INTRODUCTION
Posterior capsule opacification (PCO) is one of the most common complications after cataract surgery which could cause serious visual decrease[1]. It is well known that PCO usually result from the pathological progression of postoperative residual human lens epithelial cells (LECs),which involves in excessive LECs proliferation, migration and epithelial-mesenchymal transition (EMT)[2-4]. EMT is a transdifferentiation process by which epithelial cells lose their polarity that is marked by the loss of E-cadherin expression and acquired expression of mesenchymal components[5].The activation of EMT is triggered by various signaling molecules and transforming growth factor β (TGFβ) is one of the predominant inducers[6]. TGFβ is a multi-functional cytokine that plays a key role in embryogenesis and tissue homeostasis[7-8]. Recent studies have demonstrated the important role of TGFβ pathway in cell growth, differentiation,apoptosis, migration and EMT[8-11].
Epidermal growth factor (EGF)-like repeat and discoidin I-like domain-containing protein 3 (EDIL3), also known as developmentally regulated endothelial cell locus 1 (DEL-1), is an extracellular matrix protein, which contains 3 EGF repeat domains (E1-E3) and 2 discoidin domains (C1 and C2)[12-14].EDIL3 is initially described as an embryonic endothelial cell protein which is not expressed after birth[12]. However, recent investigations have shown that EDIL3 was overexpressed in multiple types of cancer[14-18]. EDIL3 is significantly unregulated in hepatocellular carcinoma (HCC) and participate in the regulation of EMT in HCC[16]. Therefore, it is meaningful to investigate whether EDIL3 could affect EMT in human LECs,which might reveal a novel regulator in PCO.
MATERIALS AND METHODS
Materials DMEM cell culture medium, fetal bovine serum(FBS), TRIzol®reagent, Opti-MEM™ medium, FITC-conjugated rabbit secondary antibody and horseradish peroxidase (HRP)-conjugated rabbit and mouse secondary antibodies were purchased from Thermo Fisher Scientific, Inc.(Waltham, MA, USA). X-tremeGENE™ small interfering RNA (siRNA) transfection reagent was purchased from Roche Diagnostics (Shanghai) Co., Ltd. (Shanghai, China).PrimeScript™ RT Reagent kit and SYBR®Premix Ex Taq™ were purchased from Takara Biotechnology Co., Ltd.(Dalian, China). Antibodies against EDIL3, TGFβ1, TGFβ2,α-smooth muscle actin (α-SMA), vimentin, E-cadherin and GAPDH were purchased from Proteintech (Wuhan, China).Antibodies against exracellular signal regulated kinase (ERK),phosphorylated (p)-ERK, Smad2, p-Smad2, Smad3 and p-Smad3 were purchased from Abcam (Shanghai, China).Cell-light EdU DNA cell proliferation kit was obtained from RiboBio (Guangzhou, China). Radioimmunoprecipitation assay (RIPA) lysis buffer, loading buffer and BeyoColor protein standard were purchased from Beyotime Institute of Biotechnology (Haimen, China). Immobilon Western Chemiluminescent HRP substrate was purchased from Merck KGaA (Darmstadt, Germany). Transwell inserts and cell culture plates were purchased from Corning Incorporated(Corning, NY, USA). All other reagents were analytical grade.Cell Culture Human LECs (SRA01/04) were purchased from Weitan biotechnology (Shanghai, China) and were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. The cells were maintained in a 5% CO2incubator with 100% humidity at a temperature of 37℃.
Cell Transfection and RNA Interference About 5000 LECs were seeded in 24-well plate and were transfected once they reached 30%-40% confluence. Totally 3 μL X-tremeGENE™siRNA transfection reagent and 200 ng EDIL3 siRNA were diluted to a ratio of 1:50 in Opti-MEM and incubated for 5min separately. Subsequently, the Opti-MEM containing X-tremeGENE™ siRNA transfection reagent was added to the Opti-MEM containing siRNA at a 1:1 ratio. Finally,X-tremeGENE-siRNA complexes were added to the cells and incubated for the 48h. Control group were incubated only with the transfection reagent. siRNAs targeting EDIL3 (EDIL3 siRNA) and scramble siRNAs were chemically synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The siRNA sequences were as follows: EDIL3 siRNA (human primers)forward 5’-GGUGAUAUUUGUGAUCCCATT-3’, reverse 3’-UGGGAUCACAAAUAUCACCTT-5’; and scramble (human primers) forward 5’-UUCUCCGA ACGUGUCACGUdTdT-3’and reverse 3’-ACGUGACACG UUCGGAGAAdTdT-5’.siRNA transfection was performed using X-tremeGENE™siRNA transfection reagent according to the manufacturer’s protocol, and the concentration of siRNAs used was 50 nmol.Reverse Transcription-quantitative Polymerase Chain Reaction Following siRNA transfection for 48h, total RNA was extracted from LECs using TRIzol®reagent. Total RNA was reverse transcribed into cDNA using the PrimeScript™RT Reagent kit according to the manufacturer’s protocol.qPCR was performed on cDNA using SYBR®Premix Ex Taq™ on a StepOnePlus™ Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling conditions were as follows: initial denaturation at 95℃for 3min, followed by 40 cycles at 95℃ for 5s and at 60℃ for 30s. The comparative Cq method was used for PCR quantification. Finally, the results were expressed as the mean relative value compared with control samples.The following primers were used in the present study:EDIL3, forward 5’-TACAGCAATGATGGAGAACA-3’,reverse 5’-TACCAGGACCAAGGAAGG-3’; GAPDH,forward 5’-TGGGCTACACTGAGCACCAG-3’, reverse 5’-AAGTGGTCGTTGAGGGCAAT-3’.
Cell Morphology EDIL3 transfected LECs morphology assay were carried out on a 24-well plate. The grown cultures were assessed by phase-contrast microscopy using a digital microscope system (IX81, Olympus). The changes of the cobblestone morphology LECs were observed after transfection and an incubation period of 48h.
Cell Proliferation Assay Cell proliferation was determined by 5-ethynyl-2’-deoxyuridine (EdU) assay as previous described[19]. In brief, 3×103cells were seeded in 96-well plates and incubated overnight. Then, the cells were treated with EDIL3 siRNA or controls for 48h. We then dumped the medium and added 100 μL fresh medium containing EdU(100 μmol/L). After incubation for 1.5h, the cells were stained as the following protocol: discard the EdU medium and fix the cells with 4% paraformaldehyde for 30min. Then, wash with glycine for 5min, followed by twice washes with 0.2%Trion X-100 of 10min each time. Then, the cells were stained with Apollo fluorescent azide for 30min, followed by three time washes of 0.2% Trion X-100. Furthermore, the cells were stained with Hoechst for 15min at room temperature and washed with phosphate buffered saline (PBS) for two times,and add 100 μL PBS to further analysis. The images were taken and analyzed using a digital microscope system (IX81, Olympus).Cell Migration Assay LECs were initially transfected with EDIL3 targeting or scramble siRNAs and incubated for 48h.Subsequently, 2×104cells were harvested and seeded in the upper chambers of Transwell inserts (pore size, 8 μm) in 0.5%FBS-containing medium. The lower chambers were filled with DMEM supplemented with 1% FBS as a chemoattractant.Following incubation at 37℃ for 12h, the cells on the upper membranes were removed with a cotton swab, and cells that had migrated to the lower membranes were fixed with 4%paraformaldehyde for 30min and stained with 0.1% crystal violet for 20min at room temperature. Migrated cells were observed under an IX81 inverted microscope (Olympus Corporation, Tokyo, Japan) and the data was analyzed using Image J software (1.6.0_24; National Institutes of Health,Bethesda, MD, USA).
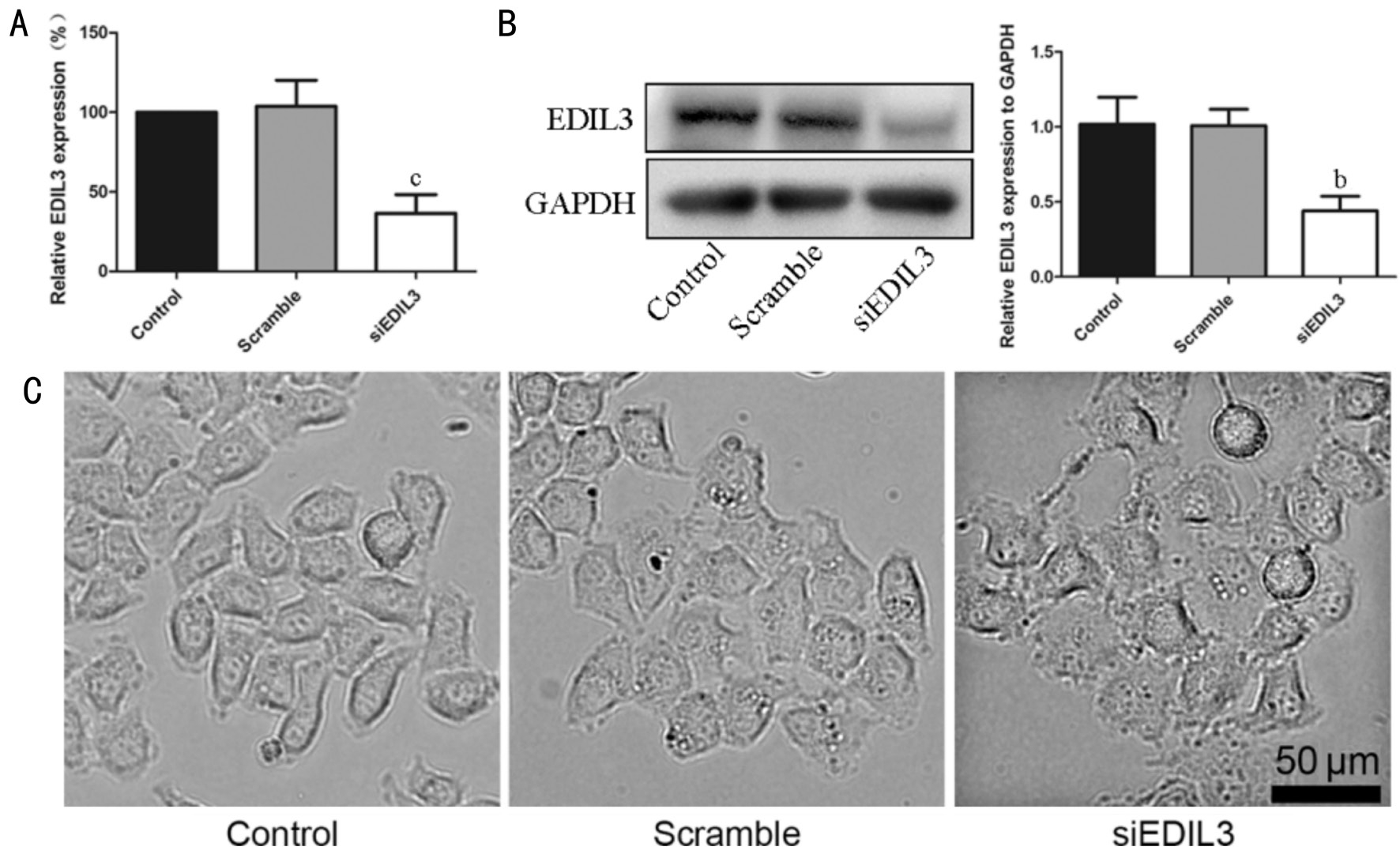
Figure 1 Silencing EDIL3 expression using siRNA alters LECs morphology A: The mRNA of EDIL3 was assessed using RT-qPCR; B: The protein of EDIL3 was detected using Western blotting; C: Morphology changes of LECs were examined using microscopy (scale bar 50 μm).bP<0.01,cP<0.001.
Confocal Microscope Analysis The E-cadherin and vimentin were stained with relevant antibodies respectively. Briefly,the cells were seeded on cover glasses, transfected with EDIL3 siRNA for 48h, washed with PBS and fixed with 4%paraformaldehyde for 20min. The cells were permeabilized with 0.1% Triton X-100 and blocked with 5% bovine serum albumin (BSA) for 1h at room temperature. After two washes with PBS, the cells were incubated with E-cadherin and Vimentin antibodies for 2h at room temperature. Then, the cells were stained with secondary fluorescence antibodies for half an hour at room temperature. Finally, the cells were stained with DAPI for 20min at room temperature. The cells were then analyzed with a Zeiss LSM-710 confocal microscope.
Western Blot Analysis Following siRNA transfection for 48h, LECs were lysed using RIPA lysis buffer containing 50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl,1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS and proteinase inhibitor cocktail. The samples were subsequently quantified using a bicinchoninic acid protein quantification kit according to the manufacturer’s protocol (Beyotime Institute of Biotechnology). Extracted protein samples(about 20 µg) were separated by 10% SDS-PAGE and transferred onto polyvinylidenedifluoride membranes.Membranes were blocked with 5% non-fat milk for 2h at room temperature. Membranes were then incubated with the relevant primary antibodies at 4℃ overnight. Then, the samples were washed three times using TBS-Tween (0.05%)and incubated with HRP-conjugated secondary antibodies for 2h at room temperature. Protein bands were visualized by enhanced chemiluminescence using Immobilon Western Chemiluminescent HRP substrate. GAPDH was used as the internal control for equal loading.
Statistical Analysis Statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Data are expressed as the mean±standard deviation (SD) of at least three independent experiments. The statistical significance of the differences between groups was assessed using one-way analysis of variance followed by a Tukey’s multiple comparison test. P<0.05 was considered a statistically significant difference.
RESULTS
Silencing EDIL3 Expression Affects Lens Epithelial Cells Morphology siRNA was used to silence the expression of EDIL3 in LECs to investigate the putative effect of EDIL3 on cell morphology. RT-qPCR and Western blot analysis showed that the mRNA and protein expression of EDIL3 was successfully suppressed (Figure 1A, 1B). Meanwhile, the cells treated with EDIL3 siRNA presented round phenotype, while the cells in control group elongated and presented tapered ends (Figure 1C). The result indicated that EDIL3 siRNA significantly suppressed the expression of EDIL3, and could alter cell morphology apparently.
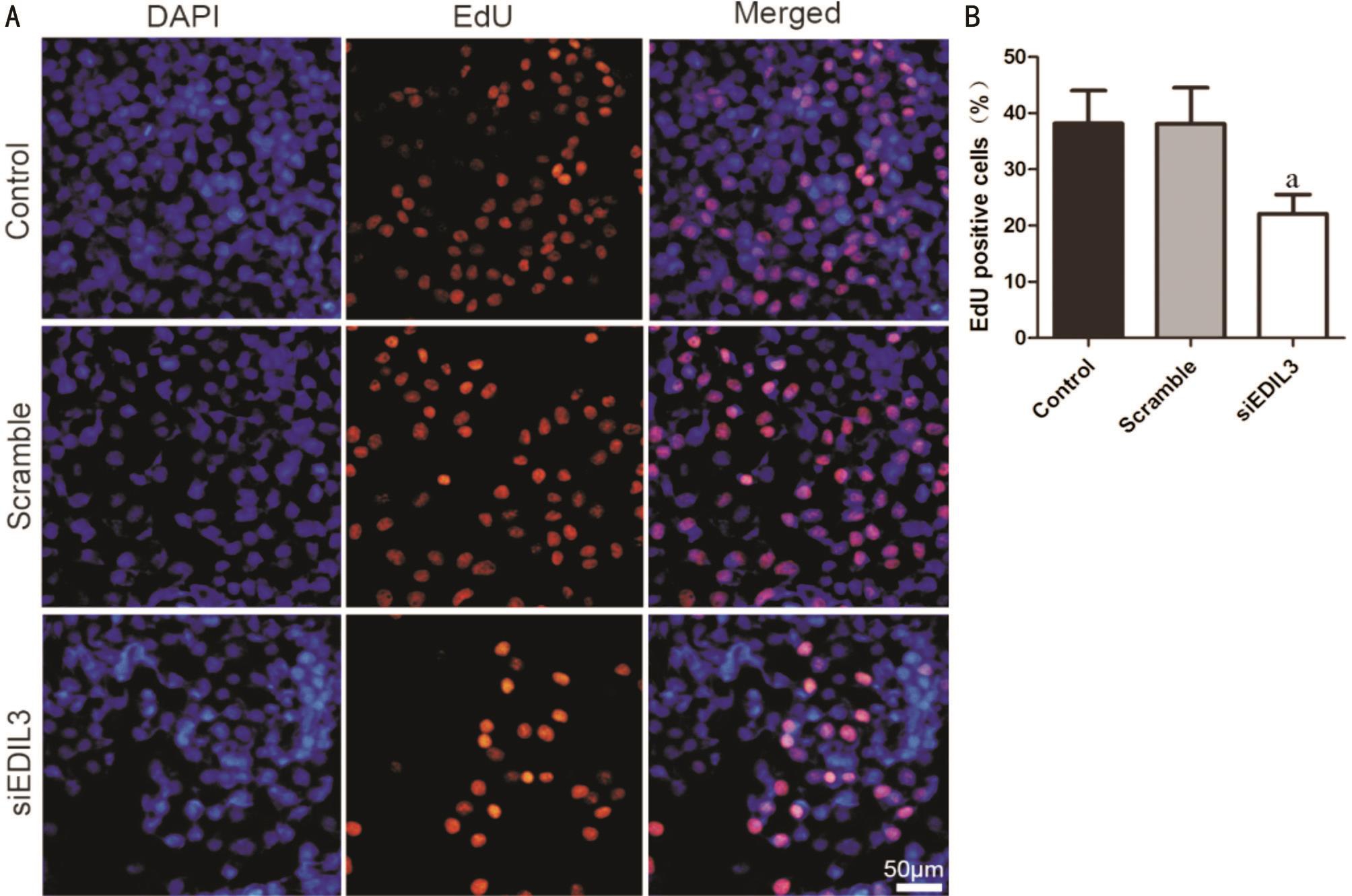
Figure 2 Silencing EDIL3 expression inhibits LECs proliferation A: LECs were transfected with EDIL3 siRNA for 48h and cell proliferation was examined using EdU assay. Representative photomicrographs of EdU staining cells in control, scramble and EDIL3 siRNA groups (scale bar 50 μm); B: Quantitative results of the percent of EdU positive cells. Data are shown as mean±SD.aP<0.05.
Silencing EDIL3 Expression Inhibits Lens Epithelial Cells Proliferation The proliferation of LECs treated with EDIL3 siRNA was evaluated using EdU cell proliferation assay. EdU is a nucleoside analog of thymidine which could be incorporated into DNA during DNA synthesis process. Thus,the ratio of EdU positive cells could be used to determine the proliferate rate of cells. As shown in Figure 2A, a significant lower ratio of red nuclei (EdU positive cell) was observed compared with the control groups. Quantitative result showed that EDIL3 depletion could significantly decrease EdU positive cells (Figure 2B). These results demonstrated that EDIL3 depletion could inhibit LECs proliferation.
Silencing EDIL3 Expression Inhibits Lens Epithelial Cells Migration LECs migration plays a vital role in PCO[20]. The effects of EDIL3 on LECs migration were measured using a Transwell migration assay. The data showed that following EDIL3 depletion, the migratory capability of LECs was significantly impaired (Figure 3A-3C). Quantitative result showed that EDIL3 depletion could suppress the migration of LECs by about 60% compared with the control and scramble groups (Figure 3D).
Silencing EDIL3 Expression Inhibits Epithelialmesenchymal Transition of Lens Epithelial Cells EMT is a transdifferentiation process that is marked by the loss of E-cadherin expression and acquired expression of mesenchymal components as vimentin and α-SMA[5]. To investigate whether EDIL3 depletion interfered with EMT progression, the markers of EMT were analyzed using confocal microscopy and Western blotting after the cells were transfected with EDIL3 siRNA for 48h. The data showed that the expression of E-cadherin was increased and the expression of vimentin was decreased in EDIL3 depletion group (Figure 4A). Furthermore, the result of Western blotting showed that EDIL3 depletion could reduce the expression of α-SMA and vimentin, while could increase the expression of E-cadherin(Figure 4B). All these findings suggested that EDIL3 silencing could inhibit the progress of EMT in LECs.
Silencing EDIL3 Expression Disrupt Transforming Growth Factor β Pathway TGFβ pathway is a key player in embryonic development, wound healing, cell proliferation,differentiation and morphogenesis[8]. TGFβ signaling is known to be mediated through Smad and non-Smad pathways[8]. Our data showed that EDIL3 depletion could inhibit the expression of TGFβ1 but had no effect on TGFβ2 (Figure 5). Furthermore,EDIL3 depletion could suppress the phosphorylation of Smad2 and Smad3 which indicated that EDIL3 siRNA could suppress the activation of TGFβ pathway via disrupting Smad mediated pathways (Figure 5). Meanwhile, EDIL3 depletion could suppress the activation of ERK, suggesting that EDIL3 siRNA could inhibit the activation of TGFβ through non-Smad pathways(Figure 5). All the data indicated that silencing EDIL3 could disrupt TGFβ pathway through both Smad and non-Smad pathways.
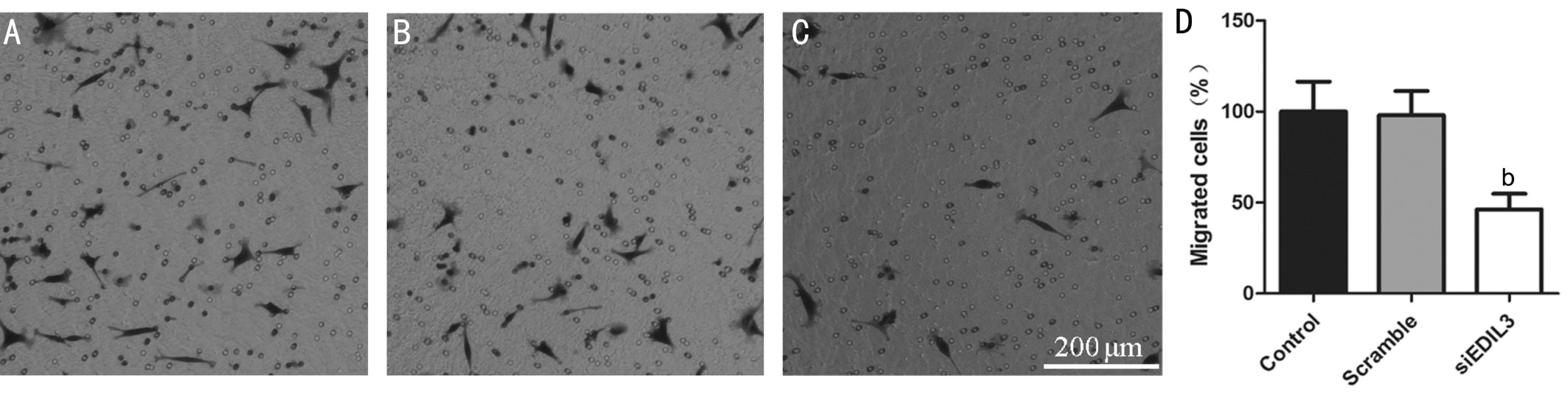
Figure 3 Silencing EDIL3 expression inhibits LECs migration LECs were transfected with EDIL3 siRNA for 48h and cell migration was examined using a Transwell assay. Representative photomicrographs of migrated cells in control (A), scramble (B) and EDIL3 siRNA (C) groups(scale bar 200 μm); D: Quantitative results of migrated cells. Data are shown as mean±SD.bP<0.01.
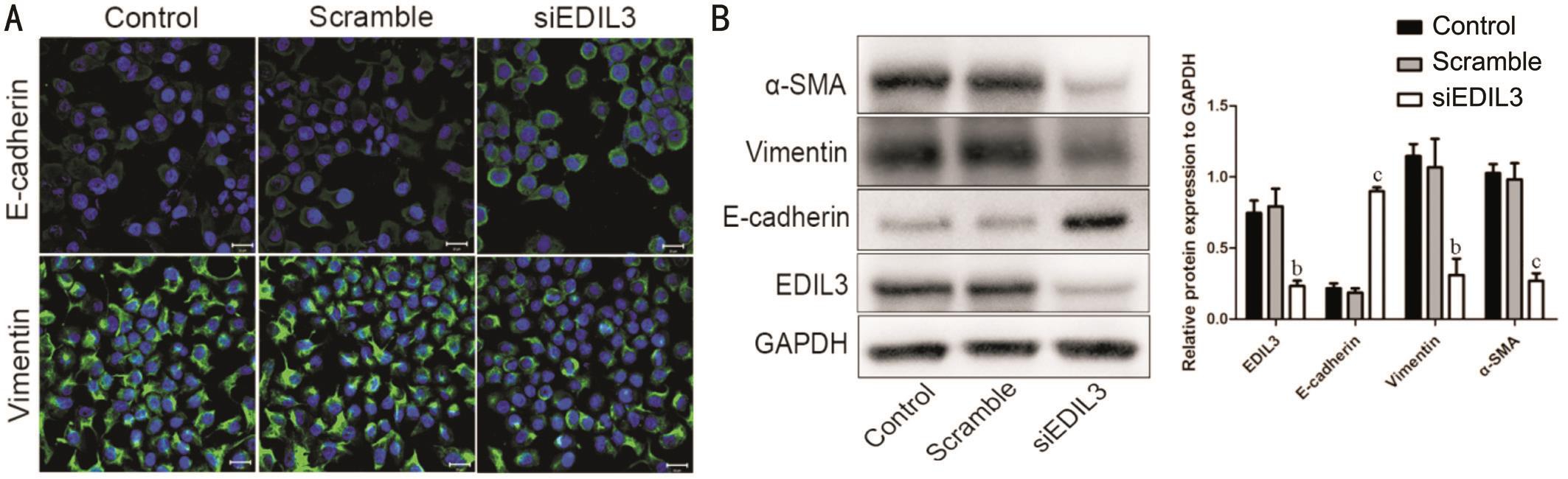
Figure 4 Silencing EDIL3 expression inhibits EMT of LECs A: Representative images of cells stained with E-cadherin and vimentin in control, scramble and EDIL3 siRNA groups (scale bar 20 μm); B: The EMT markers were detected using Western blotting. Data are shown as mean±SD.bP<0.01,cP<0.001.

Figure 5 Silencing EDIL3 disrupt TGFβ signaling pathways LECs were transfected with EDIL3 siRNA for 48h. The expression of TGFβ1 was apparently suppressed. The phosphorylation of Smad2, Smad3 and ERK were inhibited significantly. Data are shown as mean±SD.aP<0.05,bP<0.01.
DISCUSSION
PCO is a common complication of cataract surgery which can impair visual acuity in 20%-40% of patients within two to five years[21]. Cataract surgery and the implanted intraocular lens could cause tissue trauma which initiates inflammatory response and finally cause PCO[22]. Although the surgeons remove most of the LECs during cataract surgery, there are still some residual cells. The proliferation, migration and transdifferentiation of these cells are the major cellular mechanism for PCO[23]. Therefore, therapeutic methods targeting these cellular behaviors could be of great importance to prevent PCO. The results of the present study suggested that EDIL3 silencing could inhibit LECs proliferation, migration and EMT, and the molecular mechanisms might be suppressed TGFβ pathway.
It is initially demonstrated that EDIL3 is expressed during early embryogenesis by endothelial cells, and its expression is down regulated in later developmental stages[12-13]. Recent studies show that EDIL3 is over-expressed in multiple types of cancer including lung cancer, bladder cancer, breast cancer and hepatocellular carcinoma[14,16-18,24]. EMT is a vital step in cancer recurrence and metastasis and it is demonstrated that EDIL3 plays an important role in cancer cell EMT[25-26]. However, the role of EDIL3 in PCO is largely unknown so far. In the present study,we showed that disruption of EDIL3 expression using siRNA was able to suppress the proliferation, migration and EMT of LECs in vitro. These data indicated that EDIL3 might participate in PCO process, where it may act as a positive regulator.
TGFβ family proteins regulate cell physiology, proliferation,growth and differentiation, which play key roles in development and diseases[8,27]. TGFβ could bind to the type II TGFβ receptor and then lead to recruitment of the type I TGFβ receptor into a heteromeric receptor complex, which enable the type II TGFβ receptor to transphosphorylate the GS domain of the type II TGFβ receptor[26]. The activated the type I TGFβ receptor could then activate Smad2 and Smad3 by phosphorylationon C-terminalserines[26]. Furthermore, TGFβ could also activate the non-Smad pathway, which includes various branches of mitogen-activated protein kinase pathways including ERK[28].To further investigate the molecular mechanisms of EDIL3 inhibited LECs proliferation and EMT, the expression of critical signaling proteins was assessed using Western blotting analysis. The results showed that EDIL3 depletion could inhibit the expression of TGFβ1, and could suppress the phosphorylation of Smad2, Smad3 and ERK. All the data suggested that EDIL3 participate in the progress of PCO through Smad and non-Smad pathways.
In conclusion, the results of the present study indicated that EDIL3 may be implicated in PCO in vitro. Silencing EDIL3 expression was able to impair the morphology of LECs and suppress the proliferative, migratory and EMT capabilities of LECs. Furthermore, EDIL3 depletion appeared to disrupt TGFβ pathway through both Smad and non-Smad pathways.These findings suggested that EDIL3 may serve a crucial role in PCO and could be a novel therapeutic target for the prevention of PCO.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81700839); Military logistics scientific research project (No.BWS12J030); Natural Science Foundation of Shanghai (No.15ZR1413200); Research Foundation for Youth of Second Military Medical University(No.2016QN13); Research Foundation for Youth of Changhai Hospital (No.CH201712).
Conflicts of Interest: Zhang R, None; Wei YH, None; Zhao CY, None; Song HY, None; Shen N, None; Cui X, None; Gao X, None; Qi ZT, None; Zhong M, None; Shen W, None.
REFERENCES
1 Duman R, Karel F, Özyol P, Ateş C. Effect of four different intraocular lenses on posterior capsule opacification. Int J Ophthalmol 2015;8(1):118-121.
2 Wormstone IM, Eldred JA. Experimental models for posterior capsule opacification research. Exp Eye Res 2016;142:2-12.
3 Chandler HL, Gervais KJ, Lutz EA, Curto EM, Matusow RB, Wilkie DA, Gemensky-Metzler AJ. Cyclosporine A prevents ex vivo PCO formation through induction of autophagy-mediated cell death. Exp Eye Res 2015;134:63-72.
4 Kubo E, Shibata S, Shibata T, Kiyokawa E, Sasaki H, Singh DP. FGF2 antagonizes aberrant TGFβ regulation of tropomyosin: role for posterior capsule opacity. J Cell Mol Med 2017;21(5):916-928.
5 Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelialmesenchymal transition. Nat Rev Mol Cell Biol 2014;15(3):178-196.
6 Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelialmesenchymal transition in cancer progression. Curr Opin Oncol 2013;25(1):76-84.
7 Fruttiger M. Development of the retinal vasculature. genesis 2007;10(2):77-88.
8 Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol 2013;2(1):47-63.
9 Busch S, Acar A, Magnusson Y, Gregersson P, Rydén L, Landberg G.TGF-beta receptor type-2 expression in cancer-associated fibroblasts regulates breast cancer cell growth and survival and is a prognostic marker in pre-menopausal breast cancer. Oncogene 2015;34(1):27-38.
10 Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol 2014;15(9):856-865.
11 Yan F, Wang Y, Wu X, Peshavariya HM, Dusting GJ, Zhang M, Jiang F. Nox4 and redox signaling mediate TGF-β-induced endothelial cell apoptosis and phenotypic switch. Cell Death Dis 2014;5(1):e1010.
12 Aoka Y, Johnson FL, Penta K, Hirata Ki K, Hidai C, Schatzman R, Varner JA, Quertermous T. The embryonic angiogenic factor Del1 accelerates tumor growth by enhancing vascular formation. Microvasc Res 2002;64(1):148-161.
13 Pfister BE, Aydelotte MB, Burkhart W, Kuettner KE, Schmid TM.Del1: a new protein in the superficial layer of articular cartilage. Biochem Biophys Res Commun 2001;286(2):268-273.
14 Beckham CJ, Olsen J, Yin PN, Wu CH, Ting HJ, Hagen FK, Scosyrev E, Messing EM, Lee YF. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol 2014;192(2):583-592.
15 Feng MX, Ma MZ, Fu Y, Li J, Wang T, Xue F, Zhang JJ, Qin WX, Gu JR, Zhang ZG, Xia Q. Elevated autocrine EDIL3 protects hepatocellular carcinoma from anoikis through RGD-mediated integrin activation. Mol Cancer 2014;13:226.
16 Xia H, Chen J, Shi M, Gao H, Sekar K, Seshachalam VP, Ooi LL, Hui KM. EDIL3 is a novel regulator of epithelial-mesenchymal transition controlling early recurrence of hepatocellular carcinoma. J Hepatol 2015;63(4):863-873.
17 Lee JE, Moon PG, Cho YE, Kim YB, Kim IS, Park H, Baek MC.Identification of EDIL3 on extracellular vesicles involved in breast cancer cell invasion. J Proteomics 2016;131:17-28.
18 Jiang SH, Wang Y, Yang JY, et al. Overexpressed EDIL3 predicts poor prognosis and promotes anchorage-independent tumor growth in human pancreatic cancer. Oncotarget 2016;7(4):4226-4240.
19 Song H, Wang W, Zhao P, Qi Z, Zhao S. Cuprous oxide nanoparticles inhibit angiogenesis via down regulation of VEGFR2 expression.Nanoscale 2014;6(6):3206-3216.
20 Bao XL, Song H, Chen Z, Tang X. Wnt3a promotes epithelialmesenchymal transition, migration, and proliferation of lens epithelial cells. Mol Vis 2012;18:1983-1990
21 Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol 2009;127(4):555-562.
22 Nibourg LM, Gelens E, Kuijer R, Hooymans JM, van Kooten TG,Koopmans SA. Prevention of posterior capsular opacification. Exp Eye Res 2015;136:100-115.
23 Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res 2009;88(2):257-269.
24 Lee SH, Kim DY, Jing F, Kim H, Yun CO, Han DJ, Choi EY. Del-1 overexpression potentiates lung cancer cell proliferation and invasion.Biochem Biophys Res Commun 2015;468(1-2):92-98.
25 Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H,Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015;527(7579):525-530.
26 Micalizzi DS, Haber DA, Maheswaran S. Cancer metastasis through the prism of epithelial-to-mesenchymal transition in circulating tumor cells. Mol Oncol 2017;11(7):770-780.
27 Budi EH, Duan D, Derynck R. Transforming growth factor-β receptors and Smads: regulatory complexity and functional versatility. Trends Cell Biol 2017;27(9):658-672.
28 Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res 2009;19(1):128-139.