INTRODUCTION
Glaucoma is the leading cause of irreversible blindness affecting more than 70 million individuals worldwide[1].The current mainstay of treatment involves lowering the intraocular pressure (IOP) to slow or halt progression of optic nerve damage and subsequent vision loss[1-2]. First-line treatment has historically included topical ocular antihypertensive medications with escalation to oral medications, laser, or incisional surgery if conservative measures fail.
Selective laser trabeculoplasty (SLT) was first developed in dermatology for selective photothermolysis to pigmented cellular structures[3]. SLT was then introduced as a non-invasive treatment option for glaucoma in 1995 and ultimately approved by the Food and Drug Administration in 2001[4-5]. SLT uses a Q-switched, frequency-doubled, 532 nm, neodymium-doped yttrium aluminum garnet (Nd:YAG) laser that selectively targets pigmented trabecular meshwork cells without causing collateral damage to adjacent structures[4,6]. Although the mechanism by which laser trabeculoplasty reduces IOP remains uncertain, it appears to involve 3 mechanisms:mechanical distension of Schlemm’s canal, dislodging of trabecular cells, and stimulation of cellular production and turnover of extracellular matrix[7]. Argon laser trabeculoplasty(ALT) is a similar treatment modality that predates SLT,and while a recent systematic review revealed similar IOP reductions following both lasers, histologic and ultrastructural studies have shown that, unlike ALT, SLT produces less extensive damage and no coagulative effects on the trabecular meshwork (TM)[5-6,8-9]. Therefore, SLT is theoretically safer and more repeatable.
When IOP remains uncontrolled despite maximal medical therapy, surgery is required to further reduce IOP.Trabeculectomy remains the most commonly performed glaucoma surgery and produces significant IOP reductions[1,10].In conjunction with trabeculectomy, the ExPress mini shunt(Alcon, Ft Worth, TX, USA), a non-valved stainless steel implant which drains aqueous into the instrascleral space,is often used in an attempt to minimize complications of trabeculectomy[11]. Glaucoma drainage devices, such as the Ahmed valve (New World Medical, Cucamonga, CA, USA)have gained in popularity as an alternative to trabeculectomy and have been shown to result in comparable IOP reductions with fewer hypotony complications[12-14]. Phacoemulsificationtrabeculectomy is a useful option for a patient to simultaneously address cataract and glaucoma[15-18].
Prior studies have shown SLT to produce approximately a 20%-30% reduction in IOP in eyes without prior glaucoma surgery (PGS)[19-21]. To date, only one other study has investigated the utility of SLT in eyes that have previously undergone incisional glaucoma surgery[22]. Zhang et al[22]conducted a prospective study of 18 eyes whose IOP remained uncontrolled following trabeculectomy. These eyes then underwent SLT and experienced an average of 24% in IOP reduction after 9mo.The purpose of the current study is to further explore SLT as a treatment option in eyes with previous incisional glaucoma surgery whose IOP remains or becomes uncontrolled.
SUBJECTS AND METHODS
A retrospective, electronic medical record review of patients at the Medical University of South Carolina (MUSC) was conducted following study approval by the MUSC Institutional Review Board. The study adhered to the principles described in the Declaration of Helsinki. Patients were identified by reviewing scheduling logs for glaucoma laser clinic days over a 3-year period from 2013 to 2015. Clinical data were then gathered beginning at the initial SLT and followed for 1y.
Inclusion criteria for the PGS group consisted of patients who carried a diagnosis of any type of open angle glaucoma and had prior trabeculectomy with Express shunt, Ahmed valve implantation, or phacoemulsification-trabeculectomy surgery.These patients then underwent SLT to further lower IOP due to uncontrolled glaucoma despite maximum tolerated medical therapy. More than a year had transpired between incisional glaucoma surgery and SLT in all cases. A matching control group was also included consisting of patients that underwent SLT but had no prior glaucoma surgery (NPGS).
Exclusion criteria consisted of patients who were, ≤18 years old, unable to have SLT successfully performed, or lost to follow up before 1mo. Also, patients with other prior major incisional eye surgeries apart from cataract surgery alone were excluded.
SLT was performed with the Lumenis Selecta II (Santa Clara, CA, USA) which is a Q-switched Nd:YAG laser producing a single 532 nm wavelength pulse with a 400 mm spot size and 3ns pulse duration. Initial power settings varied between 0.9-1.1 mJ and was titrated until champagne bubbles were produced. Patients received 1 drop of 0.5% topical proparacaine hydrochloride and 0.5% apraclonidine hydrochloride prior to the procedure. An Ocular Hwang-Latina 5.0 single mirror SLT lens (Bellavue, WA, USA) was placed on the eye using 2.5%hydroxypropyl methylcellulose to visualize angle structures.Treatment sessions included 180° of laser performed inferiorly for a total of 50 adjacent, but not overlapping, laser spots along the TM. One drop of 0.5% apraclonidine hydrochloride was administered following the procedure. Post-procedure IOP was checked at approximately 30min to detect early postprocedure pressure spikes prior to departure. The IOP was measured by Goldmann applanation in all patients except for the immediate post-procedure IOP checks for which a Tonopen(Reichert, Depew, NY, USA) was used. If a re-treatment was subsequently performed, the laser was applied to the superior 180° of TM in the same fashion.
The primary outcome measure was IOP reduction from pre-SLT baseline. IOP was obtained pre-SLT and followed at regular intervals of 1, 6mo, and 1y following the SLT procedure. If the patient’s IOP was not at target following 180° treatment, the patient underwent a second 180° treatment at 1mo post SLT, and data were collected at 1, 6, and 12mo following the completion of the full 360° treatment when needed. Success was defined as a 20% IOP reduction compared to pre-SLT pressure, consistent with prior studies[19,21-22]. In addition, the number of medications including topical ocular antihypertensives as well as oral carbonic anhydrase inhibitors were recorded pre-SLT and at 1y post-SLT.
Results were analyzed using SPSS Statistics Premium v24(IBM Corporation, Armonk, NY, USA) and Excel 2017(Microsoft, Redmond, WA, USA). Continuous data were reported as mean±standard deviation and compared using paired t-test, unpaired t-test, or Mann-Whitney U test as appropriate. Categorical data were compared using χ2test and analysis of variance (ANOVA). Differences were considered statistically significant when the P-value <0.05.
RESULTS
A total of 106 eyes met inclusion criteria with 53 in each group. Demographics are summarized in Table 1. Mean age was higher in the PGS group at 71.8±8.6 vs 67.7±10.5y in NPGS group (P=0.03). Other baseline characteristics including sex, race, and type of glaucoma were evenly matched among groups. Mean IOP pre-SLT was 19.2±4.3 mm Hg and 20.6±6.0 mm Hg for PGS and NPGS groups, respectively (P=0.17).At each time point, the difference in mean IOP for each group was not statistically significant between the PGS and NPGS groups over 1y (P>0.17). The overall mean IOP at pre-SLT, 1,6mo, and 1y is shown in Figure 1.
For IOP reduction from baseline, the PGS and NPGS groups had a 7.11% and 4.56% decrease at 1mo and a 7.3% and 10.8% decrease at 6mo (P<0.05), respectively, as shown in Figure 2. At 1y, PGS group maintained a 3.51% reduction while the NPGS group showed a 0.66% increase in mean IOP,but neither reached statistical significance (P>0.32). Between groups, there was no statistically significant difference in IOP reductions from baseline at any time over 1y (P=0.63).
Table 1 Demographic characteristics for the PGS and NPGS groups
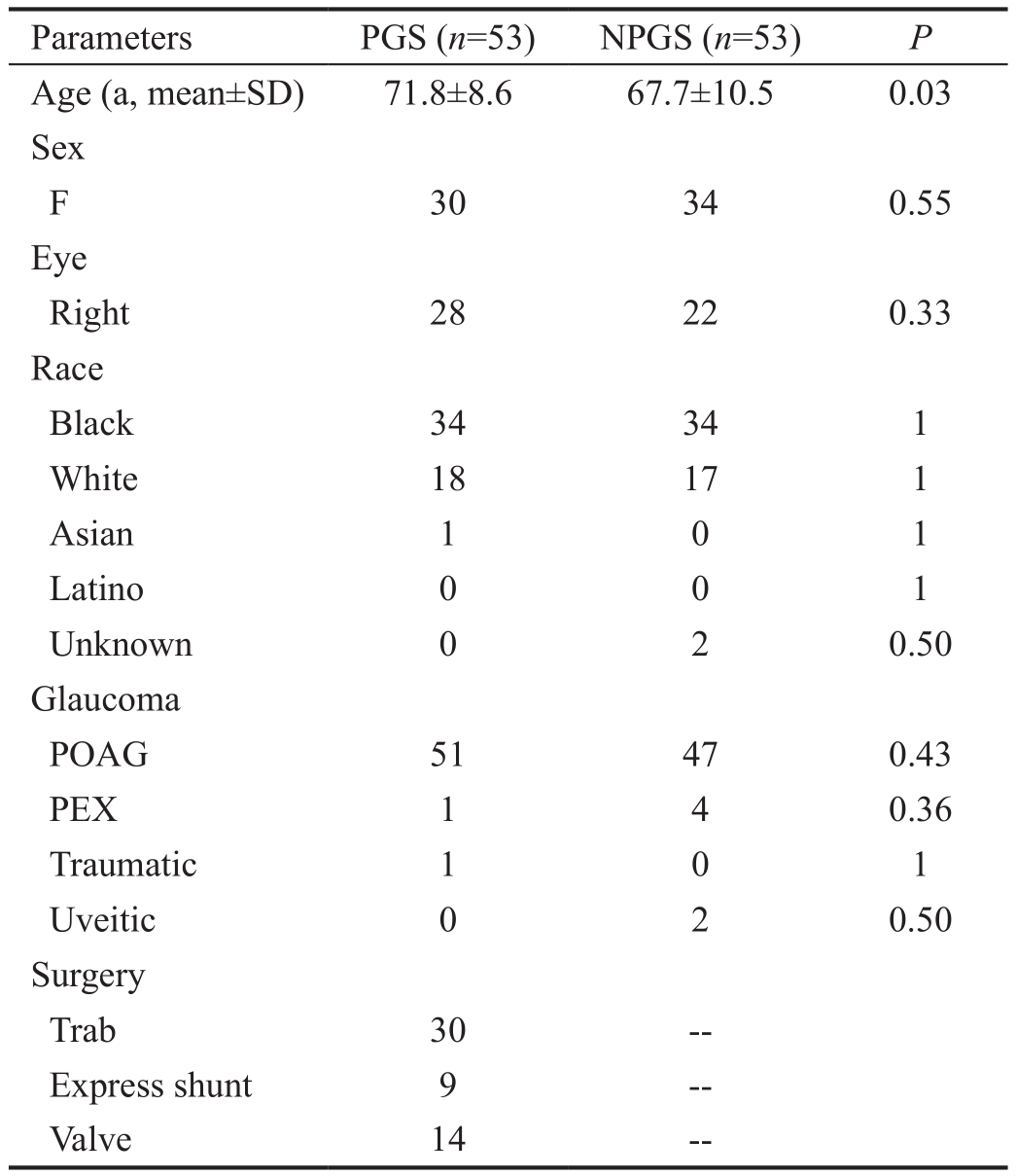
POAG: Primary openangle glaucoma; PEX: Pseudoexfoliation sydrome.
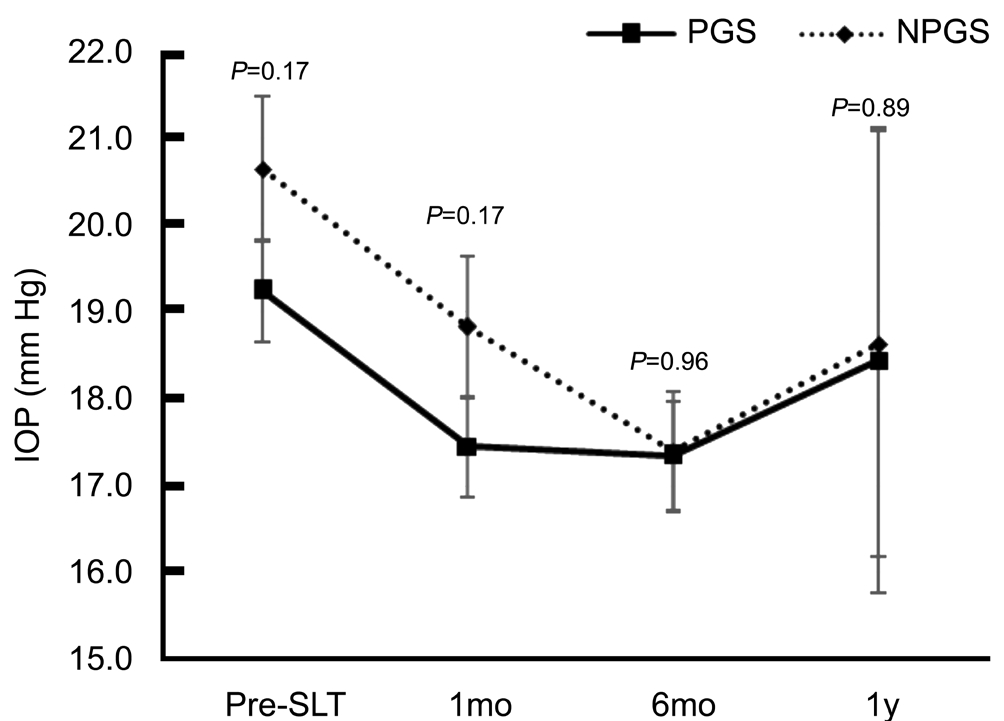
Figure 1 Mean IOP over 1y including baseline pre-SLT for PGS and NPGS groups.
Success in this study was defined as an IOP reduction of >20%.At 1mo, 28.3% and 24.5% of eyes in the PGS and NPGS groups, respectively, met this criterion for success (Figure 3).At 1y, 27.9% and 31.7% of eyes in the PGS and NPGS groups met this benchmark. There was no statistically significant difference between the groups for percentage of successful treatments at any time point (P>0.66).
The mean number of medications pre-SLT was 2.5±1.6 and 2.0±1.5 for the PGS and NPGS groups, respectively (P=0.09).Both groups were on a higher number of medications at 1y averaging 2.9±1.4 and 2.2±1.4 for PGS and NPGS groups,respectively; however, the difference for PGS group reached statistical significance (P<0.01) while the difference for the NPGS group did not (P=0.13).
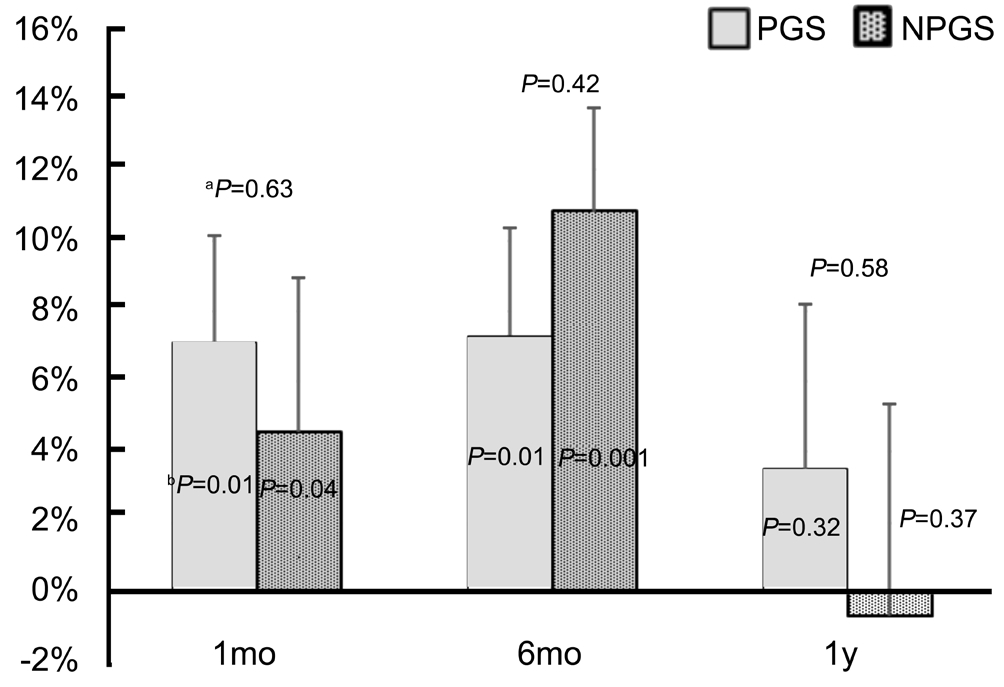
Figure 2 Mean percentage reduction in IOP for PGS and NPGS groupsaP-value from comparison of % IOP reduction in PGS versus NPGS groups at each time point;bP-value from comparison of % IOP reduction with baseline pre-SLT IOP for each group.

Figure 3 Percentage of eyes in PGS and NPGS groups meeting criteria for successful treatment of a >20 mm Hg decrease in pre-SLT IOP.
The PGS group was subdivided into eyes with initial IOP of≥21 mm Hg (n=16) or <21 mm Hg (n=37). The mean pre-SLT IOP was 24.2±3.2 and 17.1±2.6 mm Hg for the higher and lower initial IOP groups, respectively (P<0.01). The IOP trend over 1y for 2 groups based on pre-SLT IOP is shown in Figure 4. At 1, 6mo, and 1y, the group with ≥21 mm Hg pre-SLT IOP exhibited mean reductions in IOP of 18.1%, 16.7%,and 8.4%, respectively (P<0.001, P<0.01, P=0.31) as shown in Figure 5. At each of these time points, the mean percent IOP reduction was greater for the group with higher pre-SLT IOP,and this difference reached statistical significance at 1 and 6mo(P≤0.05). Furthermore, the group with initial IOP of <21 mm Hg did not exhibit significant IOP reductions at any time point.This group showed a mean IOP reduction of 2.3%, 3.4%, 1.1%at 1, 6mo, and 1y, respectively (P=0.39, P=0.19, and P=0.72).No statistically significant differences in post-SLT IOP or percent IOP reduction were observed based on gender or type of surgery within the PGS group. However, when examining racial differences, a larger mean percent IOP reduction was observed in non-Hispanic white patients compared to black patients by 15.9% (P=0.01; Figure 6). Although the mean pre-SLT IOP was higher in whites than blacks at 20.4±4.3 vs 18.8±4.1 mm Hg, respectively, the difference was not statistically significant (P=0.19). Also, the differences in mean IOP at 1, 6mo, a 1y did not exhibit statistically significant differences between white and black patients, nor did the difference in mean percent IOP reduction observed at 1mo persist at 6mo or 1y between these groups. Since there was only 1 Asian patient, no correlations based on this race were able to be explored.

Figure 4 Mean IOP within PGS group based on whether baseline IOP ≥21 mm Hg (n=16) or <21 mm Hg (n=37).
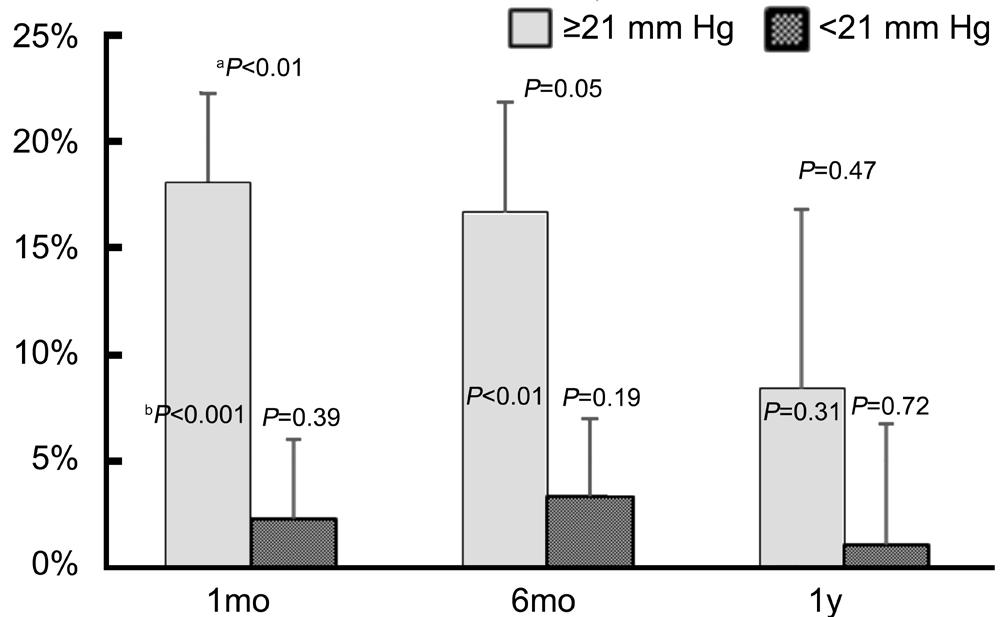
Figure 5 Reduction in IOP of eyes within the PGS group with either pre-SLT IOP ≥21 mm Hg or <21 mm HgaP values from comparison of pre-SLT IOP ≥21 mm Hg vs <21 mm Hg;bP values from comparison of pre- to post-SLT IOP reduction.
DISCUSSION
In advanced glaucoma, incisional surgery is often required to control IOP by bypassing the TM with shunting and filtering procedures[2,10]. SLT, on the other hand, reduces IOP by improving the function of the TM and is commonly employed in earlier stages of glaucoma before significant TM derangements set in. Current practice patterns suggest that if a patient required incisional surgery, the TM was deemed largely non-functional and therefore had to be bypassed. Therefore,SLT is not commonly employed following incisional glaucoma surgery as it would be assumed to have minimal effect.
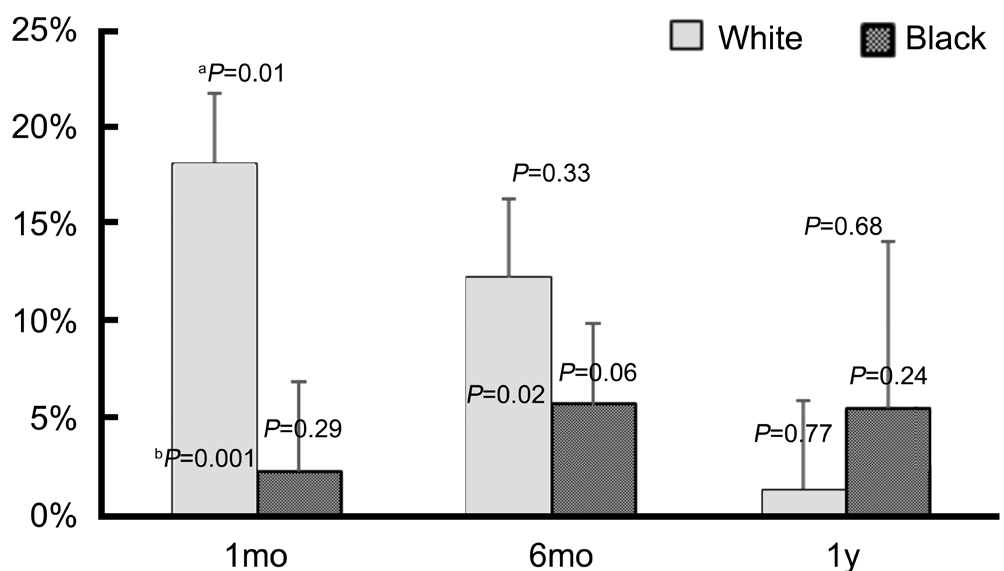
Figure 6 Percent IOP reduction from baseline between white and black patientsaP values from comparison between white and black patients;bP values from comparison of pre- to post-slt IOP reduction.
However, although the TM outflow system may be dysfunctional in eyes with severe enough glaucoma to require surgery, there appears to be a significant residual level of activity. This notion is supported by the 25%-30% of patients who underwent SLT at some point after their incisional glaucoma surgeries and exhibited at least a 20% reduction in IOP. The IOP reduction following SLT suggests some level of residual function in the TM pathway that still has potential to be modulated in a postsurgical eye.
It is particularly important to look at the decrease in IOP in the PGS group with a baseline IOP ≥21 mm Hg. At this institution,it is common to offer SLT treatment to patients on maximum tolerated medical therapy with IOP above target. Laser treatment is also commonly offered to patients who would be at target IOP on medications but have intermittent compliance or particularly poor tolerance to their medications. The primary benefit in these patients is to decrease their reliance on drops or delay starting additional drops. When the PGS group is subdivided based on initial IOP, the group with initial IOP<21 mm Hg had a significantly smaller reduction in IOP at follow up. This group subsequently skewed the overall mean IOP lowering effect toward lower mean IOP reductions.
Prior SLT studies have historically had a mean baseline IOP ranging from 23.8 to 29.3 mm Hg[19,21,23-27], which more closely approximates the initial IOP of the PGS subgroup with higher baseline ≥21 mm Hg, which was 24.2±3.2 mm Hg in this study.This cohort, therefore, is more likely to represent the group of patients who have had prior incisional glaucoma surgery but need further IOP reduction in addition to medications versus an alternative to replacing medications. This group experienced a 16.7% reduction in IOP at 6mo, which approximates published results in eyes without prior incisional glaucoma surgery. This effect, however, did seem to dissipate by 1y when only an 8.4% IOP reduction from baseline was observed.Both PGS and NPGS groups were on a higher number of mean medications at 1y compared to pre-SLT. Although this difference was statistically significant in the PGS group,the actual difference was only an average of 0.4 additional drops. This finding suggests that by 1y, there was a trend to be on more medications, which coincides with the observed tapering of IOP lowering effect from the SLT. However, an additional benefit of SLT in these patients is a decrease in diurnal IOP fluctuation[21]. IOP fluctuation has been shown to be an independent risk factor for progression of glaucoma, and although medications can reduce IOP fluctuation, compliance issues may limit their effect[21,28].
An important observation is that none of these patients required incisional surgery during that first year and that successful IOP lowering was obtained with SLT and medications changes.This finding is clinically important, especially in areas where subspecialty glaucoma care can be difficult to obtain. In current ophthalmology practice, patients who have required incisional glaucoma surgery are often referred to glaucoma subspecialists when their IOP starts to trend upwards again in anticipation of additional incisional surgery. However, SLT is a procedure commonly performed by comprehensive ophthalmologists,and this study supports a trial of SLT in patients with prior incisional glaucoma surgery who may have otherwise been referred to a subspecialist for additional incisional surgery.
The major limitation of the study is its retrospective nature.As described above, there may have been different goals for performing the SLT depending on a patient’s initial IOP.Some patients were on maximal tolerated therapy and needed adjunctive treatment while others were primarily treated with SLT to replace medications. A prospective study would be advantageous to better control for this potential selection bias.While further investigation regarding the efficacy of SLT following prior incisional glaucoma surgery is warranted, this study is consistent with Zhang et al[22]suggesting that SLT is an effective and safe treatment option to lower IOP to avoid or postpone subsequent incisional glaucoma procedures. SLT is a widely-available treatment modality and could help further lower IOP in patients who otherwise would face the burden and potential complications of additional incisional glaucoma surgery.
In conclusion, SLT may be efficacious in eyes with prior incisional glaucoma surgery and can result in similar IOP reductions compared to eyes without PGS. Therefore, it warrants consideration as treatment modality in eyes requiring further IOP reduction even in setting of prior incisional glaucoma surgery.
ACKNOWLEDGEMENTS
Authors’ contribution: Stud y design: Kammerdiener LL, Sharpe RA, Nutaitis MJ; Data collection and analysis:Kammerdiener LL, Sharpe RA, Williams DB, Das SK;Manuscript preparation: Kammerdiener LL, Sharpe RA,Williams DB, Das SK, Nutaitis MJ.
Conflicts of Interest: Sharpe RA, None; Kammerdiener LL, None; Williams DB, None; Das SK, None; Nutaitis MJ,None.
REFERENCES
1 Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311(18):1901-1911.
2 The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol 2000;130(4):429-440.
3 Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science 1983;220(4596):524-527.
4 Latina MA, Park C. Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW laser interactions. Exp Eye Res 1995;60(4):359-371.
5 Latina MA, Tumbocon JA. Selective laser trabeculoplasty: a new treatment option for open angle glaucoma. Curr Opin Ophthalmol 2002;13(2):94-96.
6 Kramer TR, Noecker RJ. Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasty in human eye bank eyes. Ophthalmology 2001;108(4):773-779.
7 Van Buskirk EM, Pond V, Rosenquist RC, Acott TS. Argon laser trabeculoplasty. Studies of mechanism of action. Ophthalmology 1984;91(9):1005-1010.
8 Cvenkel B, Hvala A, Drnovsek-Olup B, Gale N. Acute ultrastructural changes of the trabecular meshwork after selective laser trabeculoplasty and low power argon laser trabeculoplasty. Lasers Surg Med 2003;33(3):204-208.
9 McAlinden C. Selective laser trabeculoplasty (SLT) vs other treatment modalities for glaucoma: systematic review. Eye (Lond) 2014;28(3):249-258.
10 Boland MV, Ervin AM, Friedman DS, Jampel HD, Hawkins BS,Vollenweider D, Chelladurai Y, Ward D, Suarez-Cuervo C, Robinson KA. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;158(4):271-279.
11 Shaarawy T, Goldberg I, Fechtner R. EX-PRESS glaucoma filtration device: review of clinical experience and comparison with trabeculectomy.Surv Ophthalmol 2015;60(4):327-345.
12 Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol 2012;153(5):789-803.e782.
13 Tran DH, Souza C, Ang MJ, Loman J, Law SK, Coleman AL,Caprioli J. Comparison of long-term surgical success of Ahmed valve implant versus trabeculectomy in open-angle glaucoma. Br J Ophthalmol 2009;93(11):1504-1509.
14 Arora KS, Robin AL, Corcoran KJ, Corcoran SL, Ramulu PY. Use of Various glaucoma surgeries and procedures in medicare beneficiaries from 1994 to 2012. Ophthalmology 2015;122(8):1615-1624.
15 Zhang ML, Hirunyachote P, Jampel H. Combined surgery versus cataract surgery alone for eyes with cataract and glaucoma. Cochrane Database Syst Rev 2015(7):CD008671.
16 Anders N, Pham T, Holschbach A, Wollensak J. Combined phacoemulsification and filtering surgery with the 'no-stitch' technique.Arch Ophthalmol 1997;115(10):1245-1249.
17 Gimbel HV, Meyer D, DeBroff BM, Roux CW, Ferensowicz M.Intraocular pressure response to combined phacoemulsification and trabeculotomy ab externo versus phacoemulsification alone in primary open-angle glaucoma. J Cataract Refract Surg 1995;21(6):653-660.
18 Liaska A, Papaconstantinou D, Georgalas I, Koutsandrea C,Theodosiadis P, Chatzistefanou K. Phaco-trabeculectomy in controlled,advanced, open-angle glaucoma and cataract: parallel, randomized clinical study of efficacy and safety. Semin Ophthalmol 2014;29(4):226-235.
19 Goyal S, Beltran-Agullo L, Rashid S, Shah SP, Nath R, Obi A, Lim KS.Effect of primary selective laser trabeculoplasty on tonographic outflow facility: a randomised clinical trial. Br J Ophthalmol 2010;94(11):1443-1447.
20 Babighian S, Caretti L, Tavolato M, Cian R, Galan A. Excimer laser trabeculotomy vs 180 degrees selective laser trabeculoplasty in primary open-angle glaucoma. A 2-year randomized, controlled trial. Eye (Lond)2010;24(4):632-638.
21 Nagar M, Luhishi E, Shah N. Intraocular pressure control and fluctuation: the effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol 2009;93(4):497-501.
22 Zhang H, Yang Y, Xu J, Yu M. Selective laser trabeculoplasty in treating post-trabeculectomy advanced primary open-angle glaucoma.Exp Ther Med 2016;11(3):1090-1094.
23 Lai JS, Chua JK, Tham CC, Lam DS. Five-year follow up of selective laser trabeculoplasty in Chinese eyes. Clin Exp Ophthalmol 2004;32(4):368-372.
24 Damji KF, Bovell AM, Hodge WG, Rock W, Shah K, Buhrmann R,Pan YI. Selective laser trabeculoplasty versus argon laser trabeculoplasty:results from a 1-year randomised clinical trial. Br J Ophthalmol 2006;90(12):1490-1494.
25 Rosenfeld E, Shemesh G, Kurtz S. The efficacy of selective laser trabeculoplasty versus argon laser trabeculoplasty in pseudophakic glaucoma patients. Clin Ophthalmol 2012;6:1935-1940.
26 Chen E, Golchin S, Blomdahl S. A comparison between 90 degrees and 180 degrees selective laser trabeculoplasty. J Glaucoma 2004;13(1):62-65.
27 Katz LJ, Steinmann WC, Kabir A, Molineaux J, Wizov SS, Marcellino G; SLT/Med Study Group. Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma 2012;21(7):460-468.
28 Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K.Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000;9(2):134-142.