INTRODUCTION
Clonidine, is an alpha2-adrenoceptors (α2-ADRs)agonist. It exerts its effects on central nervous system via presynaptic α2-ADRs to control blood pressure[1]. Also,apraclonidine, a derivative of clonidine, has been used to reduce the intraocular pressure spike after anterior segment laser surgery[2]. Additionally, recent in vivo studies have clarified the neuroprotective and anti-vascular endothelium growth factor (anti-VEGF) effects of clonidine[3-5]. In vivo and in vitro studies has been demonstrated that clonidine is neuroprotective against retinal neuronal cell apoptosis and death induced by hypoxia and ocular hypertension[5].Furthermore, it has been shown that intraperitoneal injection of clonidine increases the level of basic fibroblast growth factor (b-FGF) mRNA in the retina of rats[3]. b-FGF impede the harmful effects of N-methyl-D-Aspartate (NMDA) in the hypoxemia and protects the neuronal tissue after hypoxemic/ischemic insults[3,6]. Moreover, it has been detected that brimonidine treatment significantly attenuates retinal and choroidal neovascularization (CNV) and vitreous VEGF concentration in animal models of retinopathy of prematurity(ROP) and CNV[7].
Neuroprotection is a challenging issue in medicine. In ophthalmology, hypoxemic, ischemic and cytotoxic insults to the optic nerve and retinal tissue leads to permanent and severe visual loss in most cases, emphasizing the importance of neuroprotection. Some drugs and cytokines including erythropoietin[8-9], brimonidine[10-11], dexmedetomidine[12],glutamate antagonists[13-14], calcium channel blocker[15], and brain-derived neurotrophic factor (BDNF)[16-17]were found to exert neuroprotective effects in in vivo and in vitro models.However, none of them have been used for neuroprotection in ophthalmology. Despite similarities between the brimonidine and clonidine effects, unlike brimonidine, clonidine has an available injecting form and can be used intravitreally. The superiority of intravitreal injection includes achieving high concentration with long term activity after single injection,while the drawbacks of intravitreal route are rare. In this study,we conducted a preclinical study to determine the maximum safe dose of intravitreal clonidine (IVC). It is certain that the results of this study can be used for investigating the neuroprotective, anti-VEGF as well as anti-glaucoma effects of IVC in future studies.
MATERIALS AND METHODS
Study Design and Animals Twenty-eight female New Zealand white rabbits weighting about 1.5 kg were used.Animals were housed in plastic cages under a 12/12-h darklight cycle, with access to the food and water ad-libitum. The rabbits were divided into 4 groups (A to D), seven rabbits in each. The groups A to C received 0.1 mL IVC (American Regent, Shirley, NY, USA) corresponding to doses of 15 μg,25 μg, and 50 μg, respectively and Group D received 0.1 mL of balanced salt solution (BSS) and considered as control group.All intravitreal injections were performed in the right eyes of rabbits. Clonidine hydrochloride with concentration of 5000 µg/10 mL was used. It was diluted as necessary with BSS in sterile condition and controlled pH to produce the required concentration. The rabbits were sedated with intramuscular ketamine (10 mg/kg) (Ketamine-Rotex, Rotexmedica, Trittau,Germany)[18]. Then injections were performed under sterile conditions and direct visualization using a surgical microscope,by an expert ophthalmologist who was blind to the study. After aspiration of 0.1 mL aqueous humor, intravitreal injection of clonidine or BSS was performed from 2 mm posterior to the limbus at the superotemporal sclera, using a 30-gauge needle.Immediately and three days after the intravitreal injection, all eyes were examined by slit lamp and indirect ophthalmoscope for developing intraocular inflammation, cataract formation,and retinochoroidal damage.
All experiments were designed and conducted in accordance with the ARVO statement for use of animals in ophthalmic and vision research and the guidelines for animal research at the Ophthalmic Research Center, Shahid Beheshti Medical University, Tehran, Iran.
Electroretinography Electroretinography (ERG) examinations were carried out at the baseline, 1, 4 and 8wk after injections.Animals were dark adapted for 12h, and all the procedures were carried out under dim red illumination. The pupils were dilated with one drop of tropicamide 1% (Mydrax,Sina Darou, Tehran, Iran) and anesthesia was achieved with intramuscular injection of ketamine hydrochloride (Ketamine-Rotex, Rotexmedica, Trittau, Germany) (50 mg/kg) and xylazine hydrochloride (Xylazin, Animedic, Germany) (5 mg/kg).The animals were placed in the recording chamber on a warm platform (38℃) to keep their body temperature constant.The ERG signals were recorded using the RETI-port/scan 21 electrophysiological diagnostic systems (Roland Consult,Brandenburg, Germany). Via a contact lens, a gold wire electrode (Roland Consult, Brandenburg, Germany) covered with one drop of 2% methylcellulose gel (EyeGel, Eyeol, UK)was placed to touch the central cornea. The ground electrode was placed on pinna and the negative electrode was positioned in forehead subcutaneous tissue. The rabbits were dark adapted again for 10min and scotopic recordings were made by using scotopic flash ERG at light intensity of 3 cd·s/m2. After 10min of light adaptation, photopic cone responses was documented by use of a photopic flash ERG at light intensity of 3 cd·s/m2.For both scotopic and photopic flash ERGs, the analogue filters of the instrument were set to the frequency ranges of 0.5 to 200 Hz. ERG analysis was made on amplitude of the b-wave.It was measured from the through of the a-wave to the pick of the b-wave. The b-wave ratio (experimental eye b-wave amplitude/control eye b-wave amplitude) was calculated for each rabbit in each ERG recording session to decrease the effect of daily and individual variability and considering the control eyes in the ERG analysis[19-20]. The mean of b-wave ratio in both photopic and scotopic conditions was calculated for any dose of IVC in every session of ERG and used as an index of retinal function in the experimental eyes[21].
Histopathological and Immunohistochemical Studies Eight weeks after intravitreal injections, the rabbits were euthanized by an intravenous injection of a lethal dose of sodium pentobarbital (0.36 mg/kg)[22]. The eyes were enucleated carefully, fixed in 10% formalin and subjected to histopathological examinations. After bisecting the eyes axially into 2 calottes, tissue processing, and embedding into paraffin blocks, 5 μm tissue sections at three different tissue planes (200 μm apart) were prepared and stained with hematoxylin & eosin. Blank sections were then subjected to immunohistochemical staining for Glial Fibrillary Acidic Protein (GFAP) (Z 0334; Dako, Glostrup, Denmark) to investigate alterations of retinal Müller cells immune reactivity for GFAP. Müller cells show increased GFAP expression in retinal damaging[23]. All the stained sections were examined under light microscopy (BX41; Olympus, Tokyo, Japan)by a masked ocular pathologist (MRK) for the presence of hemorrhage, inflammation, necrosis, atrophic changes, and GFAP immunoreactivity in the retina. The GFAP results, as described previously, were scored from 0 to 5[24]. Zero score was when there was no staining. Score 1 was when staining was limited to internal limiting membrane and nerve fiber layer. Focal and diffuse staining of Müller cells involving partial length of the cells were regarded as scores 2 and 3,respectively. Score 4 was defined as focal staining of Müller cells involving full length of the cells. When there was a diffuse staining of Müller cells involving full length of the cells, it was regarded as score 5. Mean score of 2.5 or more in each group was considered significant.
Apoptosis Assay The apoptosis assay was done using the terminal deoxynucleotidyl transferase dUTP nick end labeling(TUNEL) kit according to the manufacturer’s instructions(In Situ Cell Death Detection Kit, Fluorescein; 11684795910,Roche) to detect the presence of DNA fragments of apoptotic cells in retinal sections. The paraffin sections were de-waxed by immersing in xylene for 10min, absolute alcohol for 10min, and double distilled water. Then the sections were permeabilized in the permeabilisation solution (0.1% Triton X-100, 0.1% sodium citrate) for 8min. After washing with phosphate-buffered saline (PBS) for 5min twice, the slides were placed in citrate buffer pH 6 and microwaved (high irradiation or 750 W) for 1min. Following rapid cooling in double distilled water (20℃-25℃) and PBS (20℃-25℃), the slides were immersed in Tris-HCL 0.1 mol/L pH 7.5, 3% BSA,and 20% FBS for 45min. Subsequent to washing with PBS for 5min twice, the positive control slide from a retinoblastoma case was incubated in DNase I, Tris-HCL 50 mmoL/L pH 7.5,1 mg/mL BSA for 10min (20℃-25℃). Then 50 µL TUNEL reaction mixture (50 µL enzyme solution in 450 µL label solution)was added to each slide except negative control that received 50 µL label solution. After incubation at 37℃ for 60min in the dark, the samples were washed with PBS for 5min three times.The sections were observed and images were captured using an inverted fluorescence microscope (Olympus IX71, 515-565 nm filter). The presence of apoptotic cells in the clonidinetreated cases was assessed as compared to the control group.
Table 1 B-wave amplitude ratio in different treatment groups, photopic response mean±SD
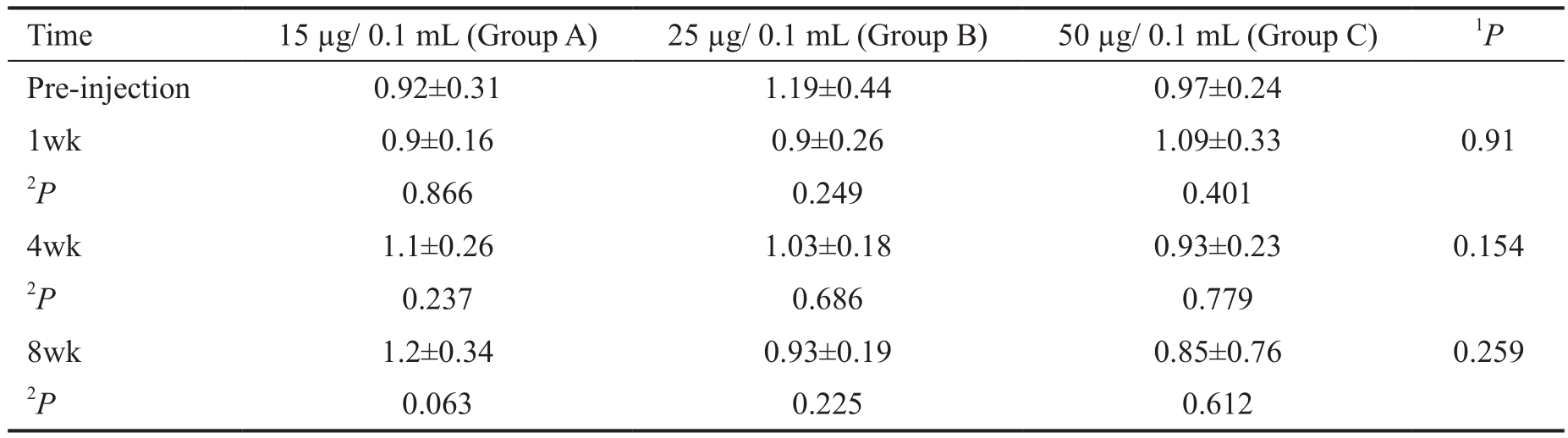
1Adjusted for the pre-injection, based on multiple linear regression. These P values show the effect of dosage increments on the b-wave amplitude decrement;2Based on Linear mixed model, adjusted for multiple comparisons by Bonferroni method.
Table 2 B-wave amplitude ratio in different treatment groups, scotopic response mean±SD
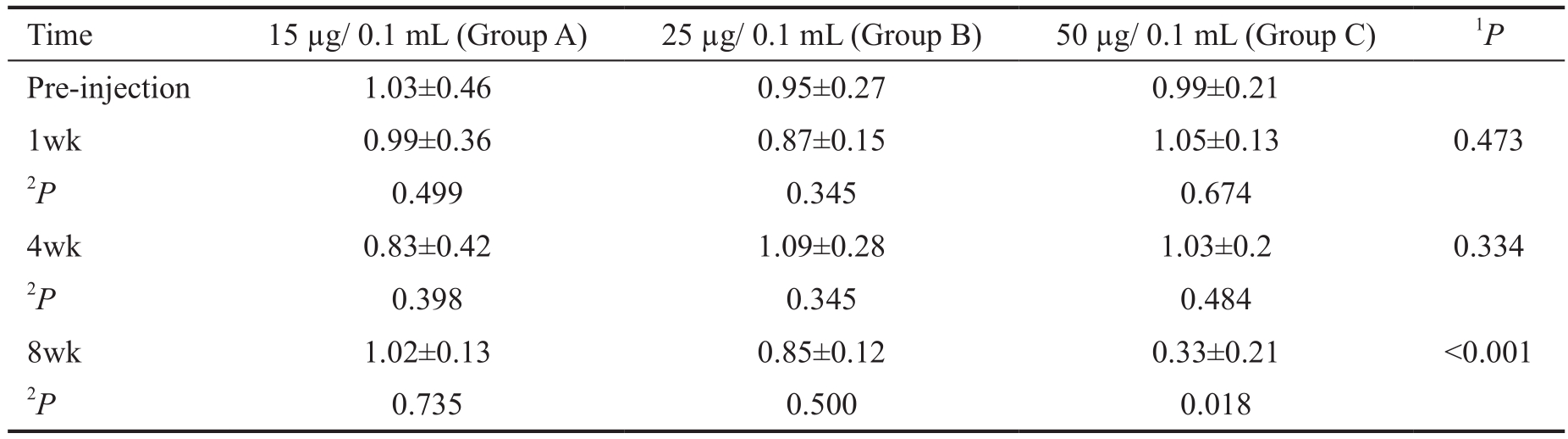
1Adjusted for the baseline, based on multiple linear regression. These P values show the effect of dosage increments on the b-wave amplitude decrement;2Based on Linear mixed model, adjusted for multiple comparisons by Bonferroni method.
Statistical Analysis All statistical analyses were performed using SPSS software (IBM Corp. Released 2014. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp., USA). All values were expressed as the mean±standard deviation. To evaluate difference of the ratio from 1 we used one sample t-test. To compare the results within each group with baseline ratio during the follow ups, we used Linear Mixed Models (LMM) option in the SPSS. The Bonferroni correction method was considered for multiple comparisons.The Mann-Whitney U test was used to compare staining intensities values between groups. To evaluate the trend of b-wave ratio values with the increment in clonidine dosage, we used multiple linear regression. P values less than 0.05 were considered statistically significant.
RESULTS
All animals tolerated the injections well. Post-IVC eye examination demonstrated no intraocular inflammation,cataract formation, retinal detachment, optic atrophy or endophthalmitis. Two rabbits (one in Group B and one in Group C) developed mild vitreous hemorrhage in their right eyes due to injection technique and were included in the analysis. One rabbit in Group B died during the study period and was excluded from the final analysis.
Electroretinography There was no difference in the ERG patterns, recorded in treated compared to the control eyes.As it was mentioned in the methods, we defined the b-wave amplitude ratio to evaluate the functional damage to the retina quantitatively. Tables 1 and 2 showed the mean of b-wave amplitude ratio in different time points (baseline, 1, 4 and 8wk after IVC) in the study groups. There were not any toxic effects related to different dosages of IVC, based on photopic ERG responses (P>0.05 for all) (Table 1). Considering the scotopic responses, while 15 µg IVC was safe, 50 µg was obviously toxic (Table 2). The mean of b-wave amplitude ratio reduced significantly eight weeks after 50 µg IVC compared to the baseline value (P=0.018). In Group B, mean of b-wave amplitude ratio decreased eight weeks after clonidine injection compared to the pre-injection value, but the decrement was not statistically significant (P=0.50). However, as an additional analysis, the mean of b-wave amplitude in the 25 and 50µg groups were significantly less than the same value in the control group at eight weeks follow up (P=0.049 and P<0.001,respectively). Also, the P value for the trend of b-wave ratio rates with the increment in clonidine dosage was significant in scotopic responses, when comparing mean of the b-wave ratio between study groups eight weeks after clonidine injection(P<0.001) (Table 2). It shows that increment of the IVC dosage significantly reduced the mean of b-wave amplitude ratio.
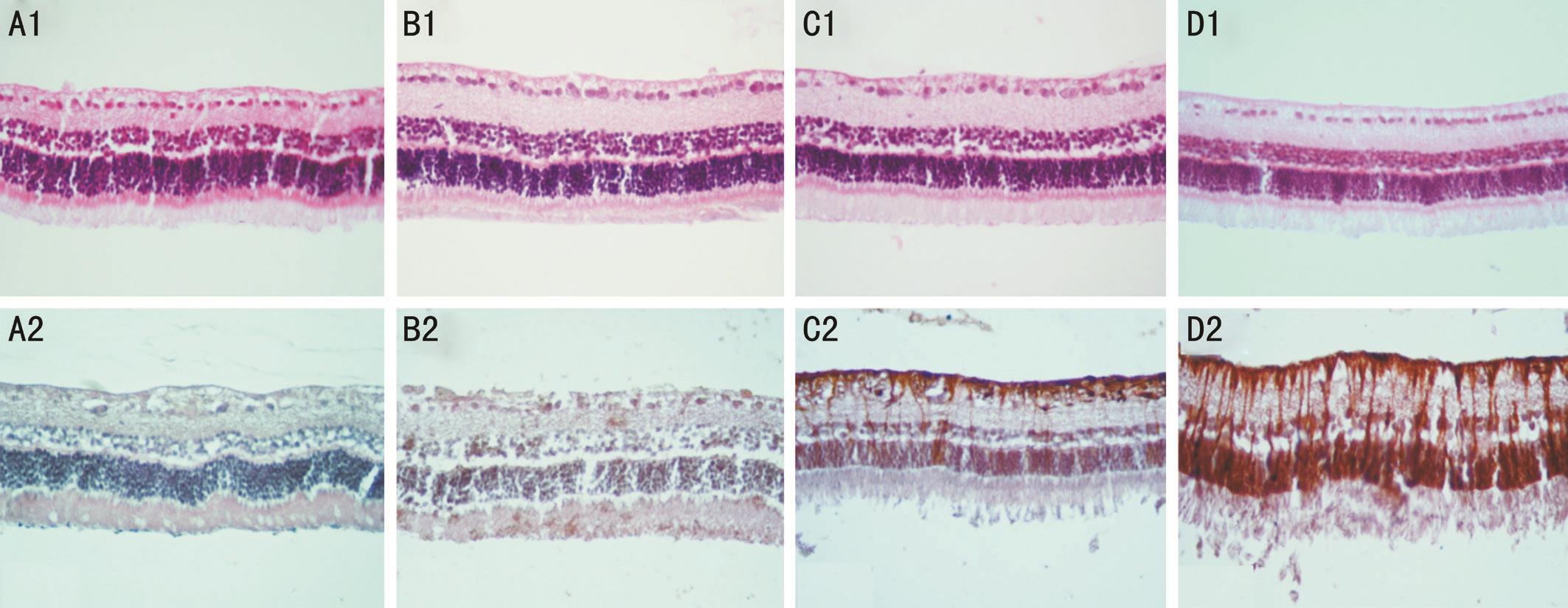
Figure 1 Representative unremarkable neurosensory retina with intravitreal injection of 15 μg (B1), 25 μg (C1), and 50 μg (D1)clonidine as compared to the BSS-injected (A1) eyes (hematoxylin & eosin stain, magnification ×400). Note non-significant GFAP immunoreactivity of the retina in a representative eye injected with 15 μg (B2) clonidine and BSS (A2) and significant retinal GFAP immunoreactivity in a representative eye injected with 25 μg (C2) and 50 μg (D2) clonidine (magnification ×400).
Light and Fluorescence Microscopy Light microscopic examinations of the eyes in the study groups disclosed no evidence of intraretinal hemorrhage, inflammation, necrosis or atrophic changes. Retinal layers integrity was not violated in any of the cases. The GFAP immunoreactivity was significantly increased in the Groups B (25 μg) and C (50 μg)with the mean scores of 2.67±1.15 and 3.33±1.53, respectively.No significant GFAP immunoreactivity was observed in the retinas of Groups A (15 μg) and D (BSS-injected eyes) with the mean scores of 1.67±0.58 and 1.33±0.58, respectively(Figure 1). The GFAP results were well correlated with the ERG results, demonstrating retinal toxicity attributable to 25 and 50 μg IVC.
TUNEL assay to characterize the retinal cells death disclosed no significant positivity in the rabbit retinas of Groups A (15 μg), B (25 μg), and C (50 μg) as compared to the control (Figure 2). Overall, given the ERG, histopathology,immunohistochemical and TUNEL assay results, 15 µg/0.1 mL IVC was determined as the maximum safe dose.
DISCUSSION
In the present study, various doses of clonidine were injected intravitreally into the rabbit eyes to determine its maximum safe dose. It was found that 15 µg IVC was the safe dose, based on, functional, histological and immunohistochemical studies.Furthermore, 15, 25 and 50 µg IVC did not cause retinal cells apoptosis using TUNEL assay. Although the TUNEL assay was unremarkable, retinal toxicity was demonstrated with the doses of 25 µg and 50 µg IVC, based on scotopic ERG and GFAP results, corresponding to the dysfunction of retinal rods and Müller cells, respectively.
Clonidine as an α2-ADRs agonist seems to have neuroprotective and anti-VEGF effects[3-4]. Yoles et al[25]found that single dose of intraperitoneal clonidine, administrated immediately after partial crush injury of the rat’s optic nerve induced neuroprotective effects compared to the controls, demonstrated by electrophysiological measurements. Weigert et al[26]used intravenous clonidine with dosage of 0.2 μg/kg/min over 10min in healthy volunteers to evaluate its effects on ocular blood flow. They found that intravenous clonidine increased the choroidal and optic nerve head blood flow, displayed by laser Doppler flowmetry. They supposed that it was due to vasodilatory effect of clonidine and concluded that it may contribute to the retinal ganglion cells (RGC) and neuronal protection. Peng et al[27]found that intraperitoneal clonidine contributed to photoreceptor protection in rat retina. They discovered that clonidine induced extracellular-signal regulatory kinases (ERKs) phosphorylation, mainly in Müller cells. Naziri et al[5]also demonstrated that intraperitoneal clonidine decreased RGCs death in an experimental-glaucoma model. It was found that clonidine reduced amyloid beta (Aβ)and amyloid protein precursor (APP) in the RGC layer and protected them from a neurodegenerative process. The anti-VEGF effects of α2-ADRs agonists have been confirmed in different in vivo and in vitro studies. Watanabe et al[4]demonstrated that clonidine had an inhibitory effect on VEGF mRNA and protein expression in the RPE cells stimulated by IL1β. Kusari et al[7]clarified that subcutaneous brimonidine significantly reduced laser induced CNV in brown Norway rats. Additionally, they found that retinal neovascularization diminished significantly in an animal model of ROP following systemic brimonidine administration. In most of these studies it was disclosed that the neuroprotective effects of α2-ADRs agonists such as clonidine, can be blocked by the selective α2-ADRs antagonist, indicating that the therapeutic effects of these agents were exerted through adrenoreceptors such as α2-receptors[25,27]. Bylund et al[28]demonstrated that these receptors(α2A subtype) are also present in the human neurosensory retina. Furthermore, Woldemussie et al[29]reported that all three subtypes of α2-ADRs (α2A, α2B and α2C) were present in human retina, differentially localized in various cellular layers.
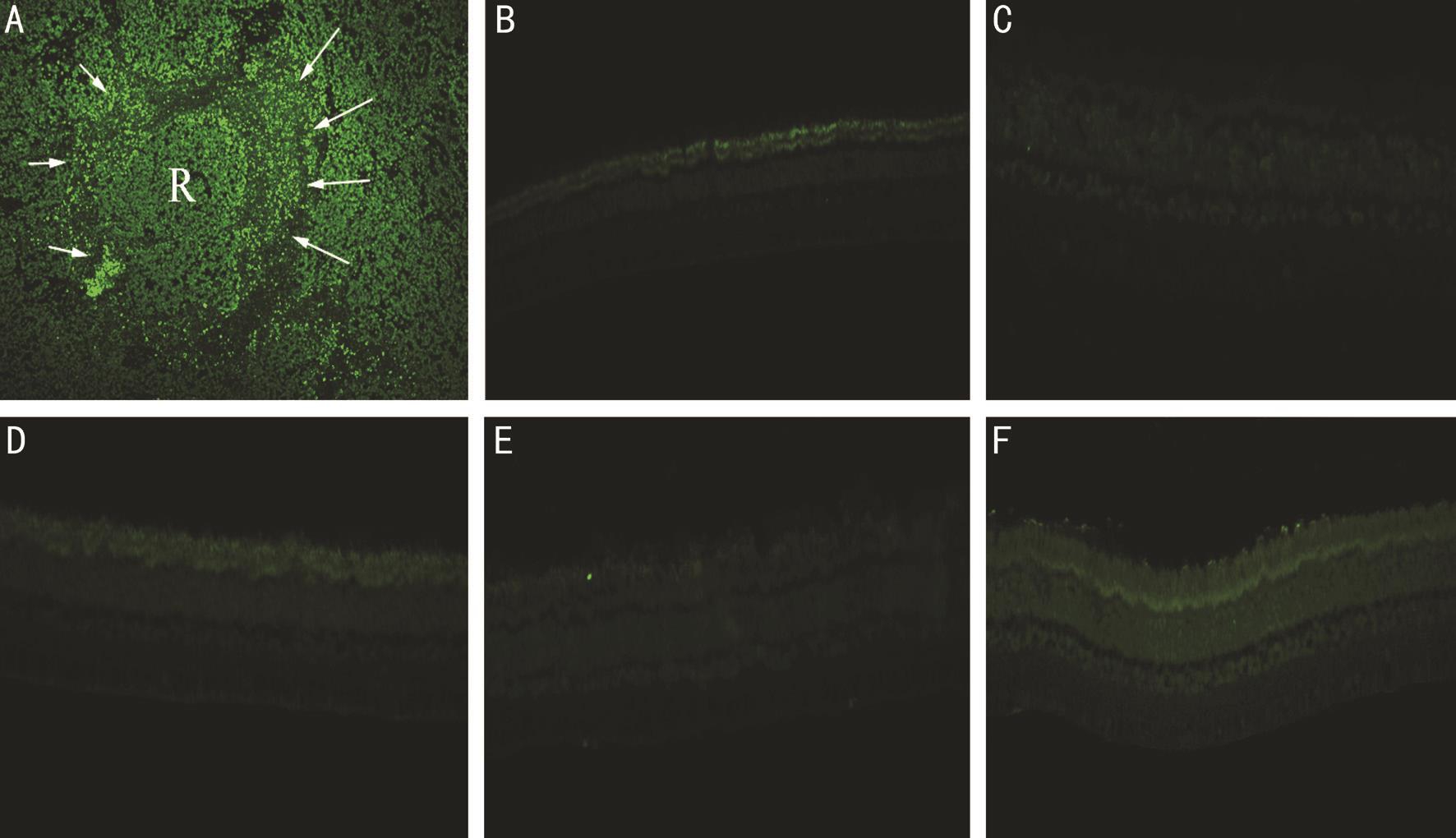
Figure 2 Determination of the apoptotic effects of 15, 25, and 50 μg of intravitreal clonidine injections on rabbit retinas by using TUNEL assay Positive control is a section of retinoblastoma with viable tumor cells (see R in A) surrounded by apoptotic cells and negative control was not treated by TUNEL reaction mixture (B). Results indicated that none of examined clonidine concentrations 15 μg (C), 25 μg (D), and 50 μg(E) imposed apoptotic effects on the treated groups when compared to the control (F) (all images magnification ×400).
ERG analysis in our study disclosed a significant decrease in b-wave amplitude and b-wave amplitude ratio in dark adapted state, detected 8wk after 25 and 50 µg IVC, respectively. More reduction of b-wave amplitude ratio was observed in those rabbits who had received 50 µg IVC compared to those who had received either 25 or 15 µg IVC. Furthermore, unlike the ERG and GFAP results, routine histopathology and TUNEL assay did not show any abnormal changes related to different dosages of IVC in the rabbit retinas. This disparity may be indicating that the 25 and 50 µg doses of IVC were sufficient for induction of retinal rods and Müller cells dysfunction but insufficient for inducing cellular apoptosis. In other words, as demonstrated by ERG and GFAP results of the current study,higher doses of IVC disturbed retinal function and induced Müller cells dysfunction but their toxic effects were not too severe to cause retinal ganglion cells apoptosis in TUNEL assay or retinal atrophic changes on light microscopy.
To the best of our knowledge, current study is the first survey in which that the maximum safe dose of intravitreal clonidine was investigated through an experimental preclinical study.The obvious advantage of intravitreal administration is that the concentration achieved with this route is many-fold greater than the one achieved with either topical or systemic administration, while inducing the least systemic side effects.In conclusion, as described before clonidine have four summarized effects including neuroprotection, anti-VEGF,increasing choroidal blood flow and anti-glaucoma. Our data suggests that 15 µg IVC is safe for intravitreal injection,however, it is still uncertain whether this dosage may also contribute to neuroprotection and/or anti-VEGF effects in animal models or in human retina, which deserves further investigations.
ACKNOWLEDGEMENTS
Conflicts of Interest: Nikkhah H, None; Garfami KH,None; Kanavi MR, None; Nashtaei EM, None; Karimi S,None; Soheilian M, None.
REFERENCES
1 Gavras I, Manolis AJ, Gavras H. The alpha2-adrenergic receptors in hypertension and heart failure: experimental and clinical studies. J Hypertens 2001;19(12):2115-2124.
2 Toris CB, Tafoya ME, Camras CB, Yablonski ME. Effects of apraclonidine on aqueous humor dynamics in human eyes. Ophthalmology 1995;102(3):456-461.
3 Chao HM, Chidlow G, Melena J, Wood JP, Osborne NN. An investigation into the potential mechanisms underlying the neuroprotective effect of clonidine in the retina. Brain Res 2000;877(1):47-57.
4 Watanabe K, Zhang XY, Kitagawa K, Yunoki T, Hayashi A. The effect of clonidine on VEGF expression in human retinal pigment epithelial cells(ARPE-19). Graefes Arch Clin Exp Ophthalmol 2009;247(2):207-213.
5 Nizari S, Guo L, Davis BM, Normando EM, Galvao J, Turner LA,Bizrah M, Dehabadi M, Tian K, Cordeiro MF. Non-amyloidogenic effects of α2 adrenergic agonists: implications for brimonidine-mediated neuroprotection. Cell Death Dis 2016;7(12):e2514.
6 Zhang C, Takahashi K, Lam TT, Tso MO. Effects of basic fibroblast growth factor in retinal ischemia. Invest Ophthalmol Vis Sci 1994;35(8):3163-3168.
7 Kusari J, Padillo E, Zhou SX, Bai Y, Wang J, Song Z, Turner LA,Bizrah M, Dehabadi M, Tian K, Cordeiro MF. Effect of brimonidine on retinal and choroidal neovascularization in a mouse model of retinopathy of prematurity and laser treated rats. Invest Ophthalmol Vis Sci 2011;52(8):5424-5431.
8 Yamasaki M, Mishima HK, Yamashita H, Kashiwagi K, Murata K,Minamoto A, Inaba T. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res 2005;1050(1-2):15-26.
9 Zhong Y, Yao H, Deng L, Cheng Y, Zhou X. Promotion of neurite outgrowth and protective effect of erythropoietin on the retinal neurons of rats. Graefes Arch Clin Exp Ophthalmol 2007;245(12):1859-1867.
10 Lambert WS, Ruiz L, Crish SD, Wheeler LA, Calkins DJ. Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons.Mol Neurodegener 2011;6(1):4.
11 Kalapesi FB, Coroneo MT, Hill MA. Human ganglion cells express the alpha-2 adrenergic receptor: Relevance to neuroprotection. Br J Ophthalmol 2005;89(6):758-763.
12 Degos V, Charpentier TL, Chhor V, Brissaud O, Lebon S, Schwendimann L, Bednareck N, Passemard S, Mantz J, Gressens P. Neuroprotective effects of dexmedetomidine against glutamate agonist-induced neuronal cell death are related to increased astrocyte brain-derived neurotrophic factor expression. Anesthesiology 2013;118(5):1123-1132.
13 Vorwerk CK, Lipton SA, Zurakowski D, Hyman BT, Sabel BA,Dreyer EB. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci 1996;37(8):1618-1624.
14 Donello JE, Padillo EU, Webster ML, Wheeler LA, Gil DW.alpha(2)-Adrenoceptor agonists inhibit vitreal glutamate and aspartate accumulation and preserve retinal function after transient ischemia. J Pharmacol Exp Ther 2001;296(1):216-223.
15 Yamada H, Chen YN, Aihara M, Araie M. Neuroprotective effect of calcium channel blocker against retinal ganglion cell damage under hypoxia. Brain Res 2006;1071(1):75-80.
16 Bonnet D, Garcia M, Vecino E, Lorentz JG, Sahel J, Hicks D. Brainderived neurotrophic factor signaling in adult pig retinal ganglion cell neurite regeneration in vitro. Brain Res 2004;1007(1-2):142-151.
17 Arango-González B, Cellerino A, Kohler K. Exogenous brain-derived neurotrophic factor (BDNF) reverts phenotypic changes in the retinas of transgenic mice lacking the BDNF gene. Invest Ophthalmol Vis Sci 2009;50(3):1416-1422.
18 Vennen KM, Mitchell MA. Rabbits. In: Manual of exotic pet practice.Mitchell MA, Tully TN Jr. Saunders, St. Louis, Missouri 2009;375-405.
19 Zemel E, Loewenstein A, Lei B, Lazar M, Perlman I. Ocular pigmentation protects the rabbit retina from gentamicin-induced toxicity.Invest Ophthalmol Vis Sci 1995;36(9):1875-1884.
20 Dib E, Maia M, Lima Ade S, de Paula Fiod Costa E, de Moraes-Filho MN, Rodrigues EB, Penha FM, Coppini LP, de Barros NM, Coimbra Rde C, Magalhães Júnior O, Guerra T, Furlani Bde A, Freymuller E,Farah ME. In vivo, in vitro toxicity and in vitro angiogenic inhibition of sunitinib malate. Curr Eye Res 2012;37(7):567-574.
21 Loewenstein A, Zemel E, Lazar M, Perlman I. Drug-induced retinal toxicity in albino rabbits: the effects of imipenem and aztreonam. Invest Ophthalmol Vis Sci 1993;34(12):3466-3476.
22 Giordano VE, Hernandez-Da Mota SE, Adabache-Guel TN,Castillejos-Chevez A, Corredor-Casas S, Salinas-Longoria SM, Romero-Vera R, Jimenez-Sierra JM, Guerrero-Naranjo JL, Morales-Canton V.Safety of intravitreal quinupristin/dalfopristin in an animal model. Int J Ophthalmol 2016;9(3):373-378.
23 Larsen AK, Osborne NN. Involvement of adenosine in retinal ischemia. Studies on the rat. Invest Ophthalmol Vis Sci 1996;37(13):2603-2611.
24 Nourinia R, Rezaei Kanavi M, Kaharkaboudi A, Taghavi SI, Aldavood SJ, Darjatmoko SR, Wang S, Gurel Z, Lavine JA, Safi S, Ahmadieh H,Daftarian N, Sheibani N. Ocular safety of intravitreal propranolol and its efficacy in attenuation of choroidal neovascularization. Invest Ophthalmol Vis Sci 2015;56(13):8228-8235.
25 Yoles E, Wheeler LA, Schwartz M. Alpha2-adrenoreceptor agonists are neuroprotective in a rat model of optic nerve degeneration. Invest Ophthalmol Vis Sci 1999;40(1):65-73.
26 Weigert G, Resch H, Luksch A, Reitsamer HA, Fuchsjager-Mayrl G, Schemetterer L, Garhofer G. Intravenous administration of clonidine reduces intraocular pressure and alters ocular blood flow. Br J Ophthalmol 2007;91(10):1354-1358.
27 Peng M, Li Y, Luo Z, Liu C, Laties AM, Wen R. Alpha2-adrenergic agonists selectively activate extracellular signal-regulated kinases in Müller cells in vivo. Invest Ophthalmol Vis Sci 1998;39(9):1721-1726.
28 Bylund DB, Chacko DM. Characterization of alpha2 adrenergic receptor subtypes in human ocular tissue homogenates. Invest Ophthalmol Vis Sci 1999;40(10):2299-2306.
29 Woldemussie E, Wijono M, Pow D. Localization of alpha 2 receptors in ocular tissues. Vis Neurosci 2007;24(5):745-756.