INTRODUCTION
Cataracts are still the most common cause of blindness worldwide[1-2]. Although cataract surgery has progressed from phacoemulsification to femtosecond laser cataract surgery[3-5]and from the placement of monofocal to multifocal intraocular lenses[6], the coverage and safety of surgical methods are still under discussion. A practical drug that prevents cataract progression is needed, and the targets are diverse. One focus for treatment is oxidative stress, and it is a major cause of human lens epithelial (HLE) cell apoptosis,which leads to cataract formation[7]. Hydrogen peroxide(H2O2) is the dominant intracellular reactive oxygen species to which the area around the lens is exposed[8]. In addition, H2O2treatment can induce HLE cell apoptosis[9].
Parthenolide is a bioactive component of feverfew (Tanacetum parthenium), and based on accumulating evidence, parthenolide has diverse functions in cell apoptosis and cell death[10-13], such as preventing or eliciting oxidative stress-induced apoptosis in different cell lines[14-15]. Parthenolide modulates apoptosis by regulating some signaling pathways, such as the nuclear factorκB (NF-κB), mitogen-activated protein kinases (MAPKs)and phosphoinositide 3-kinase (PI3K)/Akt pathways[12,16-17].Parthenolide impacts the treatment of inflammation by inhibiting NF-κB activation[18-20]. Additionally, NF-κB signaling is involved in H2O2-induced damage to HLE cells[21]. As MAPKs and extracellular signal-regulated kinases, ERK1/2 contribute to the increased proliferation observed in many tumor cells,and its inhibitors have become potential anticancer agents[22].Additionally, PI3K/Akt signaling is associated with the proliferation and migration of HLE cells, which is defined as cataractogenesis[23]. Moreover, it has been reported that parthenolide inhibits the expression of caspase-3 and caspase-9 in HLE cells to protect against H2O2-induced apoptosis[15].However, the pathways inhibiting oxidative damage in HLE cells are still unclear.
In this paper, parthenolide inhibited H2O2-induced apoptosis in HLE cells. Moreover, several molecules were found to potentially mediate this inhibitory effect, including the phosphorylated forms of NF-κB, ERK1/2 and Akt proteins;however, the total levels of these proteins did not change.Parthenolide inhibited apoptosis by decreasing the activating phosphorylation of NF-κB, MAPKs/ERK and Akt.
MATERIALS AND METHODS
Cell Culture The adherent HLE cell line (SRA 01/04), which was obtained from the Riken cell bank, was cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 100 units of penicillin, 100 µg of streptomycin, 2 mmol/L glutamine and 1% non-essential amino acids in a 37℃ incubator with a 5% CO2atmosphere. As a model to study cataract formation, an HLE cell line (SRA 01/04)was immortalized by infection with adenovirus 12-SV40, and differentiation was inhibited. For the chemical treatments, cells were uniformly distributed on 6-well or 96-well plates 24h before the experiments and cultured until the cell confluence reached 70%-80%. The culture medium was replaced, and then cells were treated with 50 µmol/L parthenolide or 200 µmol/L H2O2for an additional 24h or pretreated with different concentrations of parthenolide for 1h before the 200 µmol/L H2O2treatment.
Chemicals and Reagents H2O2, which was purchased from Sigma (Sigma, St. Louis, MO, USA), prepared immediately before use and diluted with PBS (137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 2 mmol/L KH2PO4, and pH 7.2-7.4)to 200 µmol/L. Parthenolide (Sigma, St. Louis, MO, USA)was dissolved in dimethylsulfoxide (DMSO) (Shenggong,Shanghai, China) to 100 mmol/L and stored at -20℃. Before use, parthenolide was diluted to final concentrations of 6.25,12.5, 25 and 50 µmol/L.
Cell Viability Assays Cell viability was examined using a Promega Cell Titer 96 aqueous cell viability assay (MTS) kit.Cells were plated into 96-well plates, allowed to attach and grow overnight until the cell density reached 70%-80%, and then treated with the chemicals mentioned above for 24h.The assay was performed according to the manufacturer’s instructions. First, 20 μL of an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt]/phenazine ethosulfate (PES) mixture were added to each well. Then, cells were incubated at 37℃ for 1h,and the quantity of formazan crystals produced from the MTS compound was measured by monitoring the absorbance at 490 nm,which is directly proportional to the number of living cells in culture[24]. Background absorbance from the readings from blank controls was subtracted.
Flow Cytometry Analysis Twenty-four hours after the H2O2treatment, the apoptosis of cells treated with or without parthenolide was monitored. Annexin V binding and propidium iodine staining were determined by flow cytometry.Cells were washed with PBS twice, and double stained with the FITC-conjugated Annexin V protein and propidium iodine for 20min. Flow cytometry was performed using a 488 nm laser coupled to a cell sorter (FACSC alibur; BD Biosciences,San Jose, CA, USA). Cells stained with the Annexin V protein alone were considered apoptotic cells.
Western Blot Analysis Levels of caspase-3, caspase-9,ERK1/2, p-ERK1/2, NF-κB, p-NF-κB, Akt, and p-Akt were detected using Western blotting. Antibodies used in the Western blot analysis included anti-caspase-3 and anticaspase-9 polyclonal antibodies, as well as anti-ERK1/2,anti-p-ERK1/2, anti-NF-κB, anti-p-NF-κB, anti-Akt from Chemicon (CA, USA), and an anti-p-Akt antibody from Cell Signaling Technology (Beverly, MA, USA). Secondary antibodies were purchased from Pierce. The lysis buffer contained 50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl,1% NP-40, 0.5% Triton X-100 and protease inhibitors. Protein samples were mixed with 5×SDS sample buffer, and boiled at 95℃ for 5min. Forty microliters of proteins were separated on an 8%-12% polyacrylamide gel and then blotted on a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA).The blots were blocked in blocking buffer (PBS containing 5% BSA and 0.1% Tween) for 1h at room temperature and incubated with antibodies (1:1000 in blocking buffer)overnight at 4℃. Afterwards, the blots were washed three times with washing buffer (PBS containing 0.1% Tween-20),incubated with secondary antibodies (1:10000 in blocking buffer) for 1h at room temperature, washed three times with washing buffer, and then signals were amplified using the ECL chemiluminescence detection system (Amersham).Experiments were performed at least three times.
Statistical Analysis All the experiments were performed more than three times for confirmation. Data are presented as the mean±SEM. One-way analysis of variance was used to analyze statistical significance. P values less than 0.05 were considered statistically significant differences.
RESULTS
Parthenolide Inhibited Morphological Changes Characteristic of Apoptotic Human Lens Epithelial Cells HLE cells were treated with different concentrations (50, 100, or 200 μmol/L) of H2O2to induce apoptosis. Only the 200 μmol/L H2O2treatment caused a series of morphological changes, such as cytoplasmic condensation and increased intercellular gaps (Figure 1),which are characteristic features of cell apoptosis. Meanwhile,the effects of different concentrations of parthenolide (6.25,12.5, 25 and 50 μmol/L) or a single 50 μmol/L parthenolide treatment on HLE cells were assessed. However, following treatment with higher concentrations of parthenolide (50 μmol/L)(Figure 1C), fewer HLE cells underwent H2O2-induced apoptosis and cell viability increased, as examined using the MTS assay (Figure 1H). Thus, parthenolide exerts a dosedependent anti-apoptotic effect.
Parthenolide Protected Apoptotic Human Lens Epithelial Cells from Apoptosis Caused by H2O2We used the MTSassay to assess cell viability and explore the effect on parthenolide on oxidative stress in HLE cells in vitro 24, 48,and 72h after treatment with H2O2and different concentrations of parthenolide (6.25, 12.5, 25 or 50 μmol/L). The H2O2treatment significantly decreased cell viability, particularly after 72h of treatment (Figure 2A). At 24 h, cell viability was similar to the half maximal inhibitory concentration (IC50)of parthenolide. When cells were incubated with parthenolide alone, 50 µmol/L was the optimal parthenolide concentration,inducing the greatest statistically significant increase in cell viability (Figure 2B). Moreover, cell death was blocked, even by the lowest parthenolide concentration (Figure 2A).
Additionally, Annexin V-FITC and PI staining was used to detect the apoptosis rate in response to the H2O2treatment.A significant increase in the apoptosis rate was observed in cells treated with 200 μmol/L H2O2compared to the control(Figure 3). Parthenolide reduced the percentage of apoptotic cells in a dose-dependent manner (Figure 3).
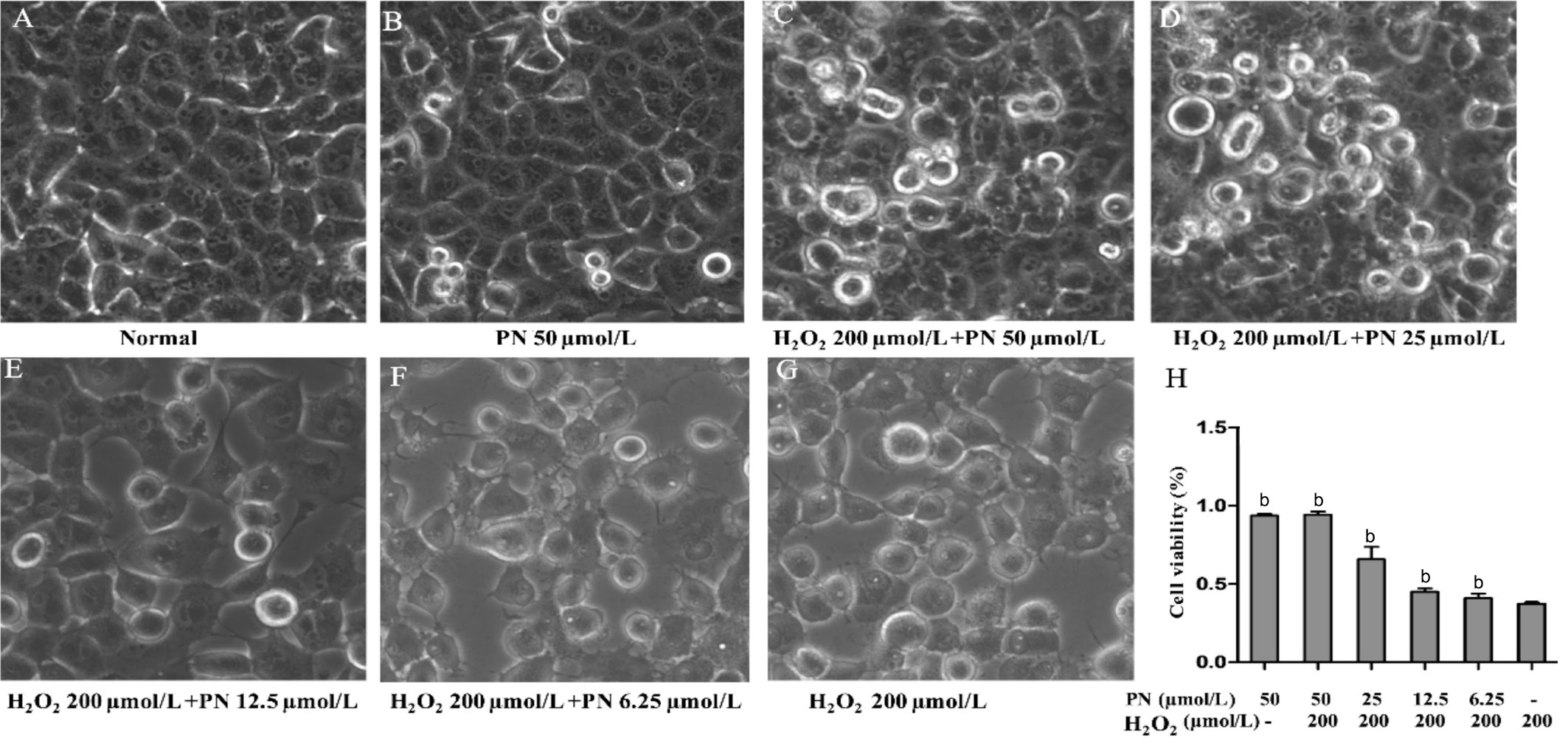
Figure 1 Parthenolide inhibited the morphological changes observed in HLE cells treated with H2O2(200 µmol/L) for 24h The ratio of cells with an abnormal morphology decreased following treatment with the higher concentrations of parthenolide A: HLE cells that were not treated with H2O2or parthenolide (Normal); B: HLE cells treated with 50 µmol/L parthenolide (PN) alone; C-F: HLE cells treated with 200µmol/L H2O2and 50, 25, 12.5, or 6.25 µmol/L parthenolide, respectively; G: HLE cells treated with 200 µmol/L H2O2alone. Magnification: 20×.H: The higher concentrations of parthenolide increased HLE cell viability.bP<0.01 compared with the H2O2treatment alone.
Parthenolide Inhibited the H2O2-induced Activation of ERK1/2, Akt and NF-κB Based on the evidence that parthenolide protect HLE cells from H2O2-induced apoptosis,we next considered the probable protective pathways. We examined the expression of the initiators and executioners of apoptosis[25], caspase-9 and caspase-3, during H2O2-induced apoptosis of HLE cells treated with various concentrations of parthenolide (6.25, 12.5, 25 or 50 μmol/L). The total expression of caspase-3 and caspase-9 was significantly increased in cells treated with 200 μmol/L H2O2compared with the control (Figure 4A, 4B). Moreover, treatment with increasing concentrations of parthenolide decreased caspase-3 and caspase-9 expression in a dose-dependent manner.
As parthenolide inhibited the expression of caspase-3 and caspase-9 during H2O2-induced apoptosis, we performed a further study to examine the potential molecular mechanisms involved in this effect. As the NF-κB, MAPK and Akt pathways are important for cell apoptosis, proliferation and signal transduction, we determined whether those pathways were involved in the inhibitory effects of parthenolide on H2O2-induced apoptosis in HLE cells.
Interestingly, according to the Western blot analysis, neither H2O2nor the parthenolide treatments changed the levels of the original form of these proteins, but both treatments altered the levels of the active, phosphorylated forms of the proteins(Figure 4C). Levels of phosphorylated ERK1/2, Akt and NF-κB were substantially increased compared to the normal control. The phosphorylation of these proteins represents their activation, indicating that parthenolide blocks the activation of ERK1/2, Akt and NF-κB, thereby reducing apoptosis.
DISCUSSION
Oxidative stress-induced apoptosis of HLE cells is confirmed as one of the most important causes of cataract formation, and it is induced by H2O2in vitro, which creates a good model to investigate possible therapies for cataract. Meanwhile, based on accumulating evidence, parthenolide has diverse functions in cell apoptosis[10-13]. Parthenolide inhibits H2O2-induced HLE cell apoptosis[15]. This study was performed to identify the molecular mechanisms involved in the inhibitory effect of parthenolide on H2O2-induced apoptosis in HLE cells.

Figure 2 Cell viability after treatment with H2O2and different concentrations of parthenolide A: Cells were incubated with the indicated concentrations of parthenolide and H2O2for 24, 48, or 72h, and cell viability was determined using the MTS assay. Cell viability was obviously altered after cells were treated for 24h. B: Cells were incubated with the indicated concentrations of parthenolide for 24 h. Cell viability was also determined using the MTS assay. Data are derived from three independent experiments (mean ±SEM).aP<0.05,bP<0.01 compared with the corresponding normal control.

Figure 3 Parthenolide inhibited HLE cells from H2O2-induced apoptosis The apoptotic cells were detected by flow cytometry. A: HLE cells without H2O2or parthenolide treatment (Normal); B: HLE cells with only 200 µmol/L H2O2treatment; C-F: HLE cells with 200 µmol/L H2O2treatment and 6.25, 12.5, 25, 50 µmol/L parthenolide treatment, respectively. Data are representative of three independent experiments with similar results.
Parthenolide inhibits apoptosis and protects against oxidative stress in HLE cells. In the present study, cell morphology and cell viability were measured in HLE cells treated with both H2O2and parthenolide. Cells treated with H2O2alone showed characteristic apoptotic features, such as cytoplasmic condensation and increased intercellular gaps. Parthenolide was added at concentrations ranging from 6.25-50 μmol/L.Even the lowest concentration of parthenolide, 6.25 μmol/L,increased cell viability compared to the H2O2group. The morphology and viability of HLE cells treated with 50 μmol/L parthenolide were similar to those of the normal control group.Moreover, this herbal constituent inhibited the expression of the caspase-3 and caspase-9 proteins in HLE cells treated with 200 µmol/L H2O2. Apoptosis is a series of proteolytic cascades in which initiator caspases mediate the activation of effector caspases[26-27]to promote the cleavage of target proteins and the orderly demise of the cell; caspase-9 is an initiator and caspase-3 is an effector[28-29]. These proteins are considered the core of the apoptotic process. Once both caspases are activated,the inevitable programmed cell death occurs. When cells were incubated with H2O2for 24h, the number of apoptotic cells was substantially increased, and the total caspase level was concomitantly changed. Thus, H2O2-induced apoptosis were blocked by parthenolide. However, parthenolide, which targets selenocysteine-containing antioxidant enzymes, has been reported to induce reactive oxygen species accumulation and apoptosis of HeLa cells[14]. Thus, the mechanism underlying the complicated regulatory functions of parthenolide in HLE cell apoptosis should be investigated in further detail.

Figure 4 Parthenolide suppressed caspase-3 and caspase-9 expression and inhibited the activation of ERK1/2, Akt and NF-κB induced by the H2O2treatment in HLE cells A: The expression of caspase-3, caspase-9 and β-actin in treated HLE cells were detected by Western blotting; B: Relative protein levels and the ratios were quantified by densitometry using Image J software,aP<0.05,bP<0.01 compared with the expression of caspase-3 in the normal control;cP<0.05,dP<0.01 compared with the expression of caspase-9 in the normal control; C: The phosphorylation of ERK1/2, Akt, and NF-κB was significantly decreased by the parthenolide treatment in a dose-dependent manner. Data are representative of three independent experiments with similar results (A and C) or were obtained from three independent experiments (B; mean ± SEM).
As the NF-κB, MAPK and Akt pathways are vital for cell apoptosis and signal transduction, we wondered whether the NF-κB, MAPK and Akt pathways were involved in the inhibitory effects of parthenolide on H2O2-induced apoptosis in HLE cells. Therefore, we examined the levels of total and phosphorylated NF-κB, ERK1/2 and Akt, which correlated with NF-κB, ERK1/2 and Akt activation[30]. Surprisingly,the results revealed a dose-dependent change in the levels of those molecules in H2O2-treated HLE cells after exposure to parthenolide. Levels of phosphorylated NF-κB, ERK1/2 and Akt were reduced, but the total levels of these proteins were unaffected. Thus, parthenolide induced a dose-dependent decrease in NF-κB, ERK1/2 and Akt activation.
In conclusion, the herbal constituent parthenolide inhibits H2O2-induced apoptosis in HLE cells, suggesting a protective effect on cataractogenesis. The mechanisms underlying the complicated regulatory effects of parthenolideon HLE cell apoptosis include the inhibition of various cell signaling pathways, including the NF-κB, ERK1/2 and Akt pathways.Therefore, parthenolide could be used as a potential treatment for cataracts in future clinical trials.
ACKNOWLEDGEMENTS
Authors' contributions: Shentu XC analyzed data and wrote the manuscript; Ping XY performed the experiments; Cheng YL performed the experiments; Zhang X performed the experiments; Tang YL performed the experiments; Tang XJ analyzed data and wrote the manuscript.
Foundations: Supported by the National Natural Science Foundation of China (No.81371000; No.81670834); the Natural Science Foundation of Zhejiang Province (No.LY17H090004); the Zhejiang Traditional Chinese Medicine Project (No.2013ZA080); the Fundamental Research Funds for the Central Universities (No.2017FZA7002).
Conflicts of Interest: Shentu XC, None; Ping XY, None;Cheng YL, None; Zhang X, None; Tang YL, None; Tang XJ, None.
REFERENCES
1 Huang S, Zheng Y, Foster PJ, Huang W, He M, Liwan Eye Study.Prevalence and causes of visual impairment in Chinese adults in urban southern China. Arch Ophthalmol 2009;127(10):1362-1367.
2 Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R,Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82(11):844-851.
3 Chen H, Lin H, Chen W, Zhang B, Xiang W, Li J, Chen W,Liu Y. Femtosecond laser combined with non-chopping rotation phacoemulsification technique for soft-nucleus cataract surgery: a prospective study. Sci Rep 2016;6:18684.
4 Chen X, Chen K, He J, Yao K. Comparing the curative effects between femtosecond laser-assisted cataract surgery and conventional phacoemulsification surgery: a meta-analysis. PLoS One 2016;11(3):e0152088.
5 Taravella MJ, Meghpara B, Frank G, Gensheimer W, Davidson R.Femtosecond laser-assisted cataract surgery in complex cases. J Cataract Refract Surg 2016;42(6):813-816.
6 Alió JL, Piñero DP, Plaza-Puche AB, Amparo F, Jiménez R, Rodríguez-Prats JL, Javaloy J. Visual and optical performance with two different diffractive multifocal intraocular lenses compared to a monofocal lens. J Refract Surg 2011;27(8):570-581.
7 Li L, Duker JS, Yoshida Y, Niki E, Rasmussen H, Russell RM, Yeum KJ. Oxidative stress and antioxidant status in older adults with early cataract. Eye (Lond) 2009;23(6):1464-1468.
8 Green K. Free radicals and aging of anterior segment tissues of the eye:a hypothesis. Ophthalmic Res 1995;27(Suppl 1):143-149.
9 Zhou YF, Guo B, Ye MJ, Liao RF, Li SL. Protective effect of rutin against H2O2-induced oxidative stress and apoptosis in human lens epithelial cells. Curr Eye Res 2016;41(7):933-942.
10 Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stressmediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem 2002;277(41):38954-38964.
11 Li-Weber M, Giaisi M, Baumann S, Treiber MK, Krammer PH. The anti-inflammatory sesquiterpene lactone parthenolide suppresses CD95-mediated activation-induced-cell-death in T-cells. Cell Death Differ 2002;9(11):1256-1265.
12 Pozarowski P, Halicka DH, Darzynkiewicz Z. Cell cycle effects and caspase-dependent and independent death of HL-60 and Jurkat cells treated with the inhibitor of NF-kappaB parthenolide. Cell Cycle 2003;2(4):377-383.
13 Li-Weber M, Palfi K, Giaisi M, Krammer PH. Dual role of the antiinflammatory sesquiterpene lactone: regulation of life and death by parthenolide. Cell Death Differ 2005;12(4):408-409.
14 Duan D, Zhang J, Yao J, Liu Y, Fang J. Targeting thioredoxin reductase by parthenolide contributes to inducing apoptosis of hela cells. J Biol Chem 2016;291(19):10021-10031.
15 Yao H, Tang X, Shao X, Feng L, Wu N, Yao K. Parthenolide protects human lens epithelial cells from oxidative stress-induced apoptosis via inhibition of activation of caspase-3 and caspase-9. Cell Res 2007;17(6):565-571.
16 Lin M, Bi H, Yan Y, Huang W, Zhang G, Zhang G, Tang S, Liu Y,Zhang L, Ma J, Zhang J. Parthenolide suppresses non-small cell lung cancer GLC-82 cells growth via B-Raf/MAPK/Erk pathway. Oncotarget 2017;8(14):23436-23447.
17 Nakabayashi H, Shimizu K. Involvement of Akt/NF-κB pathway in antitumor effects of parthenolide on glioblastoma cells in vitro and in vivo. BMC Cancer 2012;12:453.
18 Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The antiinflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol 2001;8(8):759-766.
19 García-Piñeres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, Merfort I. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem 2001;276(43):39713-39720.
20 Hehner SP, Hofmann TG, Dröge W, Schmitz ML. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J Immunol 1999;163(10):5617-5623.
21 Jin XH, Ohgami K, Shiratori K, Koyama Y, Yoshida K, Kase S, Ohno S. Inhibition of nuclear factor-kappa B activation attenuates hydrogen peroxide-induced cytotoxicity in human lens epithelial cells. Br J Ophthalmol 2007;91(3):369-371.
22 Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002;298(5600):1911-1912.
23 Kayastha F, Madhu H, Vasavada A, Johar K. Andrographolide reduces proliferation and migration of lens epithelial cells by modulating PI3K/Akt pathway. Exp Eye Res 2014;128:23-26.
24 Schumacher C, Cioffi CL, Sharif H, Haston W, Monia BP, Wennogle L. Exposure of human vascular smooth muscle cells to Raf-1 antisense oligodeoxynucleotides: cellular responses and pharmacodynamic implications. Mol Pharmacol 1998;53(1):97-104.
25 Brentnall M, Rodriguez-Menocal L, De Guevara R, Cepero E, Boise L H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Bio 2013;14(1):32.
26 Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem 1997;64(1):33-42.
27 Nuñez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene 1998;17(25):3237-3245.
28 Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome C and dATP-dependent formation of apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997;91(4):479-489.
29 Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM.Distinct caspase cascades are initiated in receptor-mediated and chemicalinduced apoptosis. J Biol Chem 1999;274(8):5053-5060.
30 Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB.NF-kappaB activation by tumour necrosis factor requires the Akt serinethreonine kinase. Nature 1999;401(6748):82-85.