
·Investigation· Current
Issue IF in JCR CiteScore ·Submission· In Press Recent Accepted PMC RSS
Citation: Yu SJ, Liu GH, Liu Y, Huang J, Han ML, Zhao BJ, Gai ZT. The evolution of
refractive status in Chinese infants during the first year of life and its
affected factors. Int J Ophthalmol 2017;10(8):1290-1294
The evolution of refractive status in Chinese
infants during the first year of life and its affected factors
Shu-Juan Yu1, Guo-Hua Liu1,
Yi Liu2, Jing Huang1, Ming-Lei Han1, Bo-Jun
Zhao3, Zhong-Tao Gai2
1Department of Ophthalmology, Qilu Children’s Hospital of Shandong
University, Jinan 250022, Shandong Province, China
2Pediatric Research Institute, Qilu Children’s Hospital of Shandong
University, Jinan 250022, Shandong Province, China
3Department of Ophthalmology, Shandong Provincial Hospital
Affiliated to Shandong University, Jinan 250021, Shandong Province, China
Correspondence
to: Zhong-Tao Gai. Pediatric Research Institute, Qilu Children’s
Hospital of Shandong University, Jinan 250022, Shandong Province, China. Gaizhongtao@sina.com;
Bo-Jun Zhao. Department of Ophthalmology, Shandong Provincial Hospital
Affiliated to Shandong University, Jinan 250021, Shandong Province, China.
15168860708@163.com
Received:
2017-01-22
Accepted: 2017-05-23
Abstract
AIM: To
study the evolution of the refractive status and examine the affected factors
in infants during the first year of life in a large sample size in China.
METHODS: A
total of 1258 babies (2516 eyes) aged 32wk gestational age to 1y participated
in the study, including 766 premature and 492 full-term infants. First, each
baby received an orthoptic examination, slit-lamp checking and fundus imaging.
Patients with diseases which might affect refractive status were excluded from
the cohort. The cycloplegia retinoscopy was performed. Their neonatal histories
were reviewed. Each measurement contained the refractive status and calculation
of the spherical equivalent (SE).
RESULTS: Refractive
state showed an average hyperopia of +0.94±1.63 D at early ages, followed by a
trend toward more hyperopia. The refractive state reached the top (+2.43±1.46
D) at the age of one to two months. Then gliding till one year old when the
refractive state reached +0.59±1.41 D. The prevalence of astigmatism was 42.17%
in the study, being 2.82% myopic astigmatism and 39.35% hyperopic astigmatism.
The 94.1% of hyperopic astigmatism was with-the-rule astigmatism and 71.83% of
myopic astigmatism was with-the-rule astigmatism. Refractive state between boys
and girls was different. The mean SE of boys was +1.97±1.57 D, while that of
girls was +1.79±1.46 D, and the difference was significant.
CONCLUSION: Before
one year old, the change of refractive status is associated with checking age
and sex. At the age of one to two months, the degree of hyperopia reaches the
top. Boys have more hyperopic degree than girls, and with-the-rule astigmatism
is predominant. Excluding premature infants with advanced retinopathy of
prematurity, premature and full-term children have same refraction status.
KEYWORDS: refractive
status; corrected age; infant, prematurity; spherical equivalent; cycloplegic
retinoscopy
DOI:10.18240/ijo.2017.08.17
Citation: Yu SJ, Liu GH, Liu Y, Huang J, Han ML, Zhao BJ, Gai ZT. The evolution of
refractive status in Chinese infants during the first year of life and its
affected factors. Int J Ophthalmol 2017;10(8):1290-1294
INTRODUCTION
Refractive
error is a major eye care problem throughout the world[1-2] and ametropia is known to increase the risk of
amblyopia and strabismus[3]. In order to early
intervene for refractive error, it is necessary to understand the starting
point of refractive error at birth and the corresponding refractive status of
different development stage. It is well-known that eye refraction changes with
age, and young children are generally hyperopic[4-11]. Compared with full-term infant, premature infants
especially low birth weight premature tent to develop myopia in the future[12-18]. However, subjects of most
studies were started measurements after one to three years of age. There were
only a few studies about refractive error especially before one year old[10]. And most of them were in small samples. Because of
the limitation of age and check condition, large samples size were few.
The
aim of our study is to find the evolvement rule of refractive status at
early development stage, by investigating the refractive status of premature
infants and full-term infants in China during the first year of life with large
samples. These data would provide essentially normative information for
screening program.
SUBJECTS AND METHODS
Subjects This is a
retrospective cohort and cross-sectional study. A total of 1258 children (2516
eyes) participated in this study. It comprised 766 premature infants (26-36wk
of gestation, the mean gestation age was 32.23wk), and 492 full-term infants
(37-44wk of gestation, the mean gestation age was 39.17wk) who were given an
eye examination at Qilu Children’s Hospital of Shandong University, from
January 2014 to August 2016. For premature infants, most of them came to the
hospital to perform retinopathy of prematurity (ROP) screening, and the rest
were for ophthalmic examination. The corrected gestation ages at the checked
point were between 32wk and one year old. Informed consent was obtained from
one or both parents before each infant was enrolled. The research was in
agreement with the Declaration of Helsinki.
Variables
and the Measurements
Common
examination First, each
baby received an orthoptic examination including cover and motility tests; and
then the eye was examined with handheld slit lamp, cycloplegia retinoscopy, and
fundus images were taken. Eyes with nystagmus, strabismus, single or double
ptosis, advanced ROP with laser-treated or intravitreal injection, or any other
retinal morbidity were excluded from the study. Eyes with spontaneously
regressed ROP were retained in the study. Children whose parents had genetic
eye diseases were also not considered.
Refractive
examination Cycloplegia
for retinoscopy was achieved with one or two drops 0.5% cyclopentolate
hydrochloride and three to five drops 0.5% phenylephrine hydrochloride every
10min. Streak retinoscopy (66 Vision Technology, Suzhou, Jiangsu Province,
China) was performed 30min afterward. All refractive measurements were
performed by the same experienced senior optometrist. An allowance of 2.0 D was
allowed for a working distance of half meter.
Data
recording The original
data comprised the date of examination, name, sex, gestational age (GA), birth
weight (BW), corrected gestational age (CGA), now weight (NW), right or left
eye, clinical diagnosis, sphere, cylinder and its axis. Refractive error was
recorded in the form of spherical equivalent (SE)=sphere+1/2cylinder.
Myopia was defined as SE less than or equal to −0.50 D, and hyperopia was
defined as SE more than or equal to +0.50 D. Astigmatism was defined as
cylindrical degree (CD) greater than or equal to ±0.50 D. In this study,
corrected age equals to postnatal age minus the difference between term (40wk)
and GA at birth. For instance, the CGA of an infant born at 28wk’ GA and tested
at postnatal age 24wk was 12wk: 24−(40−28)=12. An infant born at 38wk’ GA and
tested at postnatal age 6wk was 4wk: 6−(40−38)=4. We referred to “corrected
gestational age” as “age” in this report unless special instructions.
Statistical
Analysis In this
study, sphere, cylinder and its axis for each measurement were recorded, and
then calculated the SE for each measurement was calculated. SPSS 11.0 software
was used for all statistical analysis. Statistical significance was defined as P
value less than 0.05. Correlations between eye refraction and the different
variables were analyzed with regression and Pearson correlation tests. Because
of highly correlated with age and weight, the partial correlation analysis was
performed on the relationship between SE and body weight. The correlation
between double eyes was performed with paired samples t-test.
RESULTS
A
total of 1258 babies (2516 eyes) participated in this study, including 782 boys
(62.2%) and 476 girls (37.8%), among them there were 766 premature infants
(62.16%) and 492 full term infants (37.84%).
Comparison
of Refraction with Some Factors With
correlation and regression analysis, it was demonstrated that eye refraction
was related with its CGA and sex, but not with its birth body weight (BBW), now
body weight (NBW), right or left eye (Table 1). At the same checked age, there
was no significance between SE and birth gestational age (BGA) (P>0.05).
A high correlated coefficient was found between double eyes about SE (r=0.933)
by paired samples t-test. The difference of refraction between double
eyes is not significant (P=0.000) (Table 1).
Table
1 Correlations and differences between SE and some factors
|
Factors |
r |
P |
|
BBW |
0.032 |
0.112 |
|
NBW |
0.019 |
0.336 |
|
Right
or left |
0.001 |
0.970 |
|
CGA |
0.107 |
0.000 |
|
Sex |
-0.053 |
0.008 |
BBW:
Birth body weight; NBW: Now body weight; CGA: Corrected gestation age.
Correlation was significant at the 0.05 level (2-tailed).
Refraction
and Age All samples
were divided into eleven groups according to CGA. Refractive degree showed an
average hyperopia of +0.94±1.63 D at the beginning of the study, followed by a
trend toward more hyperopia. The refractive degree reached the top (+2.43±1.46
D) at the age of about one to two months. Then gradually decline, the
refractive degree reached +0.59±1.41 D at the age of one year old (Table 2;
Figure 1).
Table
2 Mean SE refraction in different CGA
|
CGA |
Eyes (n) |
Mean SE |
SD |
Std. Error |
95%CI for
mean |
Min |
Max |
|
|
Lower
bound |
Upper
bound |
|||||||
|
≤34wk |
26 |
+0.94 |
1.63 |
0.32 |
+0.28 |
+1.59 |
-4.75 |
+2.75 |
|
35-36wk |
130 |
+1.10 |
1.47 |
0.13 |
+0.85 |
+1.36 |
-3.00 |
+4.00 |
|
37-38wk |
386 |
+1.46 |
1.63 |
0.08 |
+1.31 |
+1.63 |
-3.75 |
+6.00 |
|
39-40wk |
438 |
+1.81 |
1.46 |
0.07 |
+1.69 |
+1.96 |
-2.25 |
+6.38 |
|
41-43wk |
392 |
+2.05 |
1.39 |
0.07 |
+1.90 |
+2.17 |
-2.00 |
+6.00 |
|
1-2mo |
582 |
+2.43 |
1.46 |
0.06 |
+2.31 |
+2.55 |
-2.50 |
+8.00 |
|
3-4mo |
270 |
+2.15 |
1.42 |
0.09 |
+1.98 |
+2.32 |
-0.75 |
+6.25 |
|
5-6mo |
154 |
+1.87 |
1.58 |
0.13 |
+1.61 |
+2.12 |
-0.75 |
+9.50 |
|
7-8mo |
74 |
+1.41 |
1.58 |
0.15 |
+1.12 |
+1.70 |
-0.50 |
+6.38 |
|
9-10mo |
42 |
+1.48 |
1.25 |
0.22 |
+1.04 |
+1.92 |
-1.75 |
+4.50 |
|
11-12mo |
22 |
+0.59 |
1.41 |
0.20 |
+0.17 |
+1.02 |
-1.13 |
+2.13 |
|
Total |
2516 |
+1.90 |
0.96 |
0.03 |
+1.85 |
+1.97 |
-4.75 |
+9.50 |
CGA: Corrected gestation age.
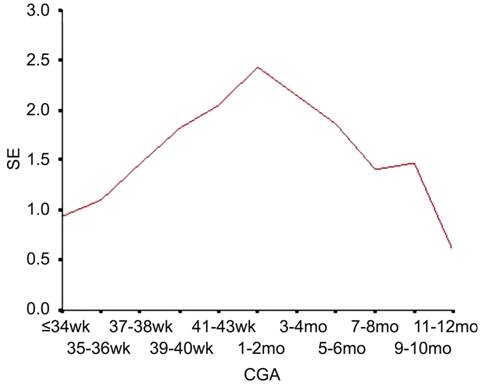
Figure 1 SE refraction in different CGA.
Refraction
and Sex Refractive
degree of different sex was different in this study. The mean SE of boys was
+1.97±1.57 D, and girls was +1.79±1.46 D. Boys had more hyperopia degree than
girls at different ages (Table 3; Figure 2).
Table 3 Mean
SE refraction of boys and girls
|
CGA |
Male |
Female |
||||
|
Eyes (n) |
Mean SE |
SD |
Eyes (n) |
Mean SE |
SD |
|
|
≤34wk |
20 |
1.07 |
1.73 |
6 |
0.50 |
1.25 |
|
35-36wk |
84 |
1.00 |
1.55 |
46 |
1.28 |
1.32 |
|
37-38wk |
232 |
1.63 |
1.67 |
154 |
1.22 |
1.55 |
|
39-40wk |
258 |
2.00 |
1.41 |
180 |
1.55 |
1.50 |
|
41-43wk |
218 |
2.02 |
1.41 |
174 |
2.07 |
1.36 |
|
1-2mo |
380 |
2.55 |
1.50 |
202 |
2.21 |
1.37 |
|
3-4mo |
172 |
2.09 |
1.39 |
98 |
2.24 |
1.46 |
|
5-6mo |
108 |
1.90 |
1.77 |
46 |
1.78 |
1.05 |
|
7-8mo |
48 |
1.24 |
1.30 |
26 |
1.72 |
1.13 |
|
9-10mo |
28 |
1.38 |
1.59 |
14 |
1.67 |
0.95 |
|
11-12mo |
16 |
0.77 |
0.94 |
6 |
0.11 |
0.90 |
|
Total |
1564 |
1.97 |
1.57 |
952 |
1.79 |
1.46 |
CGA:
Corrected gestation age.
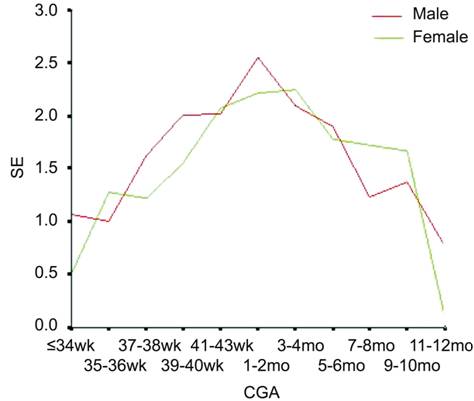
Figure 2 SE refraction of boys and girls.
Astigmatism A total of
2516 eyes were enrolled into the study. Among them 1061 eyes (42.17%) existed
astigmatism, including 71 myopic astigmatism eyes (2.82%) and 990 hyperopic
astigmatism eyes (39.35%). The minimum myopic cylindrical power was -3.50 D,
and the maximum hyperopic cylindrical power was +4.00 D. The 94.37% myopic and
93.94% hyperopic astigmatism degree were below 2.00 D (Table 4).
Table 4 Mean
cylindrical power of premature and full-term infants
n (%)
|
Parameters |
Astigmatism |
Eyes (n) |
Prevalence
(%) |
Mean (D) |
SD |
Max/Min |
≤±2.00 D
(eyes) |
≤±1.00 D
(eyes) |
|
Premature |
Myopic |
38 |
2.42 |
-1.14 |
0.59 |
-3.00 |
36 (94.74) |
24 (63.16) |
|
Hyperopic |
483 |
31.53 |
+0.99 |
0.56 |
+3.25 |
461
(95.45) |
351
(72.67) |
|
|
Full-term |
Myopic |
33 |
3.25 |
-1.23 |
0.65 |
-3.5 |
31 (93.94) |
17 (51.52) |
|
Hyperopic |
507 |
51.52 |
+1.14 |
0.63 |
+4.0 |
469
(92.50) |
318
(62.70) |
|
|
Total |
Myopic |
71 |
2.82 |
-1.19 |
0.61 |
-3.50 |
67 (94.37) |
41 (57.75) |
|
Hyperopic |
990 |
39.35 |
+1.07 |
0.60 |
+4.00 |
930
(93.94) |
669
(67.58) |
Figures
3, 4 showed the magnitude and axis of cylindrical power. The 94.1% of hyperopic
astigmatism was with-the-rule astigmatism and 71.83% of myopic astigmatism was with-the-rule
astigmatism.
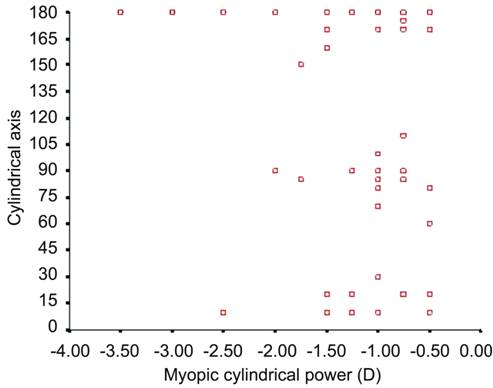
Figure 3 Myopic cylindrical power and axis.
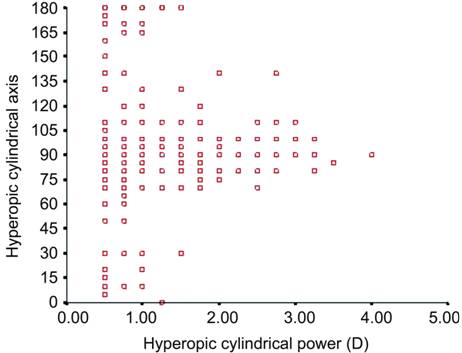
Figure 4 Hyperopic astigmatic degree and
axis.
DISCUSSION
Mean
Spherical Equivalent Refraction Our study
provided a detailed analysis with several unique findings about the refractive
status during the first year of infancy. The changes of eye refraction tend to
more hyperopic with the age, then hyperopic degree gradually declined, until
emmetropia or myopia. This rule is consistent with those former reports[3-5,17,19-20]. The crystalline lens of premature is near the cornea
and is relatively spherical in shape, which leads to shallower anterior chamber
and more highly curved cornea compared with eyes of full-term infant[5]. The lens change rapidly during the last trimester with
flattening and moving away from the cornea, which results in thinner lens
thickness, shallower anterior chamber and smaller corneal curvature. Thus, we
believe that there is a large refractive shift from myopia to hyperopia before
the time of full-term birth. Cook et al[5]
referred that the components of refractive state showed linear patterns of
growth up until 44wk postmenstrual age. In our younger patients, the
refractive was +0.94 D at the corrected gestational age of 34wk, then hyperopic
degree increased gradually. At the age of 1-2mo, the refractive reached the top
value (+2.43±1.46 D) and then declined. At the age of one year, the refractive
reached to +0.59 D. Cook et al[5] reported
that refractive state showed an average myopia of -2.00 D at the age of 32wk.
These results verified that refractive developed from myopia in embryonic
period, then followed by a trend toward hypermetropia.
Ton
et al[4] referred that the mean refractive of
infants was +1.24 D in infants aged 1mo or less and reached to +2.50 D at the
age of 4-6mo. Gunay et al[6] and Mutti
et al[7] showed a mean refractive error of
about +2.2 D from the first to the fourth month of age then declined. Wood
et al[11] showed a mean spherical equivalent
error of +1.44 D for 58 infants at 2wk of age, which increased to +2.84 D at
12wk of age. Our result was in agreement with them. Chen et al[3] found the mean cycloplegic spherical equivalent was
highly hyperopic (OD, +3.47±2.43 D; OS, +3.64±2.43 D) for full-term Chinese
neonates who were between 1d and 6d of age, whose hypermetropia was about 1 D
greater than that of our results.
Astigmatism The
prevalence of astigmatism was 42.17% in this study, including 2.82% myopic
astigmatism and 39.35% hyperopic astigmatism. It was consistent with previous
reports[13]. It was shown that the magnitude and
proportion of astigmatism and myopia of premature were greater than that of
full-term infants, especially with advanced ROP[14-15,19]. Astigmatism is mainly
influenced by corneal curve, and premature with advanced ROP has highly curved
corneas[10]. Lu et al[14]
referred that patients with aggressive posterior retinopathy of premature
(APROP) who underwent laser treatment tend to have more severe astigmatism, but
no significant differences between APROP and the non-APROP.
In
this study, premature had 2.42% myopic astigmatism and 31.53% hyperopic
astigmatism, the mean degrees were -1.14 D and +0.99 D respectively. While
full-term had 3.25% myopic astigmatism and 51.52% hyperopic astigmatism, the
mean degrees were -1.23 D and +1.14 D. The two distributions between premature
and full-term were significantly different (P=0.000). One reason was
that we excluded those premature with advanced ROP which had more risk to
develop astigmatism. Another possible reason was that some full-term infants
felt fear and crying when checking and eye speculum had to be used to open the
eyelids, which might increase the astigmatism degree.
Refraction
and Other Factors Our study
showed that refraction had a positive linear correlation with age. Many studies[4-11] referred that eye refraction was
correlated with age at the time of examination, but not with birth weight or gestational
age. However, low birth weight and ROP have long been known to be implicated in
the development of myopia, astigmatism, and anisometropia. Children with laser
treatment for ROP tend to have higher risk[10,13-16,18,20-21]. However, there were no significant differences in
the refractive status in patients with regressed ROP and in preterm infants
without ROP[14]. In this study premature infants
with advanced ROP or APROP were excluded, but regressed ROP were reserved.
Our
study found that eye refraction was connected with age at the time of
examination and sex. Boys had higher hyperopic degree than girls. The
difference between boys and girls was significant (F=8.215, P=0.004).
This result was different from most previous research results[4-11,22-24].
Kleinstein et al[25] referred that the
prevalence of myopia in boys was higher than that in girls. In China some
research showed that myopia was associated with female[26-27]. Our result showed that male had more hyperopia than
that of female, which implied that females had more feasibility to develop
myopia. But the evidence was not sufficient. Further long follow-up study will
be needed to confirm.
After
adjusting the checking age, eye refractions had no relation with birth
gestational age, birth body weight and now body weight. Excluding premature
infants with APROP or advanced ROP, premature and full-term children had same
refraction status and no significant differences between two distributions (P>0.05).
As
the samples size of different age groups in the study was not matched,
especially at the age from 7mo to one year old, this might lead to inaccuracy
of the results. Our results only showed the refractive status of infants before
one year, and it could not represented the whole refractive evolution of
children. The importance of long-term follow-up should be emphasized.
ACKNOWLEDGEMENTS
Foundation:
Supported by Shandong Nature Science Foundation (No.ZR2015HM026).
Conflicts
of Interest: Yu SJ, None; Liu GH, None; Liu Y, None; Huang
J, None; Han ML, None; Zhao BJ, None; Gai ZT, None.
REFERENCES
2 Miraldi Utz V. Nature versus nurture: a systematic
approach to elucidate gene-environment interactions in the development of
myopic refractiveerrors. Ophthalmic Genet
2017;38(2):117-121. [CrossRef]
[PubMed]
3 Chen J, Xie AL, Hou LJ, Su Y, Lu F, Thorn F.
Cycloplegic and noncycloplegic refractions of Chinese neonatal infants. Invest Ophthalmol Vis Sci
2011;52(5):2456-2461. [CrossRef] [PubMed]
4 Ton Y, Wysenbeek YS, Spierer A. Refractive error in
premature infants. J AAPOS 2004;8(6):534-538.
[CrossRef] [PubMed]
5 Cook A, White S, Batterbury M, Clark D. Ocular
growth and refractive error development in premature infants without
retinopathy of prematurity. Invest
Ophthalmol Vis Sci 2003;44(3):953-960.
[CrossRef]
6 Gunay M, Sekeroglu MA, Bardak H, Celik G, Esenulku
CM, Hekimoglu E, Bardak Y. Evaluation of refractive errors and ocular biometric
outcomes after intravitreal bevacizumab for retinopathy of prematurity. Strabismus 2016;24(2):84-88. [CrossRef]
[PubMed]
7 Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane
SL, Lin WK, Moeschberger ML, Zadnik K. Axial growth and changes in lenticular
and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci 2006;10(2):188-189. [CrossRef]
8 Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB.
Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol 2001;119(11):1625-1628. [CrossRef]
9 Scharf J, Zonis S, Zeltzer M. Refraction in
premature babies: a prospective study. J
Pediatr Ophthalmol Strabismus 1978;15(1):48-50. [PubMed]
10 Cook A, White S, Batterbury M, Clark D. Ocular growth and refractive error
development in premature infants with or without retinopathy of prematurity. Invest Ophthalmol Vis Sci 2008;49(12):5199-5207.
[CrossRef] [PubMed]
11 Wood IC, Hodi S, Morgan L. Longitudinal change of
refractive error in infants during the first year of life. Eye (Lond) 1995;9(5):551-557. [CrossRef]
[PubMed]
12 Hopkisson B, Arnold P, Billingham B, McGarrigle M,
Shribman S. Can retinoscopy be used to screen infants for amblyopia? A
longitudinal study of refraction in the first year of life. Eye (Lond)
1992;6(6):607-609. [CrossRef]
[PubMed]
13 Nissenkorn I, Yassur Y, Mashkowski D, Sherf I,
Ben-Sira I. Myopia in premature babies with or without retinopathy of
prematurity. Br J Ophthalmol 1983;67(3):170-173. [CrossRef]
15 Kuo HK, Sun IT, Chung MY, Chen YH. Refractive error
in patients with retinopathy of prematurity after laser photocoagulation or
Bevacizumab monotherapy. Ophthalmologica
2015;234(4):211-217. [CrossRef] [PubMed]
16 Özdemir Ö, Özen Tunay Z, Ergintürk Acar D. Growth
of biometric components and development of refractive errors in premature
infants with or without retinopathy of prematurity. Turk J Med Sci 2016;46(2): 468-473. [CrossRef]
[PubMed]
17 Chen TC, Tsai TH, Shih YF, Yeh PT, Yang CH, Hu FC,
Lin LL, Yang CM. Long-term evaluation of refractive status and optical
components in eyes of children born prematurely. Invest Ophthalmol Vis Sci 2010; 51(12):6140-6148. [CrossRef]
[PubMed]
18 Hsieh CJ, Liu JW, Huang JS, Lin KC. Refractive
outcome of premature infants with or without retinopathy of prematurity at 2
years of age: a prospective controlled cohort study. Kaohsiung J Med Sci 2012; 28(4):204-211. [CrossRef]
[PubMed]
19 Modrzejewska M, Grzesiak W, Karczewicz D, Zaborski
D. Refractive status and ocular axial length in preterm infants without
retinopathy of prematurity with regard to birth weight and gestational age. J Perinat Med 2010;38(3):327-331. [CrossRef]
[PubMed]
20 Roohipoor R, Karkhaneh R, Rezai Esfahani M, et al.
Comparison of refractive error changes in retinopathy of prematureity patients
treated with Diode and Red lasers. Ophthalmologica
2016;235(3):173-178. [CrossRef] [PubMed]
21 Davitt BV, Quinn GE, Wallace DK, Dobson V, Hardy
RJ, Tung B, Lai D, Good WV. Astigmatism progression in the early treatment for
retinopathy of prematurity study to 6 years of age. Ophthalmology 2011; 118(12):2326-2329. [CrossRef]
[PMC free article] [PubMed]
23 Goldschmidt E, Lam CS, Opper S. The development of
myopia in Hong Kong children. Acta
Ophthalmol Scand 2001;79(3):228-232. [CrossRef]
24 Mashige KP, Jaggernath J, Ramson P, Martin C,
Chinanayi FS, Naidoo KS. Prevalence of refractive errors in the INK area,
Durban, South Africa. Optom Vis Sci
2016;93(3):243-250. [CrossRef]
[PubMed]
25 Kleinstein RN, Jones LA, Hullett S, Kwon S, Lee RJ,
Friedman NE, Manny RE, Mutti DO, Yu JA, Zadnik K. Refractive error and
ethnicity in children. Arch Ophthalmol
2003;121(8):1141-1147. [CrossRef]
[PubMed]