INTRODUCTION
Diabetes refers to a group of systemic metabolic diseases characterized by chronic hyperglycemia. In 2013, the International Diabetes Association released results of the latest diabetes prevalence survey indicating that the global number of diabetes cases had reached 382 million, and the number of patients in China was 98.4 million and China had the highest incidence of diabetes in the world. This number is expected to reach 143 million by 2035[1]. Diabetic keratopathy is attracting increased attention from the majority of ophthalmologists.To date, however, no study investigating the risk factors for infectious keratopathy in diabetic patients has been published,and the majority of the studies constitute basic clinical research investigating pathogenesis of diabetic keratopathy[2-4]and prevention and treatment of diabetic keratopathy[5-9]. Diabetic keratopathy is one of the major ocular complications in diabetic patients. The main manifestations of diabetic keratopathy include delayed epithelial wound healing, tear film destruction,and even degradation of corneal nerve fibers. Some studies[10]have reported that corneal medial and intimal corneal optical density and central corneal thickness in diabetic patients were sensitive indicators for early diabetic keratopathy. Because of the imbalance and damage to the ocular surface environment,diabetic patients are more prone to infectious keratopathy.However, there is still a lack of research investigating risk factors for diabetic keratopathy. In this retrospective study,we compared the clinical characteristics of type 2 diabetes mellitus (T2DM) and non-diabetic mellitus (NDM) patients with infectious keratopathy, and discussed the risk factors for infectious keratopathy in T2DM patients. These results may provide a reference for the prevention, clinical diagnosis, and treatment of infectious keratopathy in diabetic patients.
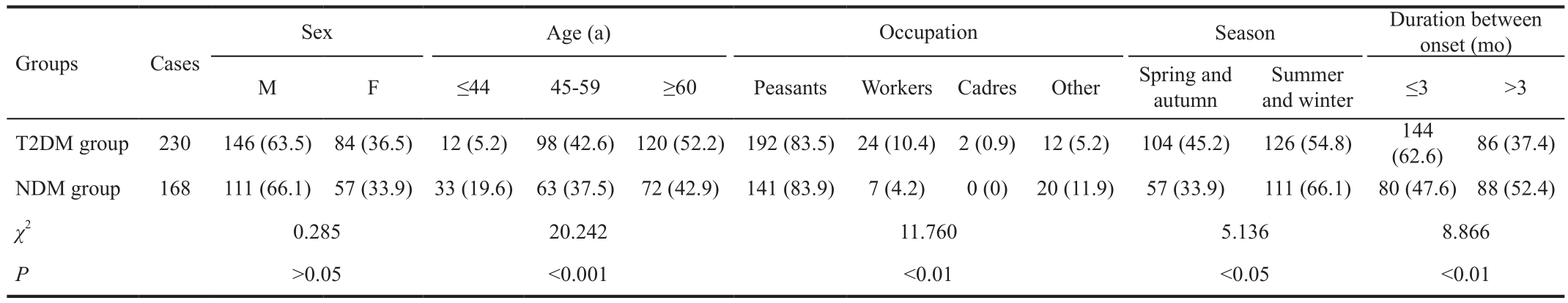
Table 1 Comparison of general characteristics of the two groups n (%)
SUBJECTS AND METHODS
Subjects A total of 230 diabetic keratopathy patients and 168 non-diabetic keratopathy patients with corneal infections treated at Qingdao Eye Hospital, Shandong Eye Institute(Qingdao, China) from 2001 to 2015 were enrolled in this study. The inclusion criteria were no history of eye surgery or chemical burns, and no eye disease caused by other systemic diseases. Patients with secondary infection caused by other diseases of the eye (e.g. palpebral fissure insufficiency, nerve paralysis keratitis, etc.) and those who were voluntarily discharged without systematic treatment were excluded. The study was approved by the Qingdao Eye Hospital Ethics Committee and was conducted in accordance with the Declaration of Helsinki.
Diabetes was diagnosed in accordance with the parameters prescribed by the World Health Organization for the diagnosis of T2DM in 1999, more specifically, weight loss, polyuria,polydipsia, polyphagia, random plasma glucose ≥11.1 mmol/L,fasting plasma glucose ≥7.0 mmol/L, and oral glucose tolerance test 2 hPG ≥11.1 mmol/L. Any one of the three numerically defined parameters above can be used as a T2DM diagnostic criterion; however, the diagnosis must be confirmed by a review performed on another day.
Protocol This retrospective study reviewed and analyzed T2DM patients and non-diabetic patients who suffered from corneal infection at Qingdao Eye Hospital. General information of the patients including medical history, sex, age, occupation,season, smoking and alcohol consumption habits, duration between onset and treatment, and inducement and glycosylated hemoglobin A1c (HbA1c) were collected. The information was acquired via medical history records included number of days hospitalized, drug treatment, and surgical treatment. Based on disease history, the results of corneal scraping, microbiology culture, and confocal microscopy[11], infectious keratopathy was divided into herpes simplex virus keratitis (HSK),bacterial keratitis, fungal keratitis, and Acanthamoeba keratitis.Statistical Analysis SPSS version 19.0 (IBM Corporation,Armonk, NY, USA) was used to construct the database and perform statistical analysis. Means were compared via the t-test, and rates were compared using the Chi-squared test.Multivariate logistic regression analysis was used to analyze the factors influencing the occurrence of diabetic corneal diseases. Factors identified as significant in the initial analysis were analyzed in the multivariate logistic regression model,with diabetic corneal disease as the dependent variable.
RESULTS
General Parameters The study included 230 diabetes patients, of which 146 (63.5%) were male and 84 (36.5%)were female. The mean age was 59.0±8.9y (range, 40-79y).There were 168 non-diabetic patients included in the study, of which 111 (66.1%) were male and 57 (33.9%) were female,with the mean age of 56.2±13.7y (range 18-84y). There were statistically significant differences in age, occupation,season, and duration between onset and treatment between the two groups (P<0.05) (Table 1). There were no significant differences in sex, smoking or alcohol consumption habits, or inducement between the two groups (P>0.05) (Tables 1, 2).HbA1c was 9.09%±2.12% in the diabetic group, which was significantly higher than normal.
Hospital Data Comparisons Between the Groups The mean durations of hospitalization were 13.15±9.51d in the diabetic group and 10.67±6.23d in the non-diabetic group; the difference was statistically significant (P<0.05). Differences in the rates of drug treatment and surgical treatment were also significant statistically between the groups (P<0.05) (Table 2).
Comparison of Infectious Keratopathy Between the Groups The incidences of bacterial keratitis and HSK differed significantly between the two groups (P<0.05), but there were no significant differences in the incidences of fungal keratitis or Acanthamoeba keratitis (P>0.05) (Table 2).
Multi-factor Analysis of Diabetic Infectious Keratopathy Factors identified as significant in the initial analysis were analyzed via a multivariate logistic regression model, with diabetic corneal disease as the dependent variable. In that analysis, age and season were identified as significant independent risk factors for diabetic keratopathy (Table 3).
Table 2 Findings for other factors, treatment data and infectious keratopathy in the two patient groups and durations of hospitalization mean±SD, n (%)
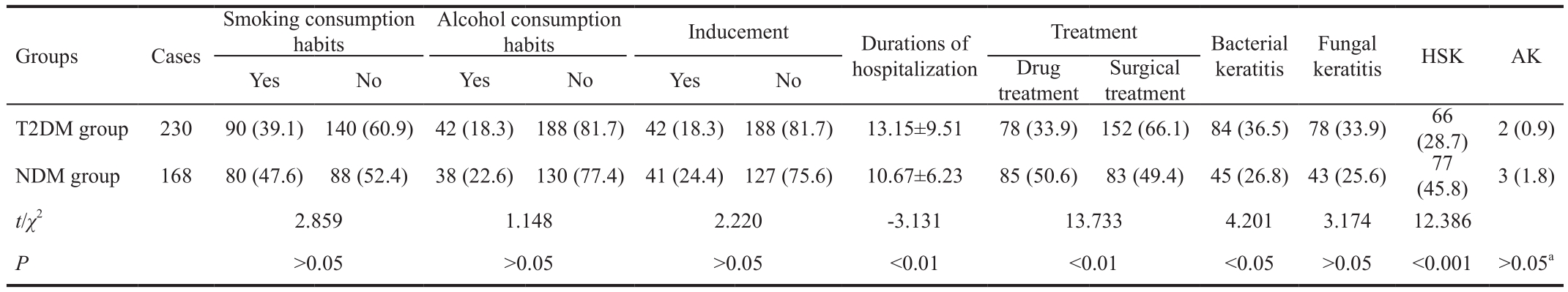
AK: Acanthamoeba keratitis;aFisher precise test.
Table 3 Multi-factor analysis of diabetic infectious keratopathy
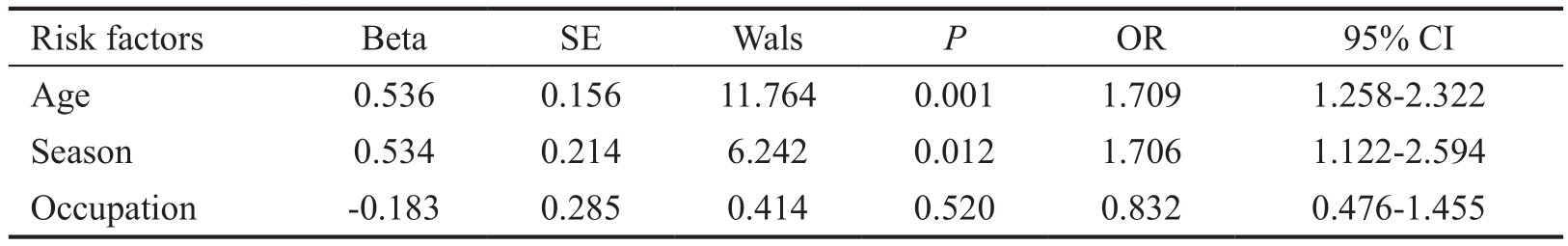
SE: Standard error; Wals: Wald statistic; OR: Odds ratio; CI: Confidence interval.
DISCUSSION
Diabetes is a group of systemic metabolic diseases characterized by chronic hyperglycemia, and it affects multiple organs and tissues of the body. Eye disease can be primarily caused by diabetes, or by various syndromes induced by other systemic diseases. To date, the study of diabetic eye disease has mainly focused on diabetic retinopathy and diabetic cataract.Additional diabetic ocular complications include neovascular glaucoma, optic neuropathy, dry eye, and diabetic keratopathy,etc[12-15]. Since Schultz et al[15]introduced the concept of diabetic keratopathy in 1981, physicians and patients have begun to recognize its importance.
In clinical practice, abnormal changes in tissue structure,metabolism, and cornea function can result from corneal epithelium erosion, corneal wound healing, corneal hypoesthesia,corneal edema, and even corneal ulcers. Usually, these symptoms have a long duration and poor prognosis[16]. To date,diabetic keratopathy research has mainly focused on aspects of pathogenesis such as diabetic corneal insulin-like growth factor 1 (IGF-1), transforming growth factor beta (TGF-β),and platelet-derived factor expression[17]. We have previously reported that diabetic keratopathy was clearly associated with corneal epithelial and neuro-metabolism irregularities such as abnormal regulation of miR-182, which is a potential target for the treatment of diabetic sensory nerve regeneration and diabetic keratopathy[18]. Substance P (SP) signaling through neurokinin-1 (NK-1) receptors contributes to the healing of diabetic corneal epithelium[19]. The application of ciliary neurotrophic factors may potentially improve limbal stem cell deficiency, and the treatment of diabetic keratopathy[20]. Silent mating type information regulation 2 homolog 1 (SIRT1)plays a significant role in diabetic keratopathy[21], and the nasal cavity to the nanomicelle curcumin can promote the healing of diabetic mouse corneal epithelium[22]. Some clinical studies have also reported that corneal changes in diabetic keratopathy patients were associated with reduced lacrimal and ocular surface function and reduced corneal sub-epithelial nerve density[23-24]. Abnormal tear quality, reduced tear secretion,and reduced corneal subepithelial nerve plexus density can lead to shallow punctate corneal lesions and persistent corneal epithelial defect, reducing the cornea’s capacity for resistance and repair, which can ultimately lead to corneal infection by pathogenic microorganisms. It has also been reported that abnormal tear secretion (e.g. uneven tear fluid lipid layer and reduced tear break-up time) and corneal sensitivity may be associated with diabetic corneal epithelial lesions[25]. Sustained corneal epithelial defect occurs mainly in corneal edema and corneal opacity to remove corneal epithelium in diabetic patients who require a vitrectomy. This is because epithelial curettage can delay epithelial repair, enhancing susceptibility to pathogenic microbial infection. This may be due to a lack of SP, and corneal epithelial cell homeostasis disorders caused by SP signaling through the NK-1 receptor to promote healing of diabetic corneal epithelium[19].
Infectious keratopathy mainly includes HSK, bacterial keratitis,fungal keratitis, and Acanthamoeba keratitis. The diagnosis of the latter three diseases mainly depends on the detection of pathogenic microorganisms, whereas HSK diagnosis still mainly relies on history of recurrence and specific clinical observations. HSK is caused by herpes simplex virus type 1 infection of the cornea. It has the highest prevalence of all the infectious keratopathies, and its prevalence in China is reportedly 11 per 10 000[26]. T2DM patients are at increased risk for bacterial keratitis and should improve their physical health, avoid trauma, and visit the hospital for regular examinations. Non-diabetic patients are more likely to suffer from HSK, which may be due to the relatively smaller number of patients hospitalized with T2DM, where the base number of diabetic patients is small.
The current study compared risk factors for infectious keratopathy in patients with T2DM to those in non-diabetic patients. We found that T2DM patients with corneal disease tended to be older than non-diabetic patients. Although the ages of the two groups differed significantly, in both groups the highest proportion of patients were aged ≥60y, with 52.2%in the diabetic group and 42.9% in the non-diabetic group.Similarly, although the occupations of the two groups differed significantly, in both groups the highest proportion of patients were peasants, with 83.5% in the diabetic group and 83.9% in the non-diabetic group. With regard to season, in both T2DM and non-diabetic patients the highest incidences of infectious keratopathy were observed during summer and winter. This may be associated with the hot weather in summer and the cold weather in winter, and implies that the temperature being too high or too low may affect the incidence of infectious keratopathy. With respect to the duration between onset and treatment, T2DM patients had a mean duration ≤3mo,whereas the non-diabetics had a mean duration ≥3mo. The shorter duration in T2DM patients may be associated with rapid progress of the disease in T2DM patients, and the longer duration in non-diabetic patients may be associated with the comparatively slow progress of the disease. With regard to the length of hospitalization, the majority of T2DM patients had longer periods of hospitalization than the majority of non-diabetic patients, indicating a longer recovery time in T2DM patients compared to non-diabetic patients. This is because diabetic patients have structural and functional corneal abnormalities, leading to delayed healing of ulcers and prolonged hospital stays[27-29]. The non-diabetic patients were more likely to undergo drug treatment, indicating that they had a better cure rate than the T2DM patients, who exhibited a higher surgery rate. This is because diabetes is an important factor in the healing of corneal ulcers because it can increase the likelihood of infection, prolong duration, greatly reduce the possibility of successful drug treatment, and necessitate surgical intervention to prevent disease progression.
Advanced age is a risk factor for T2DM in patients with infectious keratopathy. Corneal sensation has been shown to decrease with age in normal subjects[30-31]. In a recent study by Niederer et al[32], there was a linear decrease in subepithelial nerve density of 0.9% per year. In diabetic patients, reduction of corneal nerve density and its nerve branch have been reported[33-34]. The likelihood of infectious keratopathy increases with reduced nerve density and corneal sensitivity,and epithelial prone to exfoliation. Patients ≥60 years of age are more prone to infectious keratopathy, which may be related to the duration of their history of diabetes. In general, older age and longer diabetes history are positively associated with secondary corneal disease. The increase in hospitalization days in patients with T2DM indicates that infection was more difficult to control in these patients. Drug treatment requires a longer course in T2DM patients, and surgical treatment of postoperative complications is relatively extensive, thus requiring longer hospital observation time. HbA1c is one of the main indicators reflecting long-term glycemic control. In general, the control objectives of HbA1c should be less than 7%[35]. In this study, HbA1c in diabetic group was significantly higher than normal, which reflected the higher level of blood glucose and poor control in the past 2-3mo. This suggests, to some extent, that poor glycemic control may contribute to the development of corneal infection.
In summary, in the current study, infectious keratopathy was related to age and season in patients with T2DM, and T2DM patients were more prone to bacterial keratitis. Taking into account the high prevalence of diabetes and the low rate of treatment in China, individuals should increase their awareness of the condition, undergo regular physical examinations, seek early and timely standardized treatment, devote attention to eye health, especially in the summer and winter, and avoid eye trauma to prevent infectious keratopathy. Furthermore,organized efforts should be made to better educate peasants about eye health. In diabetic patients, a surgical approach should be selected with caution to minimize the risk for infectious keratopathy and diabetes associated with infection.Given the retrospective nature of the study and the limited sample size, more detailed classification and comparison among patients was not feasible, and we were able to only elaborate on the overall population. Although there were some other limitations that may have affected the general conclusions of this study, these problems will be addressed in subsequent research. T1DM primarily affects Chinese teenagers and the clinical diagnosis of T1DM is usually earlier than T2DM and has a longer duration. However, there are very few T1DM patients who require corneal patients in our hospital. Given their low numbers, they were not included in this study. The similarities and differences between the two will be further investigated and discussed in a follow-up study.
ACKNOWLEDGEMENTS
Authors’ contributions: Wang B designed, collected and analyzed the data, and prepared the manuscript. Yang S collected and prepared the manuscript. Zhai HL, Zhang YY,Cui CX and Wang JY collected the data. Xie LX designed and reviewed the manuscript.
Foundations: Supported by the National Natural Science Foundation of China (No.81500703); the Natural Science Foundation of Shandong Province (No.ZR2014HQ059;No.ZR2015YL027).
Conflicts of Interest: Wang B, None; Yang S, None; Zhai HL, None; Zhang YY, None; Cui CX, None; Wang JY,None; Xie LX, None.
REFERENCES
1 da Rocha Fernandes J, Ogurtsova K, Linnenkamp U, Guariguata L,Seuring T, Zhang P, Cavan D, Makaroff LE. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract 2016;117:48-54.
2 Bikbova G, Oshitari T, Tawada A, Yamamoto S. Corneal changes in diabetes mellitus. Curr Diabetes Rev 2012;8(4):294-302.
3 Ohashi Y. Diabetic keratopathy. Nippon Ganka Gakkai Zasshi 1997;101(2):105-110.
4 Ljubimov AV. Diabetic complications in the cornea. Vision Res 2017;pii:S0042-6989(17):30047-0.
5 Chikamoto N, Chikama T, Yamada N, Nishida T, Ishimitsu T, Kamiya A. Efficacy of substance P and insulin-like growth factor-1 peptides for preventing postsurgical superficial punctate keratopathy in diabetic patients. Jpn J Ophthalmol 2009;53(5):464-469.
6 Abdelkader H, Patel DV, McGhee CNj, Alany RG. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Exp Ophthalmol 2011;39(3):259-270.
7 Lockwood A, Hope-Ross M, Chell P. Neurotrophic keratopathy and diabetes mellitus. Eye (Lond) 2006;20(7):837-839.
8 Kaji Y. Prevention of diabetic keratopathy. Br J Ophthalmol 2005;89(3):254-255.
9 Sakamoto A, Sasaki H, Kitagawa K. Successful treatment of diabetic keratopathy with punctal occlusion. Acta Ophthalmol Scand 2004;82(1):115-117.
10 Gao F, Lin T, Pan Y. Effects of diabetic keratopathy on corneal optical density, central corneal thickness, and corneal endothelial cell counts. Exp Ther Med 2016;12(3):1705-1710.
11 Song X, Xie L, Tan X, Wang Z, Yang Y, Yuan Y, Deng Y, Fu S, Xu J,Sun X, Sheng X, Wang Q. A multi-center, cross-sectional study on the burden of infectious keratitis in China. PLoS One 2014;9(12):e113843.
12 Herse PR. A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am J Optom Physiol Opt 1988;65(3):224-230.
13 Saini JS, Khandalavla B. Corneal epithelial fragility in diabetes mellitus. Can J Ophthalmol 1995;30(3):142-146.
14 Hatchell DL, Magolan JJ Jr, Besson MJ, Goldman AI, Pederson HJ,Schultz KJ. Damage to the epithelial basement membrane in the corneas of diabetic rabbits. Arch Ophthalmol 1983;101(3):469-471.
15 Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH.Diabetic keratopathy. Trans Am Ophthalmol Soc 1981;79:180-199.
16 Wang Y, Zhou Q, Xie L. Diabetic keratopathy: new progresses and challenges. Zhonghua Yan Ke Za Zhi 2014;50(1):69-72.
17 Saghizadeh M, Chwa M, Aoki A, Lin B, Pirouzmanesh A, Brown DJ, Ljubimov AV, Kenney MC. Altered expression of growth factors and cytokines in keratoconus, bullous keratopathy and diabetic human corneas. Exp Eye Res 2001;73(2):179-189.
18 Wang Y, Zhao X, Wu X, Dai Y, Chen P, Xie L. MicroRNA-182 mediates Sirt1-induced diabetic corneal nerve regeneration. Diabetes 2016;65(7):2020-2031.
19 Yang L, Di G, Qi X, Qu M, Wang Y, Duan H, Danielson P, Xie L,Zhou Q. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor.Diabetes 2014;63(12):4262-4274.
20 Zhou Q, Chen P, Di G, Zhang Y, Wang Y, Qi X, Duan H, Xie L. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/Progenitor cells and accelerates corneal epithelial wound healing. Stem Cells 2015;33(5):1566-1576.
21 Gao J, Wang Y, Zhao X, Chen P, Xie L. MicroRNA-204-5p-mediated regulation of SIRT1 contributes to the delay of epithelial cell cycle traversal in diabetic corneas. Invest Ophthalmol Vis Sci 2015;56(3):1493-1504.
22 Guo C, Li M, Qi X, Lin G, Cui F, Li F, Wu X. Intranasal delivery of nanomicelle curcumin promotes corneal epithelial wound healing in streptozotocin-induced diabetic mice. Sci Rep 2016;6:29753.
23 Alves Mde C, Carvalheira JB, Modulo CM, Rocha EM. Tear film and ocular surface changes in diabetes mellitus. Arq Bras Oftalmol 2008;71(6 Suppl):96-103.
24 Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology 2001;108(3):586-592.
25 Inoue K, Kato S, Ohara C, Numaga J, Amano S, Oshika T. Ocular and systemic factors relevant to diabetic keratoepithelioPathy. Cornea 2001;20(8):798-801.
26 Corneal disease group of Department of Ophthalmology, Chinese Medical Association. Consensus on clinical diagnosis and treatment of infectious keratoPathy (2011). Zhonghua Yan Ke Za Zhi 2012;48(1):72-75.
27 Gekka M, Miyata K, Nagai Y, Nemoto S, Sameshima T, Tanabe T,Maruoka S, Nakahara M, Kato S, Amano S. Corneal epithelial barrier function in diabetic patients. Cornea 2004;23(1):35-37.
28 Morishige N, Chikama TI, Sassa Y, Nishida T. Abnormal light scattering detected by confocal biomicroscopy at the corneal epithelial basement membrane of subjects with type II diabetes. Diabetologia 2001;44(3):340-345.
29 Foulks GN, Thoft RA, Perry HD, Tolentino FI. Factors related to corneal epithelial complications after closed vitrectomy in diabetics. Arch Ophthalmol 1979;97(6):1076-1078.
30 Millodot M. The influence of age on the sensitivity of the cornea.Invest Ophthalmol Vis Sci 1977;16(3):240-242.
31 Roszkowska AM, Colosi P, Ferreri FM, Galasso S. Age-related modifications of corneal sensitivity. Ophthalmologica 2004;218(5):350-355.
32 Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol 2007;91(9):1165-1169.
33 Malik RA, Veves A, Walker D, Siddique I, Lye RH, Schady W, Boulton AJ. Sural nerve fibre pathology in diabetic patients with mild neuropathy:relationship to pain, quantitative sensory testing and peripheral nerve electrophysiology. Acta Neuropathol 2001;101(4):367-374.
34 Mocan MC, Durukan I, Irkec M, Orhan M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea 2006;25(7):769-773.
35 Diabetes Association of Chinese Medical Association. Guidelines for the prevention and treatment of type 2 diabetes mellitus in China (2013 Edition). Chinese Journal of Diabetes Mellitus 2014;6(7):447-498.