INTRODUCTION
Along with the development of more sophisticated intraocular lens (IOL) delivery through smaller incisions, modern cataract surgery has continually advanced.Also, cataract surgery via small incisions has resulted in better postoperative visual outcomes such as less postoperative astigmatism, reduced inflammation, a lower risk of early postoperative infection and faster would healing[1-5].
With the goal of providing better operating conditions,further advancement of injectable foldable IOLs has led to the development of a preloaded IOL delivery system.Benefits of the preloaded system include the elimination of IOL injector loading variability, avoidance of potential IOL loading errors and reduced operation time[6]. However, IOL delivery through the preloaded system has reportedly failed to meet the expectations in terms of predictability as well as particular safety issues[7-10]. According to Ong et al[8], only 45%of 85 cases showed correct IOL delivery, while 55% needed additional rotational manipulation of IOL orientation. Also,other problems related to the preloaded IOL delivery system were reported, including trapped trailing haptic, haptic-optic adhesion, overriding of plunger over optic, and trauma to optic edge[7,9-10]. Some other studies also mentioned concerns regarding the risk of bacterial contamination when using the preloaded IOL delivery system[11-13].
When ophthalmic viscosurgical devices (OVDs) are injected into a capsular bag during IOL delivery to form enough space for IOL implantation and removed via an irrigation/aspiration procedure, some residual OVD remaining after cataract surgery can cause bacterial contamination[14]. In fact, Matsuura and Inoue[11]conducted research about OVD backflow during IOL implantation, which authors insisted to be related to a lower bacterial contamination rate. The research demonstrated less OVD backflow in the preloaded IOL delivery system compared to the non-preloaded system and thus, concluded that the high bacterial contamination rate of preloaded IOL is associated with less OVD backflow during IOL implantation.Based upon previous studies, we planned to investigate delivery characteristics of preloaded IOL. Therefore, we aimed to compare preloaded IOL delivery systems with nonpreloaded systems in terms of predictability and safety. We evaluated the predictability of preloaded IOL delivery systems during loading and implantation procedures in human eyes. We also investigated dynamics of OVD during IOL implantation with colored OVD in both preloaded and non-preloaded IOL delivery systems in porcine eyes.
Table 1 Characteristics of IOLs and its cartridge

HA: Hydrophobic acrylic.
SUBJECTS AND METHODS
This study was approved prospectively by the Institutional Review Board of Severance Hospital and conducted in accordance with the Declaration of Helsinki and Good Clinical Practices. Informed consent was obtained from all patients after purposes and possible consequences of the study were explained to them.
To evaluate characteristics of various IOL delivery systems,we performed two separate studies; a human eye study and a porcine eye study. For the human eye study, patients who visited Severance Hospital, a tertiary referral center in Seoul,Korea, were included. Other inclusion criteria were 50 years of age or more, having a nuclear or cortical cataract of grade 2 to 5 according to the Lens Opacities Classification System III, and being scheduled to undergo cataract surgery with a phacoemulsification procedure by a single surgeon (Kim TI)[15].The exclusion criteria were a history of previous ocular or intraocular surgery, evidence of trauma on biomicroscopic examination, evidence of acute or chronic corneal infection,inflammatory conditions of the cornea, corneal opacity,wearing contact lenses during the preceding 6mo, preoperative astigmatism of more than 2.00 diopters, and intraoperative complications including capsular tear or rupture, and zonular dehiscence.Patients were assigned to 5 different IOL groups randomly:iSert250 NC60 (NC60, Hoya Corporation Ltd., Tokyo, Japan),EnVista MX60 (MX60, Bausch and Lomb Inc., Rochester, NY,USA), AcrySof IQ SN60WF (SN60WF, Alcon Laboratories Inc., Fort Worth, TX, USA), TECNIS ZCB00 (ZCB00, Abbott Medical Optics Inc., Santa Ana, CA, USA), and TECNIS PCB00 (PCB00, Abbott Medical Optics Inc.). Randomization occurred by using computer generated randomization envelope. All the surgeries were performed by the single surgeon (Kim TI) via a temporal clear corneal incision under topical anesthesia. Initially, one side port incisions were created with a 15-degree blade. An OVD (Healon, Abbott Medical Optics Inc., Santa Ana, CA, USA) was injected into anterior chamber before the main incision was created. Three-step clear corneal incisions were made using a 2.8-mm Meyco diamond blade (Anton Meyer & Co. Ltd., Biel, Switzerland). Whitestar Signature System (Abbott Medical Optics, Inc.) was used for phacoemulsification of nuclear fragments. Phaco tips with a 30-degree bevel and standard sleeves were used. NC60 and PCB00 were implanted in a capsular bag using a preloaded IOL delivery system without any incision tunnel enlargement. The surgeon filled a cartridge with OVD before IOL implantation.Conversely, MX60, SN60WF and ZCB00 were implanted in a capsular bag with non-preloaded IOL delivery systems. Single OVD (Healon, Abbott Medical Optics Inc.) was used both in preloaded and non-preloaded systems. Characteristics of each IOL and cartridge are shown in Table 1. Diopters of IOL was selected to achieve emmetropia. Both main incision and side ports were sealed with stromal hydration using balanced salt solution (BSS, Alcon Laboratories Inc.) at the end of surgery.Finally, all wounds were tested for leakage using a cellulose sponge.
The time from the start of IOL loading to completion of IOL implantation in the capsular bag was measured, and the number of cases requiring additional manipulation with a Sinskey hook to complete implantation of both IOL haptics in the capsular bag was recorded. Analyses were conducted using video recordings of cataract surgeries, by a single observer(Lee H).In the experimental study using excised porcine eyes, OVD(Healon, Abbott Medical Optics Inc.) mixed with 0.05%sodium fluorescein was used to analyze OVD dynamics during IOL implantation procedures. Healon was purified sodium hyaluronate with a molecular weight of 4.0×106Da. Each type of injector was prepared with colored OVD and IOL. Anterior chamber was filled with OVD. We used Ocular Barraquer 15-21 mm Hg (Phaco& SLIP) Tonometer (Ocular, Bellevue, WA,USA) to estimate IOP of experimental eyes. IOP was adjusted when it was too high or too low before IOL delivery. IOL was delivered through 2.8-mm clear corneal incision. After IOL delivery, amount of colored OVD injected into anterior chamber was analyzed via Image J (National Institute of Health, Bethesda, MD, USA) analysis of video recordings, by a single observer (Chung B). Video screen was captured at the moment of completion of IOL delivery. The observer measured area of the entire anterior chamber and area exhibiting fluorescein-stained OVD, and then calculated the ratio using Image J program. In addition to OVD influx analysis, direction of colored OVD flow during IOL delivery was also analyzed.Statistical Analysis Results are expressed as mean±standard deviation where applicable. We used the Kolmogorov-Smirnov test to confirm data normality. To statistically compare preoperative data from the eyes of each group, we used one-way analysis of variance (ANOVA) with Bonferroni adjustment for post-hoc comparisons. We used one-way ANOVA with Bonferroni adjustment for post-hoc comparisons to compare IOL delivery time and IOL power among the 5 groups. Statistical analyses were performed using SAS software (version 9.2, SAS Institute Inc., Cary, NC, USA). A P value of <0.05 was considered statistically significant.
Table 2 Patient demographics and delivery characteristics mean±SD
aP<0.05 compared with TECNIS PCB00;cP<0.05 compared with iSert250 NC60.
RESULTS
A total of 101 eyes (58 males, 43 females) that underwent phacoemulsification procedures were included in the human eye study, and mean age at the time of surgery was 65.7±13.4y.Power range of implanted IOL was between 16 to 25 diopters in both preloaded and non-preloaded groups. Mean time from the start of IOL loading to completion of IOL implantation in the capsular bag was 22.0±3.1s for NC60, 43.2±6.5s for MX60, 32.3±6.0s for SN60WF, 41.4±7.1s for ZCB00, and 14.6±2.1s for PCB00, respectively (Table 2).
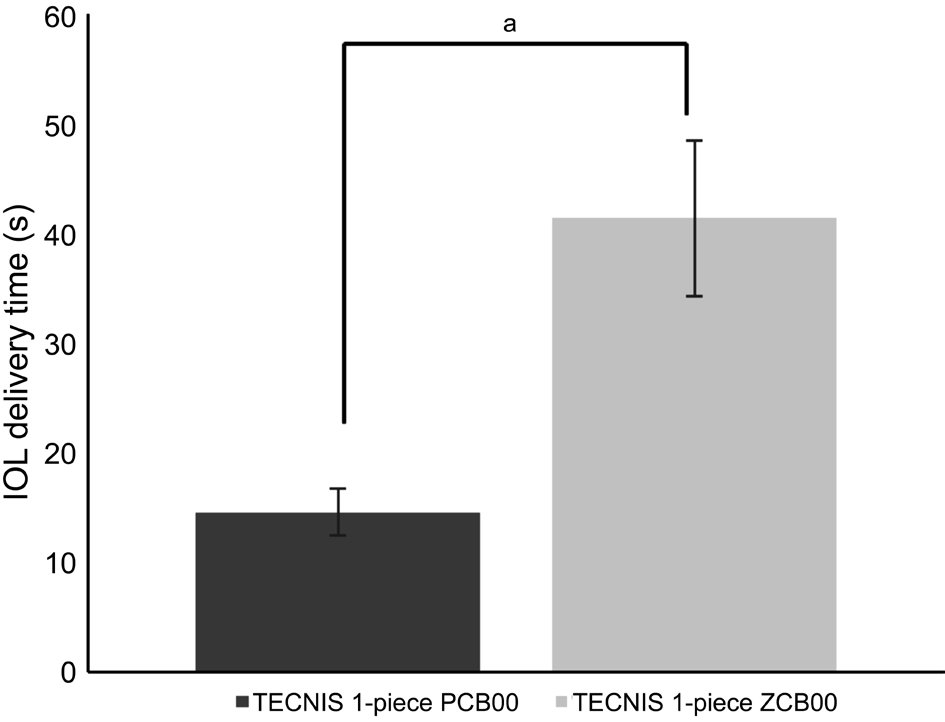
Figure 1 Comparison of delivery time of TECNIS 1-piece IOLaP<0.05.
With regards to IOL delivery time, implantation of NC60 and PCB00 using preloaded IOL delivery systems was significantly faster than other IOL groups using non-preloaded IOL delivery systems. Among non-preloaded groups, implantation of SN60WF was significantly faster than that of MX60 and ZCB00. The number of cases with additional IOL manipulation with a secondary instrument was 0/22 (0) for NC60, 0/18 (0)for SN60WF, 6/19 (31.6%) for MX60, 2/21 (9.5%) for ZCB00,and 0/21 (0) for PCB00. IOL power did not differ significantly among the groups. Surgical complications during IOL delivery,such as haptic entrapment, haptic-optic adhesion, IOL damage,and overriding of injector plunger overoptic were not reported.A total 42 eyes underwent phacoemulsification procedures using the same TECNIS IOL. IOL delivery time using a preloaded system was reduced 64.7% compared to a nonpreloaded system for the same IOL (Figure 1). In the experimental study using porcine eyes, in all cases colored OVD flowed from an injector into anterior chamber during IOL delivery without any backflow. Analysis of the video recordings demonstrated that amount of colored OVD in anterior chamber in NC60 cases was smaller than the that of other IOLs (Figure 2).Amount of OVD pushed into anterior chamber was similar in MX60, SN60WF, and ZCB00 (Figures 3-5). The percentage of colored OVD pushed into anterior chamber was 21.3%, 37.9%,31.4%, and 35.6% for NC60, MX60, SN60WF, and ZCB00,respectively.
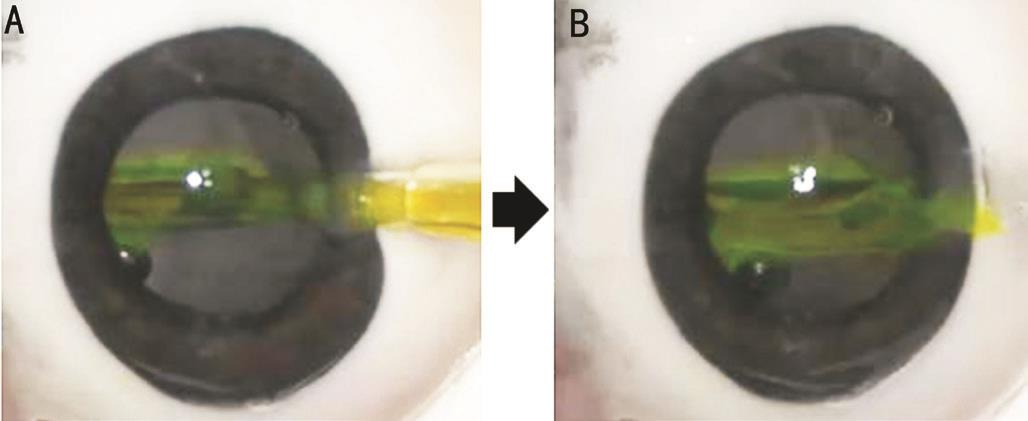
Figure 2 Characteristics of a preloaded IOL delivery system in the experimental study using excised porcine eyes A: iSert250 NC60 IOL was introduced into an eye while a cartridge was filled with colored OVD; B: After IOL delivery, colored OVD flowed from an injector into anterior chamber.
DISCUSSION
Preloaded IOL delivery systems are expected to provide effective and safe IOL delivery by reducing IOL loading errors,additional handling of IOLs, and surgical time. Our results suggest that IOL delivery time can be reduced via preloaded delivery systems by avoiding IOL loading into a cartridge, and enabling single step implantation into the capsular bag directly.As a result, IOL delivery time with preloaded systems (NC60 and PCB00) was shorter than other IOLs with non-preloaded systems. Furthermore, IOL delivery using preloaded system(PCB00) was faster than non-preloaded system (ZCB00) for the same IOL. In non-preloaded systems, it took more time to deliver MX60 and ZCB00 compared to SN60WF. MX60 is a prehydrated IOL which has higher glass transition temperature and this is reported to increase IOL unfolding time in the capsular bag[16]. This explains relatively longer IOL delivery time of MX60 compared to SN60WF. In cases of ZCB00,relatively thicker design and stiffer material of the IOL lead to prolonged IOL loading and implantation time compared to SN60WF.
Preloaded delivery systems can also reduce IOL injector loading errors, which may cause undesirable IOL behavior such as haptic entrapment or injector plunger overriding. There was no case with those undesirable IOL behaviors in this study.Previous studies have indicated that preloaded IOL delivery systems were less effective, and were associated with a greater chance of contamination[7-8,11,13]. However, predictability of IOL delivery is not solely determined by injector systems, because other factors such as surgeon’s skills, type and material of IOL also affect predictability of IOL delivery.
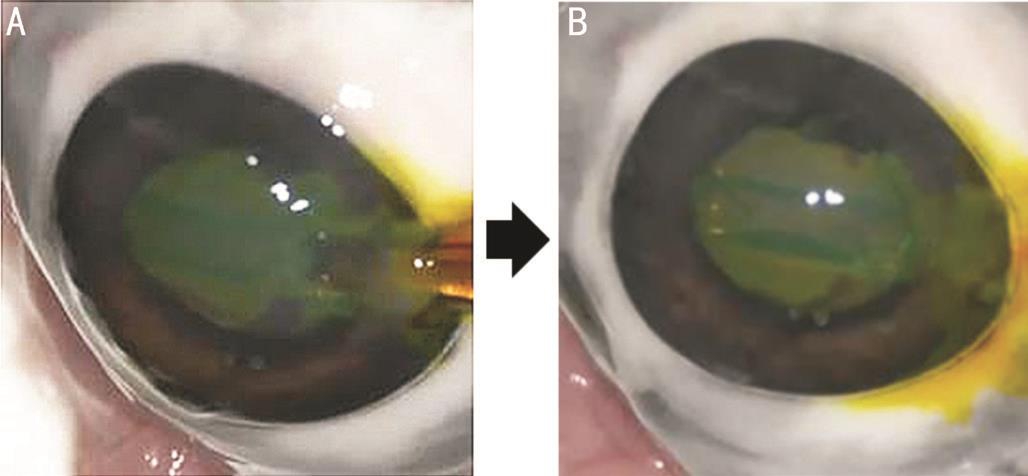
Figure 3 Characteristics of a non-preloaded IOL delivery system in the experimental study using excised porcine eyes A: EnVista MX60 IOL was introduced into an eye while a cartridge was filled with colored OVD; B: After IOL delivery, colored OVD flowed from an injector into anterior chamber.
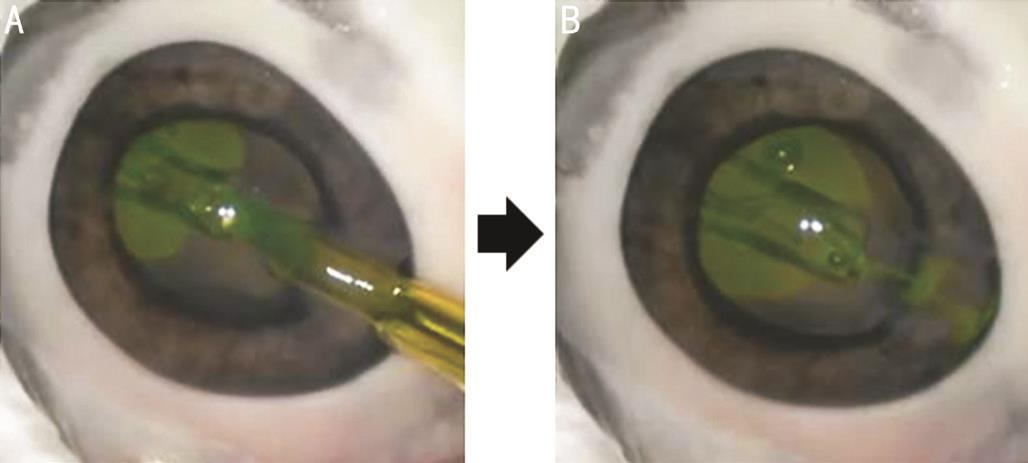
Figure 4 Characteristics of a non-preloaded IOL delivery system in the experimental study using excised porcine eyes A: AcrySof IQ SN60WF IOL was introduced into an eye while a cartridge was filled with colored OVD; B: After IOL delivery, colored OVD flowed from an injector into anterior chamber.
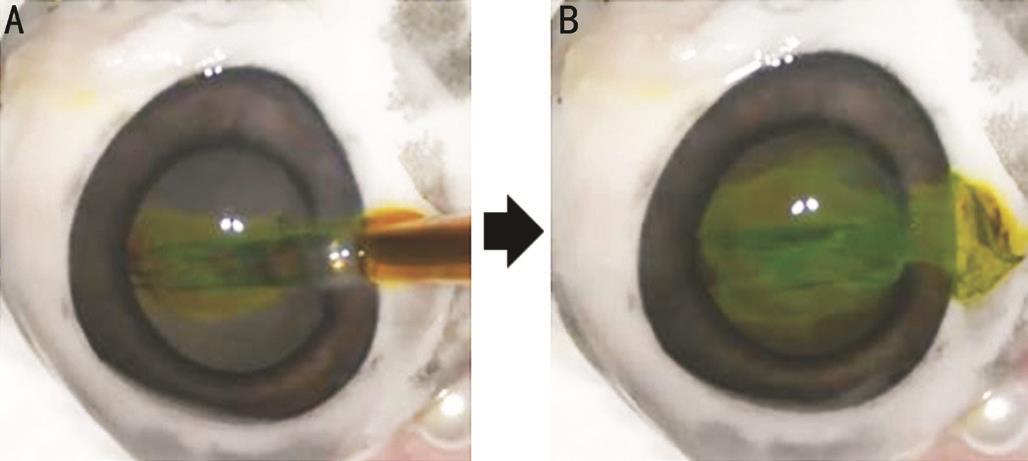
Figure 5 Characteristics of a non-preloaded IOL delivery system in the experimental study using excised porcine eyes A: TECNIS 1-piece ZCB00 IOL was introduced into an eye while a cartridge was filled with colored OVD; B: After IOL delivery, colored OVD flowed from an injector into anterior chamber.
Additional manipulation of the IOL is known to increase risk of damage to IOL, iris and lens capsule, and increase surgical time. In this study, there was no case required additional IOL handling after injection with preloaded IOL delivery systems.Compared to the previous study, our study showed much shorter IOL delivery time for preloaded systems (22.0s and 14.6s versus 47s). In addition, there were much less cases which needed additional IOL manipulation in our study (0 versus 55%).
Amount of OVD left in anterior chamber after IOL delivery could be related to various factors including structure of a cartridge, size of cartridge tip, relative difference between cartridge and plunger diameter, and degree of force used to push IOL into anterior chamber. An injector cartridge of preloaded IOL delivery system has relative small capacity to contain OVD compared to that of non-preloaded systems which has wide opening. This could be one of the factors that reduces OVD flow into anterior chamber in preloaded systems.Amount of OVD pushed into anterior chamber from an injector cartridge is possibly influenced by relative location of injector plunger tip to IOL. Placing injector plunger tip in the exact place to push an IOL into anterior chamber could facilitate injecting minimal amount of OVD into anterior chamber. In preloaded injector systems, injector plunger tip is already placed in proper location to push an IOL. On the contrary, an operator should place plunger tip manually in nonpreloaded injector systems. We suspect that non-preloaded systems not only increased risk of plunger tip misalignment,but also had chance to push relative large amount of OVD into anterior chamber.
A previous study analyzing OVD dynamics during IOL delivery reported instant OVD backflow into injector cartridge during IOL delivery in a preloaded system[11]. The authors concluded that this OVD backflow could be associated with less intraocular contamination. Compared with the previous study in which color-stained OVD was only filled at the tip of an injector cartridge, color-stained OVD was filled in a whole cartridge in our study. Instant OVD backflow was not noted at all in our study. We suspect that wound was possibly not tight enough to cause instant OVD backflow in our study based upon the fact that tightness of surgical wound can affect OVD dynamics.
According to previous experiences from the surgeon (Kim TI) who have used all 5 IOLs in the current study, IOL delivery of preloaded systems could be more effective and safe when compared with IOL delivery of non-preloaded systems. Limitations of this study includes that few types of preloaded IOL delivery system were investigated. Further study comparing various kinds of preloaded system is needed to deduce more generalized conclusion. Analysis of OVD pushed into anterior chamber in porcine study also had limitation. As we only measured volume of colored OVD by 2-dimensional images from video screenshot, the result might be only approximation of 3-dimensional volume. Additionally,the number of porcine eyes was not sufficient to generalize difference of OVD dynamics between preloaded and nonpreloaded systems.
In conclusion, faster IOL delivery and less additional IOL manipulations clearly demonstrate effectiveness of preloaded systems compared to non-preloaded IOL systems. Relatively less amount of OVD pushed into anterior chamber during IOL implantation in a preloaded delivery system could be associated with less chance of bacterial contamination.However, this experimental study seems to be insufficient to conclude safety of preloaded systems. Further study is needed to investigate factors related to efficacy and safety between preloaded and non-preloaded IOL delivery systems.
ACKNOWLEDGEMENTS
Foundation: Supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) by the Ministry of Education, Science and Technology (No.NRF-2016R1A2B4009626).
Conflicts of Interest: Chung B, None; Lee H, None; Choi M,None; Seo KY, None; Kim EK, None; Kim TI, None.
REFERENCES
1 Weston K, Nicholson R, Bunce C, Yang YF. An 8-year retrospective study of cataract surgery and postoperative endophthalmitis: injectable intraocular lenses may reduce the incidence of postoperative endophthalmitis. Br J Ophthalmol 2015;99(10):1377-1380.
2 Steinert RF, Brint SF, White SM, Fine IH. Astigmatism after small incision cataract surgery. A prospective, randomized, multicenter comparison of 4- and 6.5-mm incisions. Ophthalmology 1991;98(4):417-423;discussion 423-414.
3 Oshika T, Nagahara K, Yaguchi S, Emi K, Takenaka H, Tsuboi S, Yoshitomi F, Nagamoto T, Kurosaka D. Three year prospective,randomized evaluation of intraocular lens implantation through 3.2 and 5.5 mm incisions. J Cataract Refract Surg 1998;24(4):509-514.
4 Martin RG, Sanders DR. Visual, astigmatic, and inflammatory results with the Staar AA-4203 single-piece foldable IOL: a randomized,prospective study. Ophthalmic Surg 1992;23(11):770-775.
5 Belazzougui R, Monod SD, Baudouin C, Labbe A. Architectural analysis of clear corneal incisions using Visante OCT in acute postoperative endophthalmitis. J Fr Ophthalmol 2010;33(1):10-15.
6 Nguyen DQ, Saleh TA, Pandey SK, Bates AK. Irregularities on the surface of single-piece AcrySof SA60AT intraocular lenses. J Cataract Refract Surg 2006;32(3):495-498.
7 Tsinopoulos IT, Tsaousis KT, Symeonidis C, Dimitrakos SA. Bilateral manifestation of severe anterior capsule contraction syndrome after implantation of a preloaded intraocular lens. Clin Exp Optom 2009;92(6):503-504.
8 Ong HS, Subash M, Sandhu A, Wilkins MR. Intraocular lens delivery characteristics of the preloaded AcrySof IQ SN60WS/AcrySert injectable lens system. Am J Ophthalmol 2013;156(1):77-81.
9 Michael K, O'Colmain U, Vallance JH, Cormack TG. Capsule contraction syndrome with haptic deformation and flexion. J Cataract Refract Surg 2010;36(4):686-689.
10 Lam HH, Visvaraja S. Spontaneous dislocation of intraocular lens as a late complication of uncomplicated cataract surgery: a case series. Clin Exp Optom 2012;95(1):99-102.
11 Matsuura K, Inoue Y. Ophthalmic viscosurgical device backflow into cartridge during intraocular lens insertion using injectors. Clin Ophthalmol 2014;8:321-325.
12 Kokuzawa S, Suemori S, Mochizuki K, Hirose Y, Yaguchi T.Aspergillus tubingenesis endophthalmitis after cataract surgery with implantation of preloaded intraocular lens. Semin Ophthalmol 2014;29(4):218-221.
13 Hayashi Y, Eguchi H, Miyamoto T, Inoue M, Mitamura Y. A case of delayed-onset propionibacterium acnes endophthalmitis after cataract surgery with implantation of a preloaded intraocular lens. Case Rep Ophthalmol 2012;3(3):291-297.
14 Suzuki T, Wada T, Kozai S, Ike Y, Gilmore MS, Ohashi Y. Contribution of secreted proteases to the pathogenesis of postoperative Enterococcus faecalis endophthalmitis. J Cataract Refract Surg 2008;34(10):1776-1784.
15 Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA,Bailey IL, Friend J, McCarthy D, Wu SY. The lens opacities classification system III. The longitudinal study of cataract study group. Arch Ophthalmol 1993;111(6):831-836.
16 Eom Y, Lee JS, Rhim JW, Kang SY, Song JS, Kim HM. A simple method to shorten the unfolding time of prehydrated hydrophobic intraocular lens. Can J Ophthalmol 2014;49(4):382-387.