INTRODUCTION
Surgery plays an important role in overall management for diabetic macular edema (DME)[1-3]. For many years,conventional first-line treatment of DME has been direct photocoagulation of leaking microaneurysms combined with grid laser photocoagulation of areas of the capillary bed with diffuse leakage[4-6]. An alternative treatment is the use of vascular endothelial growth factor (VEGF) inhibitor agents[7-10].However, these agents often require multiple injections (every 4 to 8wk)[7-10]. Such repeated injections can be costly, carry a small but real risk of complications (e.g. endophthalmitis) and may be associated with a small increased risk of myocardial infarction in subjects already at risk[11]. Additionally, not all patients respond favorably to intravitreal anti-VEGF treatment[12]and some authors even suspect that chronic VEGF suppression may have a neurotoxic retinal effect[13].
It has been suggested that inflammation plays a major role in the development of DME[14-18]. Consequently, the use of intravitreal corticosteroids has been introduced as a possible therapy, with the result of DME resolution and significant improvement in BCVA, even in cases where anti-VEGF therapy has failed[1,14].
Among the intravitreal corticosteroids currently available is the sustained-delivery 0.7 dexamethasone (DEX)intravitreal implant (Ozurdex, Allergan, Inc., Irvine, CA, USA),which provides controlled release of DEX from an inactive biodegradable polymer matrix[19-22]. The DEX implant can be detected in retina and vitreous for up to 6mo after application with a maximum concentration at about 2mo[23], although some authors suggest that its clinical efficacy is limited to 4mo in most eyes[10,14]. It was approved for DME treatment by the European Medicines Agency (EMA) and by the American Food and Drugs Administration in June 2014.
In this retrospective case series study we reviewed the medical records of DME patients who had received DEX implants at least one year before.
SUBJECTS AND METHODS
Patients were diagnosed and treated consecutively as they were seen in our single-center institutional setting; the“Complejo Hospitalario Universitario de Canarias” (CHUC).All patients provided informed consent. The study adhered to the tenets of the Declaration of Helsinki. It was approved by the Ethics Review Committee of CHUC and by the Spanish drug regulatory agency (Agencia Española de Medicamentosy Productos Sanitarios) with code APR-DEX-2014-01. In this study, DEX implant was used off-label for the management of DME. This use, despite not having EMA approval at the time,was supported by published medical literature[10,14,19-23].
The study sample comprised adult diabetic patients with central fovea thickening and impaired visual acuity resulting from DME for whom previous standard treatments (laser or anti-VEGF therapy) showed no improvement in both retinal thickness and visual acuity after at least 3mo of treatment.The laser protocol involved macular focal laser or modified grid laser. Regarding anti-VEGF therapy, bevacizumab and ranibizumab were the drugs that were available at the time of the study in our setting, with a protocol consisting in a loading dose of 3 monthly injections followed by a prorenata (PRN)pattern which sometimes was supplemented with macular laser.The interval from the last laser treatment to DEX treatment was at least 6mo. The last injection of anti-VEGF was performed at least 3mo before starting treatment with a DEX implant.
DME was defined as macular thickening (≥300 µm) involving the center of the fovea that was evident on biomicroscopy and optical coherence tomography (OCT) (Cirrus HD-OCT.Carl Zeiss Meditec, Dublin, CA, USA). Exceptionally, 11 eyes of 11 naive patients (not previously treated with other clinical strategy) were included due to the presence of recent cardiovascular events or because they could not travel to the hospital monthly to receive anti-VEGF therapy due to the particular geography of this setting (Canary Islands). Patients in naïve group also met the inclusion criteria (adult diabetic patients with central fovea thickening and impaired visual acuity resulting from DME) and did not have any other ocular illness apart from DME.
DEX implant 0.7 mg was injected into the vitreous cavity using standard protocols[20]by 6 ophthalmologists belonging to the CHUC. All patients received a DEX implant at 1 to 15d after the decision to use this therapeutic option, considered as the baseline visit in this study.
Outcome data were obtained from patient visits at baseline and at months 1, 3, 5, 9 and 12 after the first DEX implant injection. At each of these visits, patients underwent measurement of best corrected visual acuity (BCVA) with Early Treatment Diabetic Retinopathy Study (ETDRS) charts,a complete eye examination and measurement of central macular thickness (CMT) and macular volume (MV) carried out with OCT images. OCT was performed with the 512×128 scan pattern of the Cirrus OCT where a 6×6 mm2area on the retina is scanned with 128 horizontal lines with spacing between lines of 47 µm, each consisting of 512 A-scans per line and with 1024 data points per A-scan (total data points > 67 million),within a scan time of 2.4s. Degradation of DEX implant was tracked by fundus. Each patient could have received up to 2 additional treatments as needed. The re-treatment criteria were based on significant loss of BCVA (>10 ETDRS letters) with respect to the best reached value after the DEX implant or CMT value back to baseline.
The main outcomes measurements were the mean change in BCVA and in CMT with respect to the baseline value.Secondary outcome measures included mean changes in MV as determined by OCT and the development of any adverse side effects resulting from the intravitreal injection of DEX implant. The response to treatment was evaluated regardless of age and sex. Results were analyzed globally as a unique group and, additionally, were also analyzed after dividing the population into three subgroups: naive, pseudophakic and phakic eyes.
Statistics was performed using Student’s t-test for comparison between visits. Between subgroups, one-way analysis of variance (ANOVA) was used. Bonferroni correction was applied when necessary. P≤0.05 were considered significant.
RESULTS
Baseline Characteristics Medical histories of 85 patients(116 eyes) were reviewed and baseline characteristics were recorded (Table 1). Patients were treated with DEX implants between January 2013 and February 2014. Except for two previously vitrectomized patients, all patients receiving a DEX implant at this hospital between these dates were included in the study. The duration of study period was 1y in all cases. The study completion rate was 97% (113 of 116), with 3 patients exiting the study early, 2 because of simply they did not want to travel between islands to attend the consultations and 1 lost to follow-up for moved to another country. Of the 113 eyes studied who completed the follow-up, 72 (63.7%) were pseudophakic, 11 (9.7%) were naive and 30 (26.6%) were phakic eyes. Previous to the DEX implant treatment, 11 eyes(9.8%) received anti-VEGF treatment, 34 eyes (30%) received laser treatment and 57 eyes (50.4%) received a combined laser and anti-VEGF treatment. No intravitreal treatments other than DEX implants were administered to any eye during the study.All patients were seen at months 1, 3, 5, 9 and 12 after the first DEX implant injection with a variation of ±2wk at months 1,3 and ±1mo at months 5, 9 and 12.
Table 1 Data of the patients at the baseline visit
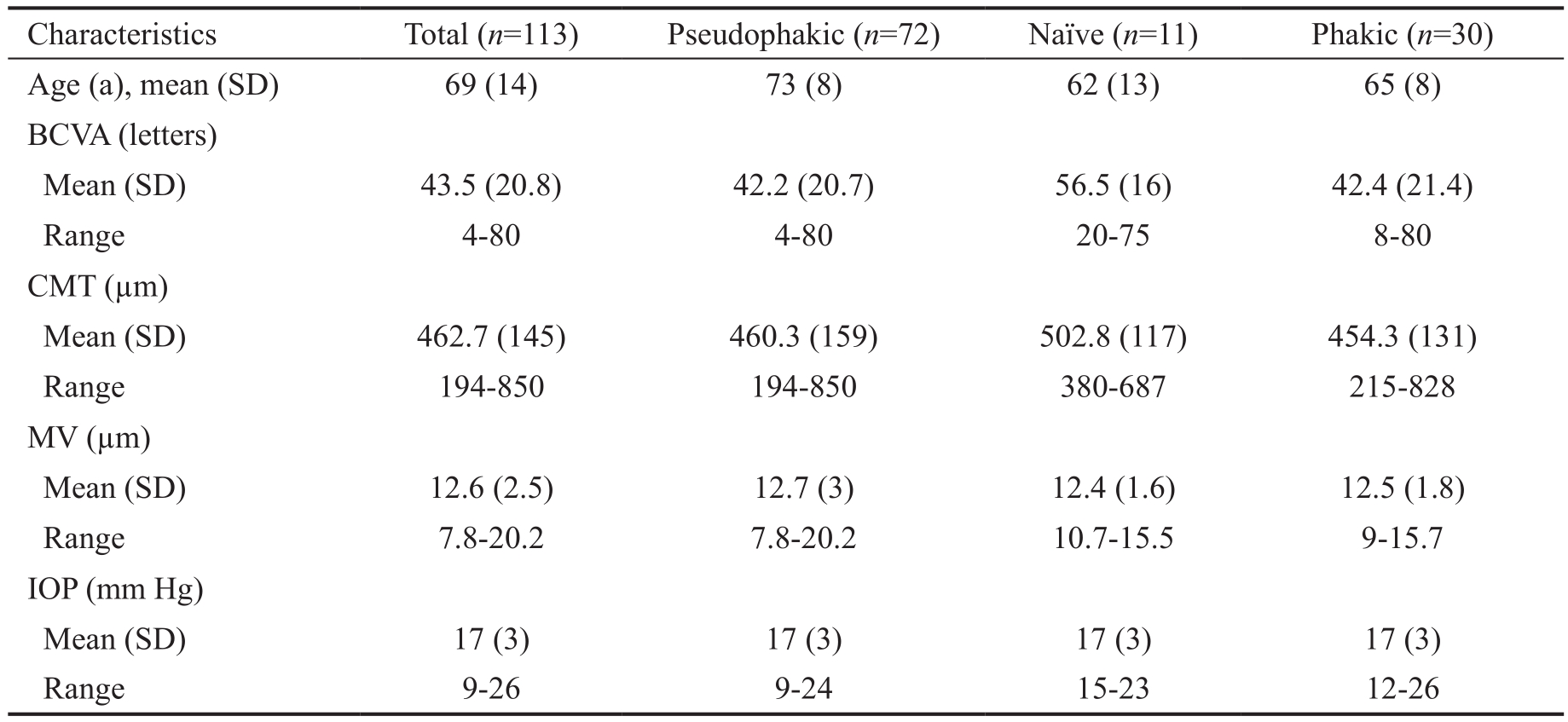
There were no statistically significant differences in clinical and demographic variables between subgroups at baseline except that BCVA was higher in the naive subgroup (P≤0.05).
Visual Acuity Mean values of BCVA improved from baseline and remained higher throughout the study in all subgroups, as shown in Table 2. The maximum mean increase in BCVA from baseline occurred at 3mo. This was +9.7 ETDRS letters for the whole sample, +6.9 ETDRS letters for the pseudophakic subgroup, +8.8 ETDRS letters for the naive subgroup and +14 ETDRS letters for the phakic subgroup. After 3mo, BCVA gradually declined as shown in Figures 1 and 2. No statistically significant difference was noted between subgroups.
Figure 3 shows the proportion of patients with BCVA worsening of at least 5 ETDRS letters from baseline at follow-up visits.Less than 5% of the whole sample showed BCVA worsening of at least 5 letters at month 3 (noticeably 0 in the naïve subgroup). This proportion increased to 15% for the whole sample at subsequent visits. Thus 85% of the whole sample did not show BCVA worsening of 5 ETDRS letters or more from baseline to the end of study period.
Central Macular Thickness As shown in Figure 4, CMT mean values at 1mo were significantly lower for the whole sample and for all the subgroups with respect to baseline values, and this improvement was also observed at month 3.Mean values remained lower than baseline values throughout the rest of the study for all subgroups except for the phakic subgroup, where an increase was noted at month 5. The results showed no significant differences between subgroups at any visit except at month 9 where CMT was significantly reduced in the pseudophakic subgroup (387.5±150 µm) with respect to the phakic subgroup (511.56±175.6 µm).
Table 2 Visual acuity of the sample
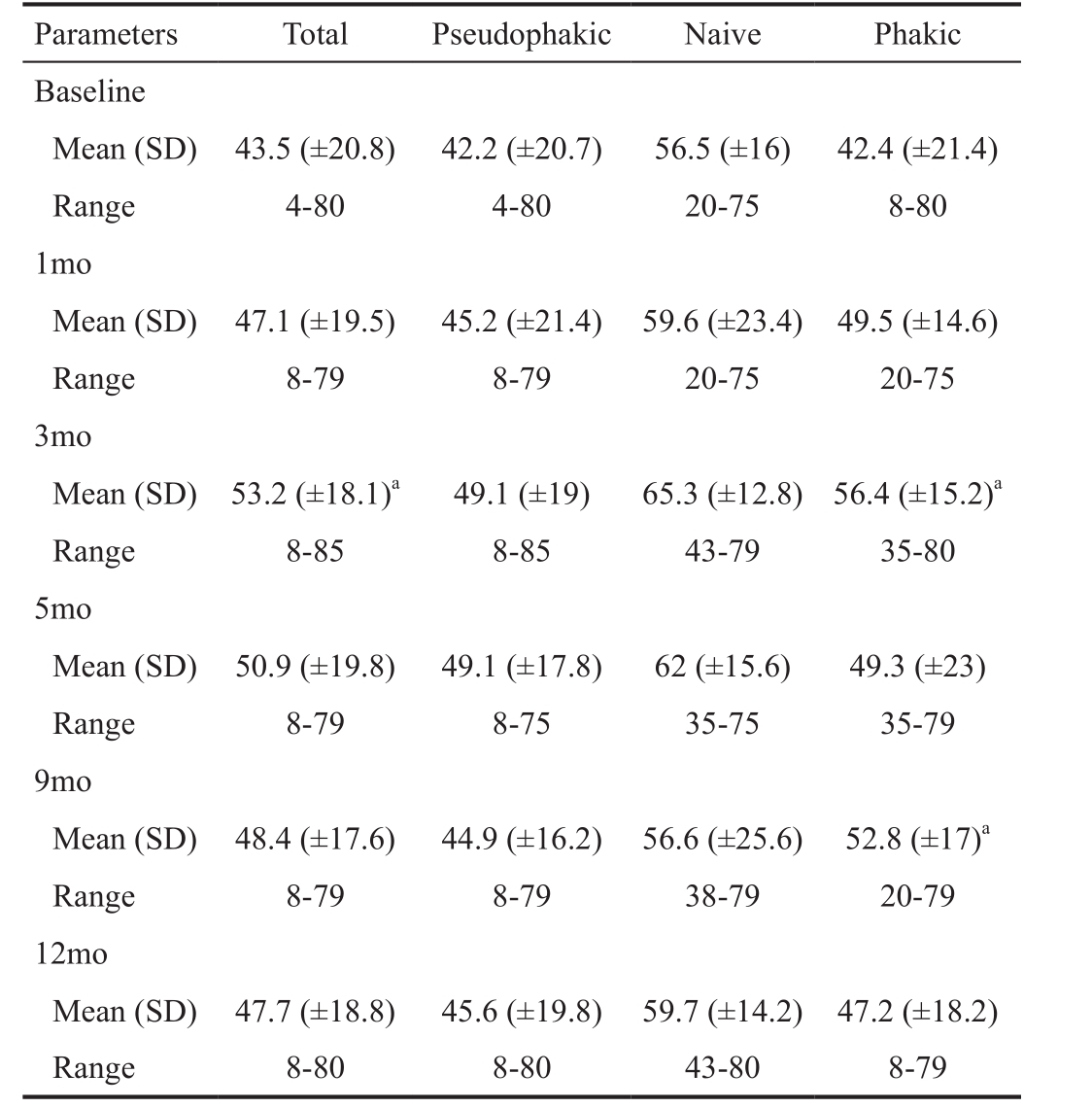
aStatistically significant difference (P≤0.05) with respect baseline value.

Figure 1 BCVA for each subgroup at baseline, months 1, 3, 5, 9 and 12.
Macular Volume As shown in Figure 5, at months 1 and 3 mean MV had decreased compared with baseline and then gradually increased during the rest of the study period. However, mean MV for the whole sample and the pseudophakic subgroup decreased statistically at months 1 and 3. MV values for each subgroup were analyzed after each follow-up visit and comparison showed no significant differences between subgroups at any of the visits.
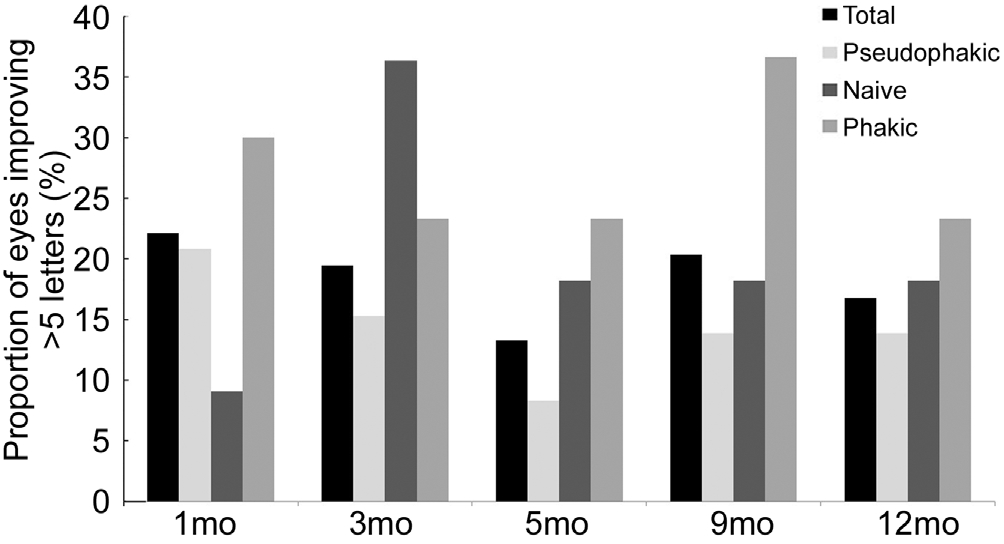
Figure 2 Proportion of eyes with BCVA improvement of at least 5 ETDRS letters at months 1, 3, 5, 9 and 12.
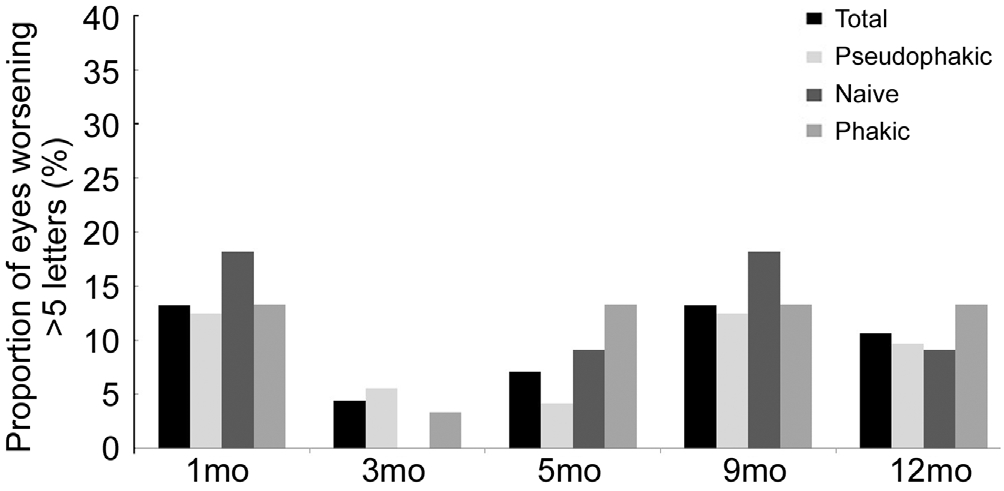
Figure 3 Proportion of eyes with BCVA worsening of at least 5 ETDRS letters at months 1, 3, 5, 9 and 12.
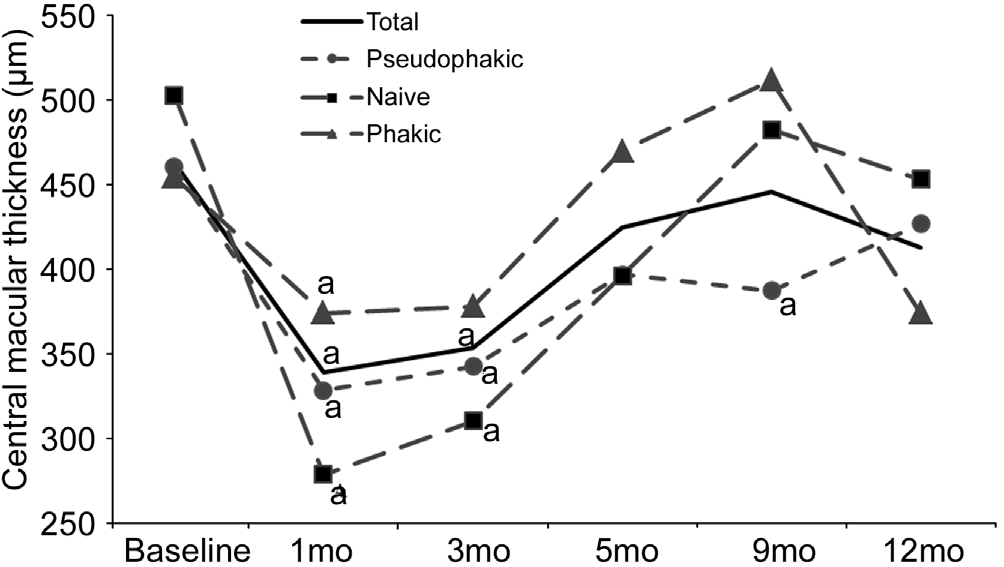
Figure 4 Mean CMT for each subgroup at baseline, months 1, 3,5, 9 and 12aP≤0.05.
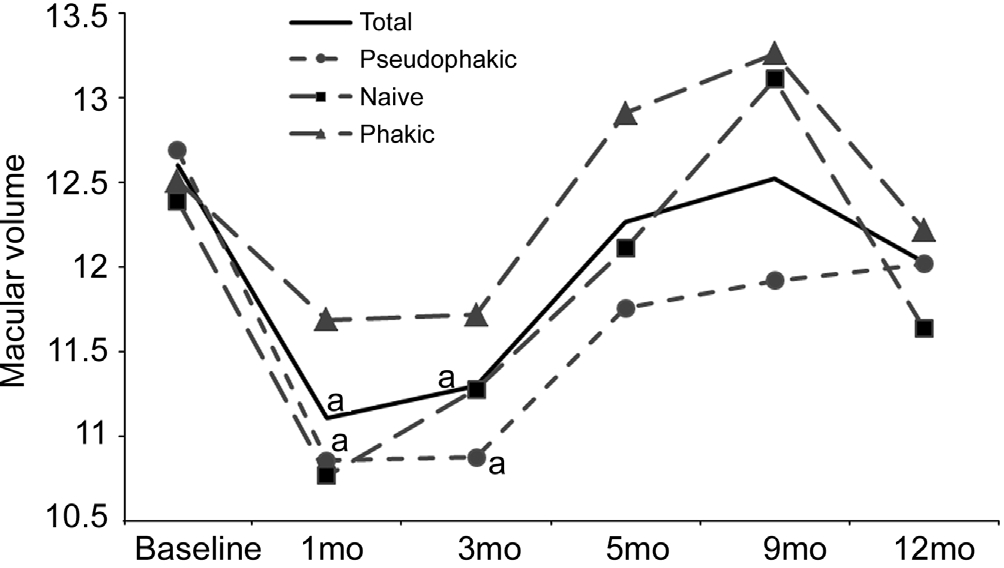
Figure 5 Mean MV for each subgroup at baseline, months 1, 3, 5,9 and 12aP≤0.05.
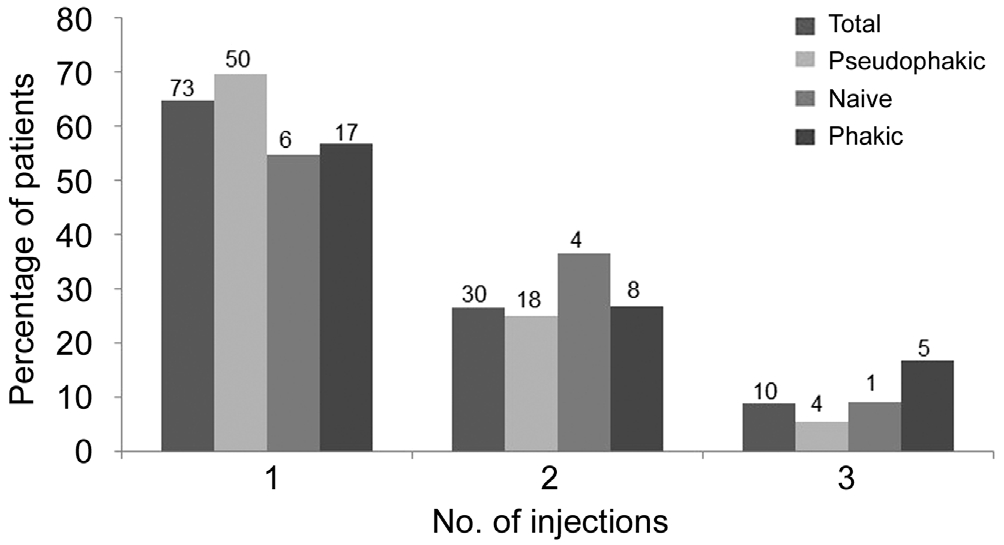
Figure 6 Proportion of eyes receiving 1, 2 or 3 injections during the 12mo study period The number of eyes is shown at the top of each bar.
Number of Treatments Patients received a mean 1.44 injections each. The percentage of patients receiving 1, 2 or 3 injections was similar between subgroups as reflected in Figure 6. Most patients (65%) received one injection, 26%received 2 injections and 9% received 3 injections. The interval between injections was at least 3mo in all cases. Time intervals(1 to 12mo) are referred from the date of the first injection,regardless of the patient may receive more than one.
Adverse Events Mild vitritis was diagnosed in one eye which required no treatment and resolved spontaneously. An increase of IOP ≥5 mm Hg was observed in 16 eyes (14%) during study period, of which 5 eyes (4%) showed an increase of IOP≥10 mm Hg with respect to baseline values. In all cases,increased IOP was managed with topical medication or observation; none required surgery. No other major ocular events or major systemic complications were observed.
DISCUSSION
The great majority of patients (90%) had been unsuccessfully treated with anti-VEGF or laser therapy, but responded satisfactorily to treatment with DEX implant, as found in previous studies[14,24]. At 1mo after treatment, the whole sample showed significant improvement in CMT and MV. At 3mo,the whole sample not only showed significant improvement in CMT and MV, but also in BCVA, suggesting that anatomic improvement preceded functional improvement in these cases.This is consistent with the findings of the Diabetic Retinopathy Clinical Research network (DRCR.net)[25].
At months 5, 9 and 12, the whole sample also showed improvement in these three parameters with respect to baseline values but the differences were not significant. Our results indicate that the greatest effectiveness of DEX implant occurred in the first 3mo after the first injection and then gradually decreased, as found in another study[26]. This may be due to decreasing vitreous concentrations of DEX over time[23].Most patients in this study received only one injection (65%).The mean number of injections per patient (1.44 in one year)was lower than that used in the BEVORDEX study[10]but similar to that used in a study by Callanan et al[22]and in the MOZART study[27]..This reflects the fact that our patients received DEX implants on a PRN basis which was probably too lax; BCVA values were allowed to decline too much before re-treatment. Currently, there is no established algorithm for the treatment of DME with DEX implants, and the optimal interval between injections or the effect of a loading dose remain unknown. We observed a clear decrease in the beneficial effect of a DEX implant at 3mo of the initial treatment. We can only speculate on what would have happened if earlier re-treatments had administered, but maybe a reinjection at 3-4mo may have helped to maintain that response. The large number of patients together with the fact that the vision had not dropped to the baseline value despite under-treatment could give information about the importance on inflammation in chronic DME and the durability of the effect is to a the benefit of sustained release drug design.
Previous studies have suggested that intravitreal corticosteroid treatment may be more effective in pseudophakic eyes than in phakic eyes[28-29]. We divided the whole sample of patients into three subgroups: pseudophakic, naive and phakic eyes. Despite marginal differences were found between subgroups as can be seen at some points in Figures 4 and 5, summarizing, we found no conclusive difference between the subgroups. All showed a similar positive response to DEX implant with a pattern that is comparable to that found in other similar studies[14,20,30]. Naive patients seemed to have a slower response to the DEX implant,as reflected in Figure 2, but the low number of patients in the naive subgroup means the results of this sub-analysis should be taken with caution.
It is noteworthy that the improvement in CMT did not manifest in improved visual outcomes, a finding also reported by other authors[10,27,29]. Despite this, we believe that follow-up with OCT images and BCVA measurement in patients with DME is needed to provide an overall view of patient evolution.
Regarding safety, DEX implants resulted in very few and mild adverse effects in this series of patients, in agreement with the findings of other similar studies[21,25]. An increase of IOP≥5 mm Hg occurred in 14% of patients and ≥10 mm Hg in 4%,also similar to the results reported in other studies[22,24]. Only one mild vitritis was diagnosed, which resolved spontaneously.There were no cases of serious local or systemic adverse events. However, the low number of re-treatments in this study and the fact that steroids cause cataract only after one or two years could have affected the incidence of cataract.
In conclusion, DEX implants in DME patients resulted in improved anatomical and functional parameters, especially evident at early stages of treatment. Re-treatment was used sparingly-only when BCVA or CMT showed signs of reverting to baseline values. Our findings suggest that this therapeutic option is capable of halting the deterioration of visual function,even in difficult cases that have failed to respond to previous therapies. In our study, this was achieved without clinically significant side effects, which supports its use in most types of DME, including naive patients.
ACKNOWLEDGEMENTS
Foundation: Supported by the Spanish Drug Regulatory Agency (No.APR-DEX-2014-01).
Conflicts of Interest: Pareja-Ríos A, None; Ruiz-de la Fuente-Rodríguez P, None; Bonaque-González S, None;López-Gálvez M, None; Lozano-López V, None; Romero-Aroca P, None.
REFERENCES
1 Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M.Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial.Ophthalmology 2006;113(9):1533-1538.
2 Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T,Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF,Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J,Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY; Meta-Analysis for Eye Disease(META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35(3):556-564.
3 Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol 2009;54(1):1-32.4 Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report no. 1. Early treatment diabetic retinopathy study research subgroup. Arch Ophthalmol 1985;103(12):1796-1806.
5 Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report no. 4. Early treatment diabetic retinopathy study research subgroup. Int Ophthalmol Clin 1987;27(4):265-272.
6 Diabetic retinopathy clinical research network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2008;115(9):1447-1449.
7 Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Sophie R, Hafiz G, Campochiaro PA; READ-2 Study Group. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol 2013;131(2):139-145.
8 Comparison of Age-related Macular Degeneration Treatments Trials(CATT) Research Group, Martin DF, Maguire MG, Fine SL, Ying GS,Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119(7):1388-1398.
9 Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD,Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, Hykin PG. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema:24-month data: report 3. Arch Ophthalmol 2012;130(8):972-979.
10 Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, Goodwin S, Aroney C, McAllister IL, Fraser-Bell S. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 2014;121(12):2473-2481.
11 Stein JD, Newman-Casey PA, Kim DD, Nwanyanwu KH, Johnson MW, Hutton DW. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology 2013;120(9):1835-1842.
12 Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L,Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS; RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119(4):789-801.
13 van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA 2005;293(12):1509-1513.
14 Lazic R, Lukic M, Boras I, Draca N, Vlasic M, Gabric N, Tomic Z.Treatment of anti-vascular endothelial growth factor-resistant diabetic macular edema with dexamethasone intravitreal implant. Retina 2014;34(4):719-724.
15 Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009;116(1):73-79.
16 Felinski EA, Antonetti DA. Glucocorticoid regulation of endothelial cell tight junction gene expression: novel treatments for diabetic retinopathy. Curr Eye Res 2005;30(11):949-957.
17 Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S, Gonder J, Kapik B, Billman K, Kane FE; FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011;118(4):626-635.
18 Tamura H, Miyamoto K, Kiryu J, Miyahara S, Katsuta H, Hirose F,Musashi K, Yoshimura N. Intravitreal injection of corticosteroid attenuates leukostasis and vascular leakage in experimental diabetic retina. Invest Ophthalmol Vis Sci 2005;46(4):1440-1444.
19 Lozano Lopez V, Serrano Garcia M, Mantolan Sarmiento C, Pareja Rios A, Losada Castillo MJ, Cordoves Dorta L, Quijada Fumero E, Virgos Aller T, Bullejos Molina M. A cost-effectiveness study of dexamethasone implants in macular edema. Arch Soc Esp Oftalmol 2015;90(1):14-21.
20 Haller JA, Dugel P, Weinberg DV, Chou C, Whitcup SM. Evaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edema.Retina 2009;29(1):46-51.
21 Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA,Weinberg DV, Chou C, Whitcup SM; Dexamethasone DDS Phase II Study Group. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol 2010;128(3):289-296.
22 Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, Liu CC, Li XY, Hollander DA, Schiffman RM, Whitcup SM;Ozurdex PLACID Study Group. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013;120(9):1843-1851.
23 Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM,Kuppermann BD, Welty D. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci 2011;52(1):80-86.
24 Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, Liu CC, Lou J, Whitcup SM; Ozurdex CHAMPLAIN Study Group.Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina 2011;31(5):915-923.
25 Diabetic Retinopathy Clinical Research Network, Browning DJ,Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, Bressler NM, Danis RP, Kinyoun JL, Nguyen QD, Bhavsar AR, Gottlieb J,Pieramici DJ, Rauser ME, Apte RS, Lim JI, Miskala PH. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 2007;114(3):525-536.
26 Pacella E, Vestri AR, Muscella R, Carbotti MR, Castellucci M, Coi L,Turchetti P, Pacella F. Preliminary results of an intravitreal dexamethasone implant (Ozurdex) in patients with persistent diabetic macular edema.Clin Ophthalmol 2013;7:1423-1428
27 Guigou S, Hajjar C, Parrat E, Merite PY, Pommier S, Matonti F, Prost-Magnin O, Meyer F. Multicenter Ozurdex assessment for diabetic macular edema: MOZART study. J Fr Ophtalmol 2014;37(6):480-485
28 Diabetic Retinopathy Clinical Research Network, Elman MJ, Qin H,Aiello LP, Beck RW, Bressler NM, Ferris FL 3rd, Glassman AR, Maturi RK, Melia M. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology 2012;119(11):2312-2318.
29 Blumenkranz MS, Haller JA, Kuppermann BD, Williams GA,Ip M, Davis M, Weinberg DV, Chou C, Whitcup SM. Correlation of visual acuity and macular thickness measured by optical coherence tomography in patients with persistent macular edema. Retina 2010;30(7):1090-1094.
30 Boyer DS, Yoon YH, Belfort R Jr, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM; Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014;121(10):1904-1914.