INTRODUCTION
Neuromyelitis optica (NMO) is a serious immunemediated, idiopathic, demyelinating and necrotic disease that is often found in Asian (e.g. Chinese and Japanese)populations[1]; it mainly affects the optic nerve and spinal cord.After penetrating the lamina cribrosa sclerae, the retinal nerve fiber layer (RNFL) forms the optic nerve. Therefore, RNFL thickness measurements are a common in vivo method used to investigate optic nerve function. The RNFL is composed of axons of retinal ganglion cells. Recent studies have suggested that the ganglion cell-inner plexiform layer (GCIPL) is composed of ganglion cell layer and inner plexiform layer; it does not include the RNFL. The GCIPL becomes significantly thinner in glaucoma patients and GCIPL measurements have the same reliability and sensitivity as RNFL measurements in diagnosing optic nerve damage in glaucoma[2]. Optical coherence tomography (OCT) is a non-contact, noninvasive and highly sensitive ophthalmic diagnostic imaging technique that provides high-resolution images of the retinal anatomy and measures RNFL thickness. In this study, we aimed to use OCT to observe changes in the RNFL and GCIPL of NMO patients to evaluate the extent of damage to the optic nerve.
SUBJECTS AND METHODS
Subjects We conducted a cross-sectional study and enrolled 30 NMO cases that were diagnosed by the Department of Neurology in our hospital between January 2013 and November 2015, according to the revised version of the 2006 Wingerchuk diagnostic criteria[3]. The duration of disease in these patients ranged from one to eight years; all were in clinical remission. The following inclusion criteria were utilized: 1) recruitment three months after the onset of NMO;2) direct ophthalmofundoscopic examination showing no papilledema or hemorrhage adjacent to the optic disc; 3)negative for relative afferent pupil defect (RAPD), with or without drug treatment. The following exclusion criteria were utilized: 1) diagnosed or suspected glaucoma with an intraocular pressure greater than 21 mm Hg (1 mm Hg=0.133 kPa)and a cup/disc ratio greater than 0.6; 2) non-inflammatory optic neuropathy causing optic atrophy (e.g. ischemic optic neuropathy); 3) visual system tumors; 4) a history of intraocular surgery; 5) novel optic neuritis (ON) within the prior three months; 6) a diopter measurement greater than-6.00 D. Among these patients, 20 cases had binocular ON and 10 cases had monocular ON. The patients were divided into the following two groups: 1) the NMO-ON group (n=30) included one eye from each patient with binocular ON (randomly selected) and one eye from each patient with monocular ON(affected eye); and 2) the NMO-ON contra group (n=10)included the contralateral eyes of patients with monocular ON.The control group consisted of 30 age- and gender-matched healthy individuals. We randomly selected one eye from each control subject, for a total of 30 eyes examined.
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University,and informed consent was obtained from all subjects in this study according to the Declaration of Helsinki.
Examination Methods
Routine examination Anterior segment slit lamp examination,anterior chamber angle and fundus examinations (+90 D),intraocular pressure measurements and automatic computer refractometry were conducted.
Optical coherence tomography scanning Cirrus highdefinition optical coherence tomography (HD-OCT, Carl Zeiss, Germany) uses its own ganglion cell analysis (GCA)algorithm to accurately calculate the thickness of the GCIPL in the macular area with good reproducibility[2,4]. The GCIPL consists of the ganglion cell and inner plexiform layers; it does not contain the RNFL and is therefore capable of excluding the interference caused by the RNFL thickness. During the examination, the patient was seated, and the height of the instrument was adjusted appropriately. The subject’s jaw was then placed on the jaw brackets and their forehead was attached to the forehead stent. Subsequently, the height of the eye was adjusted so that the subject was in a comfortable position while the scanning probe was focused onto the pupil of the subject’s eye. The same highly experienced clinician conducted the fundus OCT scan for each of the subjects.
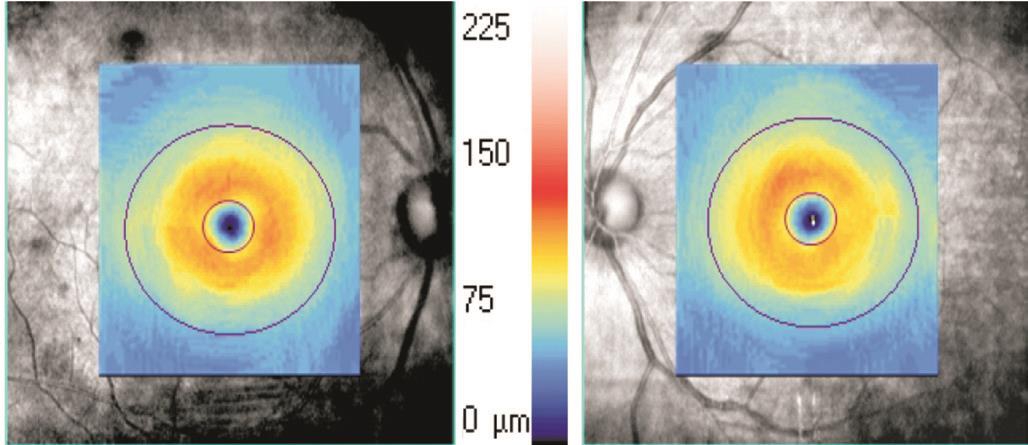
Figure 1 Scanning range of the macular cube 512×128 scanning mode and the distribution of GCIPL thicknesses in the 14.13 mm2elliptical area centered on the macular fovea.
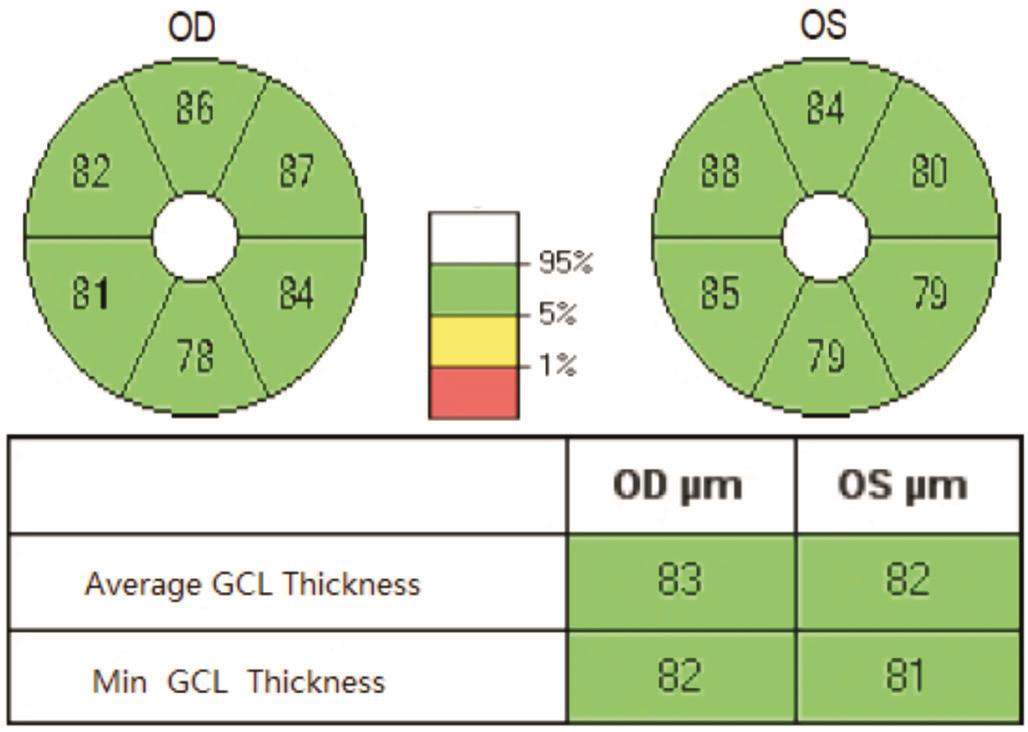
Figure 2 GCIPL thickness ellipsogram, the average and minimum GCIPL thickness values.
Macular ganglion cell-inner plexiform layer thickness scanning The patient was asked to focus on an internal fixed point of view. The macular area of the patient was targeted as the scanning site and was centered on the lowest point of the macular fovea. The macular cube 512×218 (Figure 1) scanning mode was utilized with a scanning area of 6.0 mm×6.0 mm,a scanning depth of 2 mm, an axial resolution of 5 μm and a lateral resolution of 20 μm. We used the GCA algorithm that was native to the HD-OCT system, which made it possible to measure the GCIPL thickness of a 14.13 mm2elliptical region that is centered on the macular fovea. The horizontal and vertical diameters of the elliptical inner ring were 1 and 1.2 mm, respectively; the horizontal and vertical diameters of the outer ring were 4 and 4.8 mm, respectively. The ganglion cell layer of the inner ring region was very thin and difficult to measure; therefore, it was excluded. The outer ring size measurement was based on the distribution characteristics of the ganglion cells in the macular region. We found that the area between the inner and outer rings was the thickest normal ganglion cell layer. We obtained the mean and minimum(i.e. the lowest GCIPL thickness on a meridian through the elliptical ring) GCIPL thickness measurements (Figure 2). The scan was repeated three times; the scan with the clearest image and strongest signal was selected for this study.
Table 1 Basic patient characteristics of the NMO patient groups and healthy control group
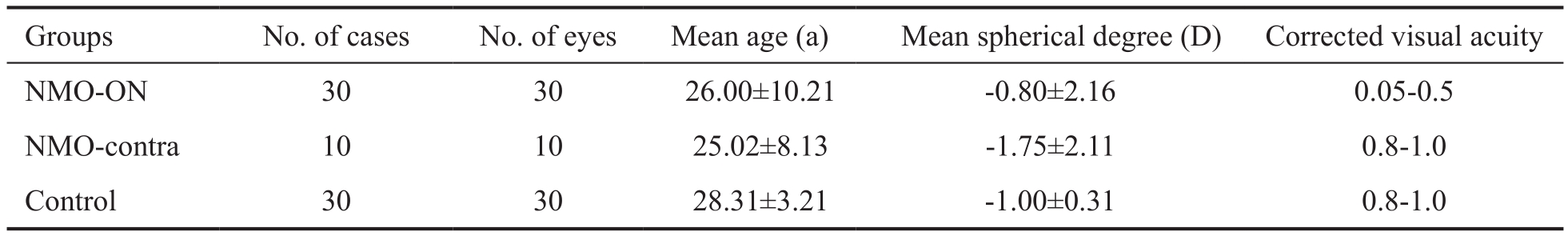
ANOVA showed no significant differences in mean age, mean spherical degree or corrected visual acuity between the NMO-contra and control groups but revealed differences in mean age and corrected visual acuity between the NMO-ON and control groups.
Table 2 GCIPL and RNFL thicknesses for the two NMO groups and the normal control group: comparisons and correlations with findings from visual field analysis mean±SD, μm
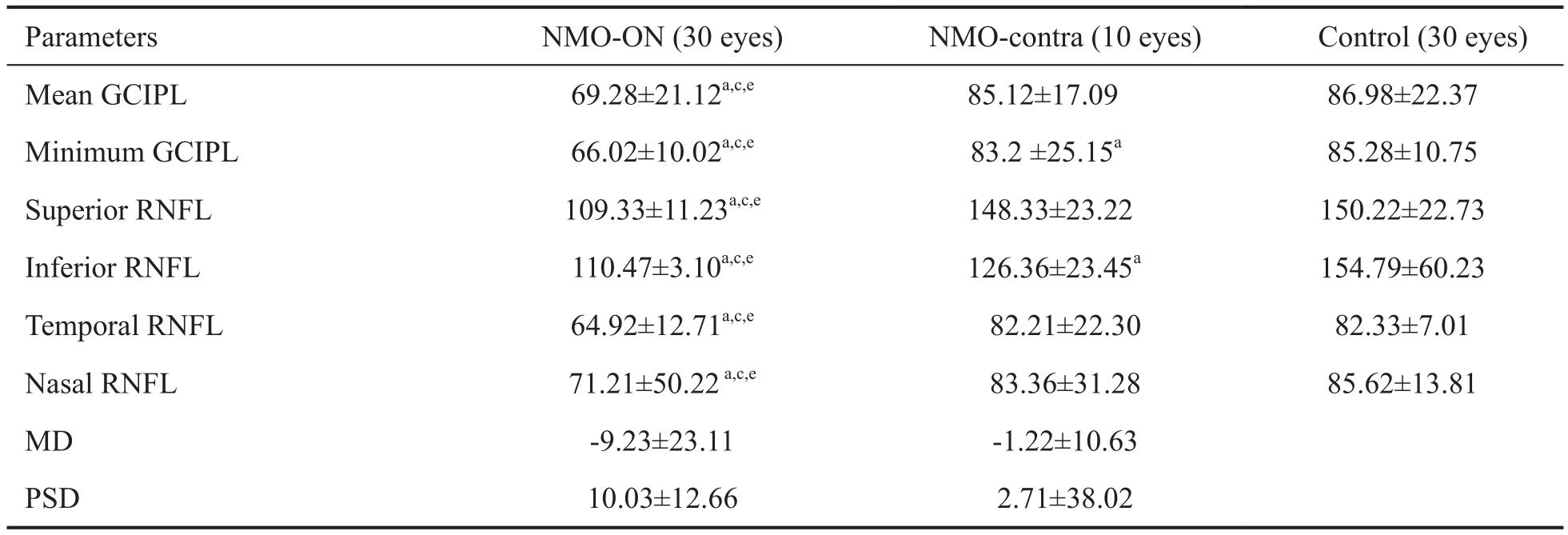
aP<0.05 compared with the control group;cP<0.05 for MD;eP<0.05 with respect to correlations with visual field analysis findings.
Optic disc retinal nerve fiber layer thickness scanning The patient was asked to focus on an internal fixed point of view.The optic disc cube 200×200 scanning mode was utilized with a scanning area of 6.0 mm×6.0 mm and a scanning depth of 2 mm. The HD-OCT system automatically analyzed RNFL thickness in the superior, temporal, inferior and nasal quadrants. The scan was repeated three times; the scan with the clearest image and strongest signal was used for this study.
Visual field inspection We utilized the 30°-2 program of the Humphrey 750 visual field analyzer. Each patient underwent at least two visual field tests until the loss rate of the fixed point of view (a measure of detection reliability), false positive rate and false negative rate were all <25%. We recorded the mean deviation (MD) and the corrected pattern standard deviation(PSD) of the visual field measurements.
Statistical Analysis All statistical tests were conducted using SPSS 17.0 software (IBM Corp., Armonk, NY, USA), and parameters are expressed as mean±SD. One-way analysis of variance (ANOVA) was used to compare the differences in GCIPL and RNFL thickness measurements between the two NMO groups and the control group. Spearman’s correlation analysis was performed on GCIPL thickness, RNFL thickness and visual field parameters. P<0.05 was considered statistically significant.
RESULTS
Basic Study Participant Characteristics A total of 30 patients were enrolled in the NMO group, which included 12 males and 18 females. Patients were between 16 and 34 years of age (mean 26.00±10.21y). The degree of refractive error in this patient group was between +1.5 D and -3.0 D, and their corrected visual acuity was between 0.05 and 1.0. The control group included 12 men and 18 women who were between 17 and 36 years of age (mean 28.31±3.21y) and had corrected visual acuities ≥0.8 and refractive errors between -2.0 D and+2.0 D (Table 1).
In the NMO-ON group, the thickness of the GCIPL in the macular area was significantly lower than that in the healthy control group (P<0.05). There were no significant differences between the NMO-contra and healthy control groups (Table 2).The RNFL thicknesses of the mean and all four quadrants in the NMO-ON group as well as the inferior quadrant in the NMO-ON contra group were significantly thinner than those in the healthy control group (P<0.05; Table 2).
DISCUSSION
The typical clinical manifestations of NMO include visual impairment and periorbital pain that is associated with eye movement; furthermore, symmetrical paraplegia or limb paralysis due to myelitis may be involved. At onset, the clinical symptoms of NMO are severe, and the prognosis for recovery is poor. Many patients are left with significant visual impairment or even total blindness[5]. The pathophysiology of NMO involves severe optic nerve injury and can cause permanent blindness[6]. Currently, there are no reliable and sensitive methods to express the degree of optic nerve damage due to NMO[7]. OCT provides a rapid and quantitative method to detect the extent of NMO-related visual impairment and changes in disease condition; additionally, it can be applied to patients who are unable to tolerate long periods of examination(e.g. children). The latest generation of HD-OCT is the best choice for detecting microstructural changes in the retina due to its high-frequency scanning (27 000 scans/s) and precise(5 μm) axial resolution, thus enabling more accurate scanning and quantification of RNFL and GCIPL changes with higher reproducibility[8]. The application of OCT to study NMO patients has been rarely reported in the literature.
The RNFL and GCIPL models are derived from the study of glaucoma. In glaucoma-induced optic neuropathy, both layers exhibit reduced thickness, and the RNFL is commonly recognized as an indicator of damage in glaucoma[9-10]. Tan et al[11]conducted a multi-center study showing that the combination of the three innermost layers of the macular region (i.e. the ganglion, inner plexiform and inner nuclear layers) was the best site to measure for the diagnosis of glaucoma; its diagnostic ability is comparable to the RNFL.The macular ganglion cell layer may have up to 8 to 10 layers of cells, whereas there is only one layer outside of the macular area. About one-third of ganglion cells are present in the posterior pole retina and the diameter of macular ganglion cells is approximately 10 to 20 times larger than the diameter of ganglion axons; therefore, the detection of macular ganglion cell loss can sensitively reflect damage to the optic nerve[12].
Our study utilized an RNFL and GCIPL HD-OCT scanning program to evaluate optic nerve injury in NMO patients. In the NMO-ON group, the RNFL thickness was significantly thinner in each quadrant compared to the control group, indicating that ON in NMO patients may significantly damage the RNFL layer. In the NMO-ON contra group, we found that the RNFL layer in the inferior quadrant was thinner compared to the control group. It is possible that the optic nerve fiber layer was also damaged in the nonsymptomatic, contralateral eyes of NMO patients; however, due to the mild severity of and limited extent of the affected areas, this type of injury did not cause clinical symptoms. An explanation for this phenomenon remains to be clarified through long-term observation. The GCIPL was similar to the RNFL; namely, the thickness of these layers in the NMO-ON group was significantly thinner compared to the control group. This finding suggests that recurrent episodes of ON in NMO patients can significantly damage optic ganglion cells and affect the visual function of these patients. The thickness of these layers in the NMO-ON contra group was also thinner compared to the control group;however, the differences were not statistically significant.
Multiple sclerosis (MS) and NMO have similar pathology.In recent years, there have been many studies on optic nerve injury in MS. Huang et al[13]investigated the RNFL and GCIPL in MS patients with monocular ON and found that the RNFL measurements in the nasal, temporal and temporal quadrants of contralateral normal eyes were thinner compared to the diseased eye. Fisher et al[14]reported a large study that included 90 MS patients and 36 case controls; their results showed that in ON, the average RNFL thickness of the diseased eye was significantly reduced compared to the contralateral and normal eyes. In addition, Fisher et al[14]found that the RNFL thickness of the eyes of patients without a history of ON were also decreased to some extent compared to the eyes of healthy controls. It is speculated that the process of chronic axonal loss in MS patients may not be entirely similar to the acute process of ON and that the visual function and morphological structure of the eyes of patients without a history of ON may have already been altered to some extent.
Cheng et al[15]found that the RNFL and GCIPL thickness of eyes at the first onset and early stages of ON were thicker compared to controls and was associated with acute papilledema; however, the RNFL and GCIPL thickness measurements in the early stages of recurrent ON were found to be thinner than in controls. This finding suggests that the OCT-measured RNFL thickness is consistent with the development of axonal loss in the course of NMO disease.Therefore, OCT can be used as a rapid, noninvasive and standardized imaging modality, with good prospects in the clinical application of axonal loss detection, early examination and disease monitoring[16]. The correlation between the RNFL and GCIPL thickness measurements and visual field parameters was significant in the NMO-ON group. The RNFL has a damage threshold of approximately 75 μm, in other words,visual function will decrease only when axon loss reaches a certain extent (i.e. RNFL damage thickness >75 μm)[17-19].Therefore, RNFL measurements are more sensitive than visual field parameters. The minimum GCIPL value and the inferior quadrant thickness of the RNFL in the NMO-contra group were correlated with visual field testing; these findings support the statement above.
Traditional methods of assessing optic nerve damage include visual evoked potentials and head MRI, which indicate the function and visualize the morphology of the optic nerve,respectively. However, these two examinations provide only a general assessment of the optic nerve and the entire visual system. OCT is a new examination modality that can perform a detailed assessment of NMO-related optic nerve damage at the level of optic nerve fibers and optic ganglion cells.The pathological process of NMO-induced ON eventually leads to significantly reduced RNFL and GCIPL thicknesses;accordingly, this degradation indicates that ON in NMO patients is a manifestation of extensive optic nerve axon injury[20]. Further studies and larger sample sizes are required to confirm this effect.
ACKNOWLEDGEMENTS
Foundation: Supported by Science and Technology Bureau Project Fund of Wenzhou, China (No.Y20160460).
Conflicts of Interest: Hu SJ, None; Lu PR, None.
REFERENCES
1 Li R, Qiu W, Lu Z, Dai Y, Wu A, Long Y, Wang Y, Bao J, Hu X. Acute transverse myelitis in demyelinating diseases among the Chinese. J Neurol 2011;258(12):2206-2213.
2 Jeoung JW, Choi YJ, Park KH, Kim DM. Macular ganglion cell imaging study: glaucoma diagnostic accuracy of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2013;54(7):4422-4429.
3 Wingerchuk DM, Lennon VA, Pittock SJ, Pittock SJ, Lucchinetti CF,Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. J Neurol 2006;66(10):1485-1489.
4 Mwanza JC, Oakley JD, Budenz DL, Chang RT, Knight OJ, Feuer WJ. Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci 2011;52(11):8323-8329.
5 Jarius S, Wildemann B. Neuromyelitis optica. Nervenarzt 2007;78(12):1365-1377.
6 Monteiro ML, Fernandes DB, Apóstolos-Pereira SL, Callegaro D.Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2012;53(7):3959-3966.
7 Fernandes DB, Raza AS, Nogueira RG, Wang D, Callegaro D, Hood DC, Monteiro ML. Evaluation of inneoretinal layers in patients with multiple sclerosis or neuromyelitis optica using optical coherence tomography. Ophthalmology 2013;120(2):387-394.
8 Kallenbach K, Simonsen H, Sander B, Wanscher B, Larsson H, Larsen M, Frederiksen JL. Retinal nerve fiber layer thickness is associated with lesion length in acute optic neuritis. Neurology 2010;74(3):252-258.
9 Costello F, Hodge W, Pan YI, Metz L, Kardon RH. Retinal nerve fiber layer and future risk of multiple sclerosis. Can J Neurol Sci 2008;35(4):482-487.
10 Merle H, Olindo S, Donnio A, Richer R, Smadia D, Cabre P. Retinal peripapillary nerve fiber layer thickness in neuromyelitis optica. Invest Ophthalmol Vis Sci 2008;49(10):4412-4417.
11 Tan O, Li G, Lu AT, Varma R, Huang D; Advanced Imaging for Glaucoma Study Group. Mapping of macular sub-structures with optical coherence tomography for glaucoma diagnosis. Ophthalmology 2008;115(6):949-956.
12 Ganekal S. Ganglion cell complex scan in the early prediction of glaucoma. Nepal J Ophthalmol 2012;4(8):236-241.
13 Huang J, Dai H, Zhang H, Wang X, Chen T. Clinical investigation of optic coherence tomography in evaluating the impairment of optic nerve secondary to multiple sclerosis. Zhonghua Yan Ke Za Zhi 2014;50(12):900-905.
14 Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC,Calabresi PA, Maguire MG, Cutter GR, Balcer LJ. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis.Ophthalmology 2006;113(2):324-332.
15 Cheng G, Zhao J, Liang Z, Zhang H, Ma JM, Sui RF, Mao J. Analysis of optical coherence tomography in early stage of optic neuritis in multiple sclerosis patients. Zhonghua Yan Ke Za Zhi 2011;47(10):913-919.
16 Tian G, Li Z, Zhao G, Fenq C, Li M, Huang Y, Sun X. Evaluation of retinal nerve fliber layers and ganglion cell complex in patients with optic neuritis or neuromyelitis optica spectrum disorders using optical coherence tomography in a Chinese cohort. J Ophthalmol 2015;2015:832784.
17 Sato S, Hirooka K, Baba T, Tenkumo K, Nitta E, Shiraga F. Correlation between the ganglion cell-inner plexiform layer thickness measured with cirrus HD-OCT and macular visual field sensitivity measured with microperimetry. Invest Ophthalmol Vi Sci 2013;54(4):3046-3051.
18 Takayama K, Hangai M, Durbin M, Nakano N, Morooka S, Akagi T,Ikeda HO, Yoshimura N. A novel method to detect local ganglion cell loss in early glaucoma using spectral-domain optical coherence tomography.Invest Ophthalmol Vis Sci 2012;53(11):6904-6913.
19 Lee J, Hangai M, Kimura Y, Takayama K, Kee C, Yoshimura N.Measurement of macular ganglion cell layer and circumpapillary retinal nerve fiber layer to detect paracentral scotoma in early glaucoma. Graefes Arch Clin Exp Ophthalmol 2013;251(8):2003-2012.
20 de Seze J, Blanc F, Jeanjean L, Zéphir H, Labauge P, Bouyon M,Ballonzoli L, Castelnovo G, Fleury M, Defoort S, Vermersch P, Speeg C. Optical coherence tomography in neuromyelitis optica. Arch Neurol 2008;65(7):920-923.