 |
|
Month 24 |
2 |
-0.04
(-0.21 0.12) |
0.05 |
0.82 |
0 |
0.52 |
0.60 |
|
Month 36 |
1 |
-0.07
(-0.29, 0.15) |
NA |
NA |
NA |
0.63 |
0.53 |
|
Comparisons
of logMAR improvement between the two groups (combined therapy group vs
anti-VEGF monotherapy group) |
|||||||
|
Month 1 |
4 |
0.01
(-0.07, 0.10) |
8.35 |
0.04 |
64% |
0.25 |
0.80 |
|
Month 3 |
7 |
0.08
(-0.00, 0.17) |
23.55 |
0.0006 |
75% |
1.86 |
0.06 |
|
Month 6 |
7 |
0.12
(0.06, 0.18) |
7.58 |
0.27 |
21% |
3.89 |
<0.0001 |
|
Month 9 |
4 |
0.09
(-0.01, 0.19) |
0.23 |
0.97 |
0 |
1.78 |
0.07 |
|
Month 12 |
8 |
0.10
(-0.01, 0.22) |
20.16 |
0.005 |
65% |
1.76 |
0.08 |
|
Month 24 |
2 |
0.25
(0.12, 0.38) |
0.35 |
0.55 |
0 |
3.81 |
0.0001 |
|
Month 36 |
1 |
0.28
(0.13, 0.43) |
NA |
NA |
NA |
3.57 |
0.0004 |
BCVA:
Best corrected visual acuity; WMD: Weighted mean difference; CI: Confidence
interval; Combined therapy: Intravitreal anti-VEGF agents plus PDT; PDT: Photodynamic
therapy.
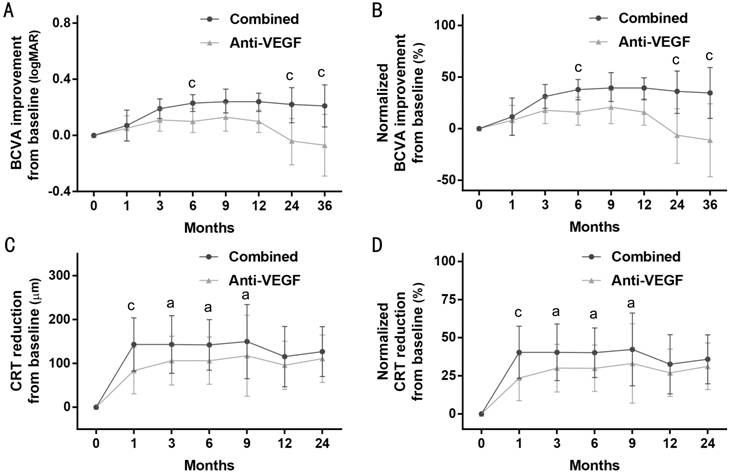
Figure
2 LogMAR BCVA improvement and CRT
reduction from baseline A: LogMAR BCVA improvement from baseline; B:
Normalized logMAR BCVA improvement from baseline; C: CRT reduction from
baseline; D: Normalized CRT reduction from baseline. Outcomes are presented as
WMD with 95% CI. Comparisons between the two groups (combined therapy group vs
anti-VEGF monotherapy group) by Meta-analysis: aP<0.05, cP<0.001.
In
combined therapy group, the mean BCVA improved continuously from month 3 to 36
compared with baseline BCVA. The pooled WMDs at 3, 6, 9, 12, 24 and 36mo were
0.19 (0.12, 0.26), 0.23 (0.17, 0.29), 0.24 (0.16, 0.33), 0.24 (0.17, 0.30),
0.22 (0.09, 0.34) and 0.21 (0.06, 0.36), respectively. In anti-VEGF monotherapy
group, the mean BCVA only improved at month 3, 6, 9 and 12 after initial
treatment, with pooled WMDs of 0.11 (0.03, 0.19), 0.10 (0.02, 0.19), 0.13
(0.03, 0.23) and 0.10 (0.02, 0.18), respectively. Furthermore, it deteriorated
at month 24 and month 36. There was no evidence of heterogeneity across the
above trials.
Comparisons
between the two groups showed that the treatment effects in combined therapy
group were significantly better than those of anti-VEGF monotherapy group at
month 6, 24 and 36, with pooled WMDs of 0.12 (0.06, 0.18), 0.25 (0.12, 0.38)
and 0.28 (0.13, 0.43), respectively. No significant difference was found at
other months. There were significant heterogeneities at month 1, 3 and 12, so
the random-effect models were used to combine data.
After
being normalized to the baseline before treatment, logMAR BCVA increased by
8.0%-39.4% in combined treatment group in 36mo, but, in anti-VEGF monotherapy
group, it only showed 7.3%-20.9% increase from month 1 to 12, and even a 6.4%
decrease at month 24 and a 11.2% decrease at month 36 (Figure 2B).
Anatomical
Outcomes The pooled
WMDs of CRT reductions from the baseline and the comparisons between the two
groups by Meta-analysis are presented in Table 5 and Figure 2C. In both groups,
the CRT reductions from the baseline are statistically significant during the
36 months’ follow-up. But the CRT reductions in the combined therapy group were
higher than that in the anti-VEGF monotherapy group in early stages, and the
differences were statistically significant at month 1, 3, 6 and 9, with pooled
WMDs of 63.90 (20.41, 107.38), 33.47 (4.69, 62.24), 30.57 (0.12, 60.01) and
28.00 (2.51, 53.49), respectively.
After
being normalized to the baseline before treatment, CRT reduced by 40.1%-42.3%
in combined treatment group at month 1, 3, 6 and 9, but it only showed
23.5.2%-29.9% reduction in anti-VEGF monotherapy group at those time points.
The differences of CRT reduction between the two groups at month 12 and 24 were
not significant (Figure 2B).
Four
studies reported the data for regression rate of polyps at month 3. Analysis of
these data showed that the regression rate in combined therapy group was much
higher than that in anti-VEGF monotherapy group [RD: 0.47 (0.26, 0.68); P<0.0001](Table
5; Figure 3).
Table
5 Comparisons of anatomical outcomes and dverse event by Meta-analysis
|
Outcomes
of interest |
No. of
studies |
WMD or RD
(95% CI) |
Heterogeneity |
Z |
P |
||
|
Chi2 |
P |
I2 |
|||||
|
Anatomical
outcomes |
|||||||
|
CRT
reduction |
|||||||
|
Mean CRT
reduction in combined therapy group (follow-up vs baseline) |
|||||||
|
Month 1 |
4 |
143.07
(82.44, 203.70) |
10.15 |
0.02 |
70% |
4.63 |
0.00001 |
|
Month 3 |
6 |
143.13
(77.38, 208.87) |
51.88 |
<0.00001 |
90% |
4.27 |
0.0001 |
|
Month 6 |
6 |
142.18
(84.52, 199.83) |
42.14 |
<0.00001 |
88% |
4.83 |
<0.00001 |
|
Month 9 |
4 |
149.72
(65.13, 234.31) |
39.11 |
<0.0001 |
92% |
3.47 |
0.0005 |
|
Month 12 |
6 |
115.46
(46.71, 184.22) |
48.49 |
<0.00001 |
90% |
3.29 |
0.001 |
|
Month 24 |
1 |
126.96
(70.08, 183.84) |
NA |
NA |
NA |
4.37 |
<0.0001 |
|
Mean CRT
reduction in anti-VEGF monotherapy group (follow-up vs baseline) |
|||||||
|
Month 1 |
4 |
83.43
(30.87, 135.99) |
12.12 |
0.007 |
75% |
3.11 |
0.002 |
|
Month 3 |
6 |
106.33
(50.94, 161.71) |
23.83 |
0.0002 |
79% |
3.76 |
0.0002 |
|
Month 6 |
6 |
106.19
(52.37, 160.00) |
23.94 |
0.0002 |
79% |
3.87 |
0.0001 |
|
Month 9 |
4 |
117.41
(25.08, 209.73) |
25.62 |
0.0001 |
88% |
2.49 |
0.01 |
|
Month 12 |
6 |
95.71
(40.89, 150.53) |
29.99 |
0.0001 |
83% |
3.42 |
0.0006 |
|
Month 24 |
1 |
110.68
(56.39, 164.97) |
NA |
NA |
NA |
4.00 |
<0.0001 |
|
Comparisons
of CRT reduction between the two groups (combined therapy group vs
anti-VEGF monotherapy group) |
|||||||
|
Month 1 |
4 |
63.90
(20.41, 107.38) |
7.23 |
0.06 |
58% |
2.88 |
<0.004 |
|
Month 3 |
6 |
33.47
(4.69, 62.24) |
7.66 |
0.18 |
35% |
2.28 |
0.02 |
|
Month 6 |
6 |
30.57
(0.12, 60.01) |
5.57 |
0.35 |
10% |
1.97 |
<0.05 |
|
Month 9 |
4 |
28.00
(2.51, 53.49) |
4.24 |
0.24 |
29% |
2.15 |
0.03 |
|
Month 12 |
6 |
11.90
(-23.39, 47.19) |
5.63 |
0.34 |
11% |
0.66 |
0.51 |
|
Month 24 |
1 |
16.28
(-44.35, 76.91) |
NA |
NA |
NA |
0.53 |
0.60 |
|
Regression
of polyps (combined therapy group vs anti-VEGF monotherapy group) |
|||||||
|
Month 3 |
4 |
0.47
(0.26, 0.68) |
7.77 |
0.05 |
61% |
4.40 |
<0.0001 |
|
Incidence
of adverse event (combined therapy group vs anti-VEGF monotherapy
group) |
|||||||
|
Retinal
hemorrhage |
6 |
0.01
(-0.05, 0.07) |
2.42 |
0.79 |
0 |
0.25 |
0.80 |
CRT:
Central retinal thickness; WMD: Weighted mean difference; RD: Risk difference;
CI: Confidence interval; Combined: Intravitreal anti-VEGF inhibitors plus PDT;
PDT: Photodynamic therapy.
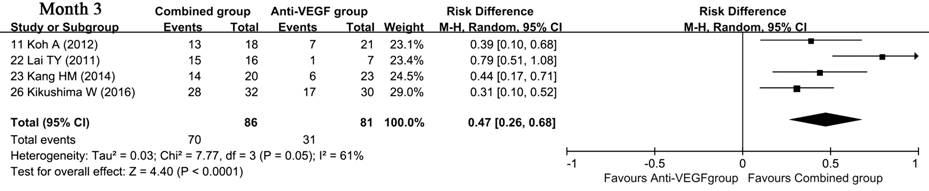
Figure
3 Forest plot displaying the pooled estimate of regression rate of polys Combined
therapy group vs anti-VEGF monotherapy group.
Adverse
Events Retinal
hemorrhage was the most common complication associated PCV treatment. Six
studies including 218 patients reported the frequency of retinal hemorrhage,
and the pooled data showed no significant difference between the two groups
[RD: 0.01 (-0.05, 0.07); P=0.80] (Table 5; Figure 4).
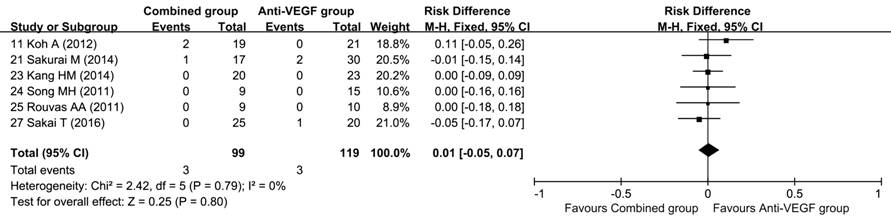
Figure
4 Forest plot displaying the pooled estimate of retinal hemorrhage Combined therapy group vs
anti-VEGF monotherapy group.
Subgroup
Analysis, Sensitivity Analysis and Publication Bias There was no
statistically significant difference in all available subgroup analyses except
the comparison at month 3 and 6. The results of sensitivity analyses showed
that 76.3% (29/38) of the Meta-analysis results were stable, and 23.7% (9/38)
of the results were not stable and the patterns of difference were changed when
a certain study was excluded (Table 6).
Table
6 Results of sensitivity analyses
|
Outcomes
of interest |
A certain
exclued study |
Original
significance |
Significance
after a certain study was exclued |
|
Mean
logMAR improvement in anti-VEGF monotherapy group (follow up vs
baseline) |
|||
|
Month 6 |
[26] |
S |
NS |
|
Month 9 |
[26] |
S |
NS |
|
Month 12 |
S |
NS |
|
|
Comparisons
of logMAR improvement between the two groups (combined therapy group vs anti-VEGF
monotherapy group) |
|||
|
Month 3 |
NS |
S |
|
|
Month 12 |
[22] |
NS |
S |
|
Mean CRT
reduction in anti-VEGF monotherapy group (follow up vs baseline) |
|||
|
Month 9 |
[24] |
S |
NS |
|
Comparisons
of CRT reduction between the two groups (combined therapy group vs
anti-VEGF monotherapy group) |
|||
|
Month 3 |
S |
NS |
|
|
Month 6 |
S |
NS |
|
|
Month 9 |
S |
NS |
|
Combined:
Intravitreal anti-VEGF inhibitors plus PDT; PDT: Photodynamic therapy; S: With
significance; NS: No significance.
We
only tried to evaluate the publication bias of the comparisons between the two
groups when the number of studies is no less than four. Begg’s tests (P>0.05)
and Egger’s tests (P>0.05) showed no evidence of publication bias.
DISCUSSION
This
Meta-analysis of two RCTs and five non-randomized comparative studies including
317 cases, showed that combined therapy (anti-VEGF combined with PDT) was
superior to anti-VEGF monotherapy in terms of visual and anatomical outcomes.
No significant difference was found in retinal hemorrhagic complication between
the two groups. Thus, the combined treatment seems to be a rational approach
for PCV.
Treatment
strategies for PCV include thermal laser photocoagulation, verteporfin PDT,
anti-VEGF therapies, and combination of these[27].
Although several treatment modalities for PCV are available currently and
several relevant studies with small samples were conducted, more reliable
evidences are still needed for ophthalmologists to make the best choice.
Recently,
several Meta-analyses, comparing these treatment modalities for PCV, were
publish and some consensuses were reached. Two Meta-analyses, comparing
combined therapy with PDT monotherapy, confirmed that combined therapy resulted
in better visual acuity[2,28].
But, three Meta-analyses, comparing anti-VEGF with PDT, got conflicting
conclusions[28-30]. Tang et
al[28] and Yong et al’s[29] results showed that anti-VEGF and PDT appeared to be
comparable in terms of visual acuity improvement. On the contrary, Liu et al’s[30] Meta-analysis suggested that anti-VEGF (intravitreal
ranibizumab) had better effect on the improvement of visual acuity in PCV.
Furthermore, none of the Meta-analyses compared the efficacy between combined
therapy and anti-VEGF monotherapy. Therefore, we performed this Meta-analysis
of the available literature to compare the outcomes of combined therapy with
anti-VEGF monotherapy.
BCVA
is one of the most important criterions for evaluating the efficacy on PCV. Our
results showed that the mean BCVA in combined therapy group improved
continuously from month 3 to 36 compared with the baseline BCVA. However, the
mean BCVA in anti-VEGF monotherapy group just improved from month 3 to 12 after
initial treatment and deteriorated from month 24 to 36. These results indicated
that the treatment effects of combined therapy lasted longer than those of
anti-VEGF monotherapy.
Comparisons
between the two groups showed that the treatment effects in combined therapy
group at month 6, 24 and 36 were significantly better than those of anti-VEGF
monotherapy group, and no significant difference was found at other months.
This suggested that combined therapy may be much better than anti-VEGF
monotherapy in early and long-term treatment for PCV.
The
normalized analyses of the two groups showed that logMAR BCVA increased by
8.0%-39.4% in combined treatment group during the 36 months’ follow-up.
However, in anti-VEGF monotherapy group only 7.3%-20.9% increase from month 1
to 12, and even a 6.4% decrease at month 24 and a 11.2% decrease at month 36
were observed. These results showed that the BCVA improved more in combined
therapy group.
Taken
together, the above results showed that the BCVA improvement in combined
therapy group not only lasted longer but also was much better than that in
anti-VEGF monotherapy group.
CRT
is defined as the distance between the internal limiting membrane and the inner
surface of the retinal pigment epithelium at the fovea, and it can be
non-invasively, accurately, rapidly and conveniently measured by OCT, so CRT
has been widely used in evaluating the anatomical changes of PCV. Our results
showed that the CRT reduced from the baseline in both groups during 24 months’
follow-up, but combined treatment had better effects during the first 9 months’
follow-up.
Regression
rate of polyps is another important indicator in evaluating the anatomical
changes of PCV. Our results showed that the regression rate of polyps in
combined treatment group was much higher than that in anti-VEGF monotherapy
group at month 3. This suggested that combined treatment had better effect in
regression of polyps at early stage. Various trials have also shown that
anti-VEGF treatments are effective in improving visual acuity, reducing leakage
and resolving fluids, but ineffective in polyp regression[13-15,17,22,31],
which is consistent with our results.
Retinal
hemorrhage is one of the major sight-threatening problems related to PCV
treatment[15,17,20-21,32-38]. In this
Meta-analysis, our data showed no significant difference between combined
therapy and anti-VEGF monotherapy. Several studies have reported that PDT
usually cause more complications of retinal hemorrhage[35,39]. But a recent Meta-analysis demonstrated that
combined therapy appeared to result in lower rate of retinal hemorrhage
compared with PDT, which is due to the fact that anti-VEGF agents could block
the increased VEGF expression induced by PDT[2].
This may explain why combined therapy did not bring more changes of retinal
hemorrhage than anti-VEGF monotherapy in our study.
Heterogeneity
is often a concern in Meta-analysis. Substantial heterogeneity was observed in
some analyses, especially in the comparison of BCVA improvement between the two
groups, and the comparison of CRT follow-up with the baseline, which is not
surprising and can be partially explained by the following facts: most of the
included studies are non-randomized; various matching criterions were
different; measurements of outcomes were non-standardized; patients were from
different population including Asians and Europeans. Using random-effect models
in pooling the data might reduce the effect of heterogeneity.
To
assess the impact of a certain single study on the estimates, we performed a
sensitivity analysis by iteratively excluding each study to assess stability of
the Meta-analysis results. Our results showed that most of the Meta-analyses
were stable. We also tried to evaluate potential publication bias with Begg’s
and Egger’s tests in comparisons between the two groups when number of studies
is no less than 4, which showed no evidence of publication bias. This showed
that our results have certain reliability.
A
number of strengths can be found in this Meta-analysis. Firstly, to our
knowledge, this is the first Meta-analysis comparing combined therapy with
anti-VEGF monotherapy in treatment of PCV. Secondly, the Meta-analysis was a
direct comparison between combined therapy and anti-VEGF monotherapy, rather
than an indirect comparison. Thirdly, the Meta-analysis had strict inclusion
and exclusion criteria. Fourthly, we strictly followed the guideline of PRISMA
statement and Cochrane Handbook for Systematic Reviews of Interventions,
including literature search, data extraction, and statistical analysis, thereby
making our results more scientific and reliable. Thus, our study might provide
the most up-to-date information in this area.
This
Meta-analysis has some limitations that should be taken into account. Firstly,
most of the included studies were NRSs, which might result in selection bias.
Nonetheless, the major baseline characteristics of the two groups were
comparable, therefore, selection bias was less likely to occur. Secondly,
included studies used ranibizumab, bevacizumab or aflibercept as anti-VEGF
agent, so there might be a difference between the three agents in treating PCV.
However, recent studies have demonstrated that ranibizumab and bevacizumab have similar efficacy in treating
age-related macular degeneration and PCV[40-43], and that ranibizumab and aflibercept have similar
efficacy in BCVA improvement in PCV[44]. Thirdly,
“grey literature” was not included in this study, which might result in
publication bias. Fourthly, substantial heterogeneity was observed in some
analyses. Using random-effects models in pooling data might reduce, but will
not abolish, the effect of heterogeneity. Fifthly, sensitivity analysis showed
that a minority of the Meta-analyses were not stable, which might reduce the
reliability of the results. Sixthly, the longest follow-up duration of included
studies was only 36mo. Also, there were only two studies which had 24-month
follow-up and there was only one study which had 36-month follow-up, which
could result in bias in functional and anatomical outcomes. So more data of
longer duration are needed to determine the efficacy and safety of combined
treatment over long term. Finally, only 9 studies with small sample size were
included in this Meta-analysis, and more large-sample-sized studies are needed
to evaluate the efficacy of the treatments in PCV.
In
conclusion, to our knowledge, this is the first Meta-analysis comparing
combined therapy with anti-VEGF monotherapy for PCV. Our findings clearly
document that anti-VEGF combined with PDT is a more effective therapy for PCV
compared with anti-VEGF monotherapy. Furthermore, combined therapy does not
increase the incidence of retinal hemorrhage.
ACKNOWLEDGEMENTS
Conflicts
of Interest: Han LH, None; Yuan LF, None; Liang X, None;
Jia X, None; Zhang ML, None.
REFERENCES
1 Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA. Polypoidal
choroidal vasculopathy. Surv Ophthalmol
2004;49(1):25-37. [CrossRef] [PubMed]
2 Wang W, He M, Zhang X. Combined intravitreal anti-VEGF and photodynamic
therapy versus photodynamic monotherapy for polypoidal choroidal vasculopathy:
a systematic review and meta-analysis of comparative studies. PLoS One 2014;9(10):e110667. [CrossRef] [PMC free article] [PubMed]
3 Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine
green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995;15(2):100-110. [CrossRef]
4 Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I,
Takahashi K, Matsumura M. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 2002;133(5):639-648. [CrossRef]
5 Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, Lam DS. Aqueous
humor levels of vascular endothelial growth factor and pigment
epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal
neovascularization. Am J Ophthalmol
2006;141(3):456-462. [CrossRef] [PubMed]
6 Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M.
Expression of pigment epithelium derived factor and vascular endothelial growth
factor in choroidal neovascular membranes and polypoidal choroidal
vasculopathy. Br J Ophthalmol
2004;88(6):809-815. [CrossRef] [PMC free article] [PubMed]
7 Song JH, Byeon SH, Lee SC, Koh HJ, Kwon OW. Short-term safety and
efficacy of a single intravitreal bevacizumab injection for the management of
polypoidal choroidal vasculopathy. Ophthalmologica
2009;223(2):85-92. [CrossRef] [PubMed]
8 Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, Kamei M, Tano Y. Efficacy
of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 2008;92(1):70-73. [CrossRef] [PubMed]
9 Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K,
Ohtsuka H, Ariga H. One-year results of three monthly ranibizumab injections
and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese
patients. Am J Ophthalmol
2012;154(1):117-124.e1. [CrossRef] [PubMed]
10 Tsujikawa A, Ooto S, Yamashiro K, Tamura H, Otani A, Yoshimura N.
Treatment of polypoidal choroidal vasculopathy by intravitreal injection of
bevacizumab. Jpn J Ophthalmol
2010;54(4):310-319. [CrossRef] [PubMed]
11 Nowak-Sliwinska P, van den Bergh H, Sickenberg M, Koh AH.
Photodynamic therapy for polypoidal choroidal vasculopathy. Prog Retin Eye Res 2013;37:182-199. [CrossRef] [PubMed]
12 Spaide RF, Donsoff I, Lam DL, Yannuzzi LA, Jampol LM, Slakter J,
Sorenson J, Freund KB. Treatment of polypoidal choroidal vasculopathy with
photodynamic therapy. Retina
2002;22(5):529-535. [CrossRef] [PubMed]
13 Oishi A, Kojima H, Mandai M, Honda S, Matsuoka T, Oh H, Kita M, Nagai
T, Fujihara M, Bessho N, Uenishi M, Kurimoto Y, Negi A. Comparison of the
effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy:
12-month LAPTOP study results. Am J
Ophthalmol 2013;156(4):644-651. [CrossRef] [PubMed]
14 Inoue M, Arakawa A, Yamane S, Kadonosono K. Long-term outcome of
intravitreal ranibizumab treatment, compared with photodynamic therapy, in
patients with polypoidal choroidal vasculopathy. Eye (Lond) 2013;27(9):1013-1020. [CrossRef] [PMC free article] [PubMed]
15 Rouvas AA, Papakostas TD, Ntouraki A, Douvali M, Vergados I, Ladas
ID. Photodynamic therapy, ranibizumab, and ranibizumab with photodynamic
therapy for the treatment of polypoidal choroidal vasculopathy. Retina 2011;31(3):464-474. [CrossRef] [PubMed]
16 Schmidt-Erfurth U, Schlötzer-Schrehard U, Cursiefen C, Michels S,
Beckendorf A, Naumann GO. Influence of photodynamic therapy on expression of
vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment
epithelium-derived factor. Invest
Ophthalmol Vis Sci 2003; 44(10):4473-4480. [CrossRef]
17 Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S,
Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH. EVEREST study: efficacy and
safety of verteporfin photodynamic therapy in combination with ranibizumab or
alone versus ranibizumab monotherapy in patients with symptomatic macular
polypoidal choroidal vasculopathy. Retina
2012;32(8):1453-1464. [CrossRef] [PubMed]
18 Lim JY, Lee SY, Kim JG, Lee JY, Chung H, Yoon YH. Intravitreal
bevacizumab alone versus in combination with photodynamic therapy for the
treatment of neovascular maculopathy in patients aged 50 years or older: 1-year
results of a prospective clinical study. Acta
Ophthalmol 2012;90(1):61-67. [CrossRef] [PubMed]
19 Sakurai M, Baba T, Kitahashi M, Yokouchi H, Kubota-Taniai M, Bikbova
G, Oshitari T, Yamamoto S. One-year results of intravitreal ranibizumab
combined with reduced-fluence photodynamic therapy for polypoidal choroidal
vasculopathy. Clin Ophthalmol 2014;8:235-241.
[CrossRef] [PMC free article] [PubMed]
20 Lai TY, Lee GK, Luk FO, Lam DS. Intravitreal ranibizumab with or
without photodynamic therapy for the treatment of symptomatic polypoidal
choroidal vasculopathy. Retina
2011;31(8):1581-1588. [CrossRef] [PubMed]
21 Kang HM, Koh HJ. Two-year outcome after combination therapy for
polypoidal choroidal vasculopathy: comparison with photodynamic monotherapy and
anti-vascular endothelial growth factor monotherapy. Ophthalmologica 2014;231(2):86-93. [CrossRef] [PubMed]
22 Song MH, Ryu HW, Roh YJ. One-year results of intravitreal ranibizumab
with or without photodynamic therapy for polypoidal choroidal vasculopathy. Ophthalmologica 2011;226(3):119-126. [CrossRef] [PubMed]
24 Sakai T, Okano K, Kohno H, Tsuneoka H. Three-year visual outcomes of
intravitreal ranibizumab with or without photodynamic therapy for polypoidal
choroidal vasculopathy. Acta Ophthalmol
2016;94(8): e765-e771. [CrossRef] [PubMed]
25 Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred
reporting items for systematic reviews and meta-analyses: the PRISMA statement.
PLoS Med 2009;6(7):e1000097. [CrossRef] [PMC free article] [PubMed]
26 Downs SH, Black N. The feasibility of creating a checklist for the
assessment of the methodological quality both of randomised and non-randomised
studies of health care interventions. J
Epidemiol Community Health 1998;52(6):377-384. [CrossRef] [PMC free article] [PubMed]
27 Imamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA. Polypoidal
choroidal vasculopathy: a review. Surv
Ophthalmol 2010;55(6):501-515. [CrossRef] [PubMed]
28 Tang K, Si JK, Guo DD, Cui Y, Du YX, Pan XM, Bi HS. Ranibizumab alone
or in combination with photodynamic therapy vs photodynamic therapy for
polypoidal choroidal vasculopathy: a systematic review and Meta-analysis. Int J Ophthalmol 2015;8(5):1056-1066. [PMC free article] [PubMed]
29 Yong M, Zhou M, Deng G. Photodynamic therapy versus anti-vascular
endothelial growth factor agents for polypoidal choroidal vasculopathy: a meta-analysis.
BMC Ophthalmol 2015;15:82. [CrossRef] [PMC free article] [PubMed]
30 Liu L, Ma J, Duan P, Liu Y, Yin ZQ. Practicability confirmation by
meta-analysis of intravitreal ranibizumab compared to photodynamic therapy to
treat polypoidal choroidal vasculopathy. Mol
Vis 2015;21: 1130-1141. [PMC free article] [PubMed]
31 Lai TY, Chan WM, Liu DT, Luk FO, Lam DS. Intravitreal bevacizumab
(Avastin) with or without photodynamic therapy for the treatment of polypoidal
choroidal vasculopathy. Br J Ophthalmol
2008;92(5):661-666. [CrossRef] [PubMed]
32 Lee MY, Lee WK, Baek J, Kwon OW, Lee JH. Photodynamic therapy versus
combination therapy in polypoidal choroidal vasculopathy: changes of aqueous
vascular endothelial growth factor. Am J
Ophthalmol 2013;156(2):343-348. [CrossRef] [PubMed]
33 Saito M, Iida T, Kano M, Itagaki K. Two-year results of combined
intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal
vasculopathy. Graefes Arch Clin Exp
Ophthalmol 2013;251(9): 2099-2110. [CrossRef] [PubMed]
34 Sakurada Y, Iijima H. Two-year results of photodynamic therapy with
or without intravitreal ranibizumab for polypoidal choroidal vasculopathy. J Ocul Pharmacol Ther
2013;29(9):832-836. [CrossRef] [PubMed]
35 Lee YA, Yang CH, Yang CM, Ho TC, Lin CP, Huang JS, Chen MS.
Photodynamic therapy with or without intravitreal bevacizumab for polypoidal
choroidal vasculopathy: two years of follow-up. Am J Ophthalmol 2012;154(5):872-880.e2. [CrossRef] [PubMed]
36 Kim SJ, Yu HG. Efficacy of combined photodynamic therapy and
intravitreal bevacizumab injection versus photodynamic therapy alone in
polypoidal choroidal vasculopathy. Retina
2011;31(9):1827-1834. [CrossRef] [PubMed]
37 Maruko I, Iida T, Sugano Y, Saito M, Sekiryu T. Subfoveal retinal and
choroidal thickness after verteporfin photodynamic therapy for polypoidal
choroidal vasculopathy. Am J Ophthalmol
2011;151(4):594-603.e1. [CrossRef] [PubMed]
38 Gomi F, Sawa M, Wakabayashi T, Sasamoto Y, Suzuki M, Tsujikawa M.
Efficacy of intravitreal bevacizumab combined with photodynamic therapy for
polypoidal choroidal vasculopathy. Am J
Ophthalmol 2010; 150(1):48-54.e1. [CrossRef] [PubMed]
39 Hirami Y, Tsujikawa A, Otani A, Yodoi Y, Aikawa H, Mandai M,
Yoshimura N. Hemorrhagic complications after photodynamic therapy for
polypoidal choroidal vasculopathy. Retina
2007;27(3):335-341. [CrossRef] [PubMed]
40 Krebs I, Schmetterer L, Boltz A, Told R, Vécsei-Marlovits V, Egger S,
Schönherr U, Haas A, Ansari-Shahrezaei S, Binder S; MANTA Research Group. A
randomised double-masked trial comparing the visual outcome after treatment
with ranibizumab or bevacizumab in patients with neovascular age-related
macular degeneration. Br J Ophthalmol
2013;97(3):266-271. [CrossRef] [PubMed]
41 Kodjikian L, Souied EH, Mimoun G, Mauget-Faÿsse M, Behar-Cohen F,
Decullier E, Huot L, Aulagner G; GEFAL Study Group. Ranibizumab versus
bevacizumab for neovascular age-related macular degeneration: results from the
GEFAL Noninferiority Randomized Trial. Ophthalmology
2013;120(11):2300-2309. [CrossRef] [PubMed]
42 Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ,
Culliford LA, Reeves BC; IVAN study investigators. Alternative treatments to
inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of
the IVAN randomised controlled trial. Lancet
2013;382(9900):1258-1267. [CrossRef]
43 Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth
C, Redford M, Ferris FL 3rd. Ranibizumab and bevacizumab for treatment of
neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119(7):1388-1398. [CrossRef] [PMC free article] [PubMed]
44 Cho HJ, Kim KM, Kim HS, Han JI, Kim CG, Lee TG, Kim JW. Intravitreal
aflibercept and ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol 2016;165:1-6. [CrossRef] [PubMed]