INTRODUCTION
Glaucoma is an important cause of blindness worldwide.In 2013, 64.3 million patients had glaucoma, and this number will reach 111.8 million in 2040 according to WHO estimates[1]. Primary angle opened glaucoma (POAG) is the most common type of glaucoma[2]. Intraocular pressure(IOP) is the critical modifiable factor that can prevent POAG progression using therapeutic interventions[3-4]. However, for some patients with POAG, disease progression continues even if the IOP is controlled to normal levels because IOP fluctuations area risk factor for POAG progression[5-6].IOP readings involve many kinds of tonometries, such as Goldmann applanation tonometry or non-contact tonometry,which are obtained in a seated posture at a hospital clinic.The IOP is typically assessed every two hours for one day,but nocturnal measurements in the seated position may not reflect the full range of IOP rhythms, which change with sleep posture, body posture and physical activity[7]. The IOP has been shown increase by more than 6 mm Hg between the seated and the supine position[8], and large fluctuations in IOP
have negative effects on patients with glaucoma[9]. When a substantial difference in IOP between eyes is observed, the prognosis for each eye may be different. Asymmetric eye damage is common in patients with glaucoma[10]. The exact same situation is rarely observed in both eyes of patients with POAG, and one eye is almost always worse than the other eye in patients with glaucoma. Thus, studies exploring why and how asymmetric damage occurs in patients with POAG are important.
Humans spend approximately 8h sleeping per day; therefore,we hypothesized that nearly one-third of the lives of patients with glaucoma is affected by the patients’ sleeping behaviors.According to some studies, sleeping position may be a disease mechanism because it results in fluctuations in IOP[11], and Kaplowitz et al[12]suggests that the side of the worse eye is related to the preferred sleeping position. Nevertheless, precise evidence proving the role of life style in glaucoma progression is insufficient[13].
The current study attempted to investigate the differences in sleep laterality between patients with POAG and healthy people and to explore the most appropriate sleep position for patients with asymmetric POAG according to IOP variations measured in different sleeping positions. If changes in IOP following a change in body position are correlated with sleep laterality, then the relationship may explain the pathogenesis of asymmetric POAG.
SUBJECTS AND METHODS
Subjects Selection This prospective, observational study was performed in the Department of Ophthalmology, West China Hospital, Sichuan University. All procedures reported in this study were performed in accordance with the principles of the Declaration of Helsinki, and ethical permission was obtained from Chinese Clinical Trial Registry (ChiCTRROC-16010264). The patients were diagnosed by an experienced glaucoma specialist (Chen XM) based on slit lamp microscope and ophthalmoscope exams as well as a visual field test (OCTOPUS 101; HAAG-STREIT AG Company,Switzerland) and optic nerve analysis (Cirrus HD OCT; Carl Zeiss Meditec, CA, USA).
Clear inclusion criteria were utilized. Patients were required to have normal open angles on gonioscopy, significant optic disc changes in the form of focal or diffuse neuroretinal rim thinning, and visual field defects corresponding to a retinal nerve fiber layer defect. Patients were diagnosed asymmetric POAG if the difference in the binocular C/D ratio was greater than 0.2. Patients with a binocular C/D ratio of less than 0.2 were diagnoses with relatively symmetric POAG.Healthy volunteers were observed in this study to compare the differences in sleeping positions between patients with POAG and healthy people. Participants with other ocular diseases such as a history of trauma, ocular tremor that cannot be corrected, ocular infection, optical neuropathy not resulting from glaucoma, or other systematic diseases, were excluded. A few individuals who could not report their sleep position were also excluded.
Measurements All measures were performed by a single operator between 9 a.m. and 11 a.m. A single iCare rebound tonometer (Care Pro; TiolatOy, Finland) was used to measure the IOP of both eyes in each posture. First, participants were guided into a quiet, dimly lit room and then allowed to rest for five minutes in a chair. During that time, the operator checked most of the basic information and sleep habits. Then,the subjects were asked to maintain their IOP by changing positions: sitting, supine, and lying on the right and left sides. Measurements were collected in each posture at fiveminute intervals. A built-in incline sensor in the iCare system measures the supine IOP. During the examination, a pillow was used to support the patient’s neck and head in the supine position (Figure 1). The subject was asked to look straight ahead, and the IOP was measured in the center of the cornea using a touching transducer. The left eye was always checked after the right eye. Each eye was measured five times, and the average value was used for the statistical analysis.

Figure 1 Sleeping position included supine, right lateral decubitus posture and left lateral decubitus posture When the participant face to right side, the right eye was the dependent eye and left eye was the independent eye, while reversely when he/she faces to left side.
When the participants were lying in the lateral decubitus posture, the lower eye was defined as the dependent eye, and the other eye was the independent eye. The better and worse eyes were defined by the C/D ratio and visual field analysis.
Statistical Analysis The statistical analysis was performed using SPSS Version19.0 (SPSS Inc., Chicago, Illinois, USA).Figures were created in Origin (Origin Lab Corporation, USA).The sample size was calculated using the following formula:![]() , α=0.05, β=0.1, δ=0.05, Q1=n1/N,and Q2=n2/N, where n1=n2and Q1=Q2=1. Seventy-five patients were calculated as the minimum sample size. All values are presented as mean±standard deviations. The independentsample t-test was used to compare the mean values of the data,and a paired t-test was used to compare values obtained from each eye. Percentages were used to describe the results of the questionnaire, and the relationship between the worse eye and the predominant sleeping position was determined using a Chi-square test. A P value less than 0.05 was considered to represent a significant difference.
, α=0.05, β=0.1, δ=0.05, Q1=n1/N,and Q2=n2/N, where n1=n2and Q1=Q2=1. Seventy-five patients were calculated as the minimum sample size. All values are presented as mean±standard deviations. The independentsample t-test was used to compare the mean values of the data,and a paired t-test was used to compare values obtained from each eye. Percentages were used to describe the results of the questionnaire, and the relationship between the worse eye and the predominant sleeping position was determined using a Chi-square test. A P value less than 0.05 was considered to represent a significant difference.
RESULTS
Analysis of Sleep Laterality Forty-six volunteers and 131 patients with POAG were included in this study. The average age of the healthy population was 56.8±17.93y and included 29 males (63%) and 17 females (37%), where as the average age of patients with POAG was 44.8±16.69y and included 83 males (63%) and 48 females (37%).
All 177 participants completed a survey to assess their predominant sleep position. Most healthy individuals(45.65%) preferred the right lateral decubitus (RLD) posture.Approximately 21.74% preferred the supine posture, and nearly 13.04% preferred the left lateral decubitus (LLD) posture and lateral decubitus posture, respectively. Only 3 people claimed no preference (non-fixed; Figure 2). Most patients with POAG(48.09%) preferred the RLD, followed by the LLD (20.61%),non-fixed (12.98%), supine (12.21%), and lateral decubitus postures (6.11%; Figure 3). The greatest proportions of patients with POAG and healthy volunteers preferred the RLD posture (48.09% and 45.65%); the non-fixed posture was not the least common posture when bilateral alternative sleeping positions and non-fixed sleeping positions were included in this group. The sleep laterality of patients with POAG was not significantly different from the healthy population (Table 1).
Effect of the Sleeping Position on Intraocular Pressure Variations
Ipsilateral intraocular pressure of all patients with primary open angle glaucoma As shown in Table 2, the difference in IOP between eyes between any two positions was statistically significant. For the right eye, the IOP was highest when the patient laid in the RLD position, followed by the LLD and supine positions, and the lowest IOP was measured in the seated position. The IOP of the left eye was similar across individuals because the highest IOP was detected in the LLD position. Based on these results, the dependent eye always had the higher IOP.
Compared to the IOP in sitting position, that of the right eye in the RLD position was increased to 7.44±4.72 mm Hg, and the maximum variation was 25 mm Hg, whereas the left eye increased to 5.64±4.42 mm Hg, for a maximum value of 18 mm Hg. In the left lateral position, the IOP of the right eye increased to 5.77±5.04 mm Hg, and the IOP of the left eye increased to 7.34±4.93 mm Hg; the maximum values were 20 mm Hg and 27 mm Hg, respectively. All these values were obviously greater than the IOP ranges measured in the supine position, which were 2.80±4.34 mm Hg for the right eye and 2.89±3.99 mm Hg for the left eye, with maximum values of no more than 20 mm Hg. Hence, IOP exhibited the least variation in the supine position, and the independent eye always experienced a greater IOP fluctuation during the transition into the lateral position.
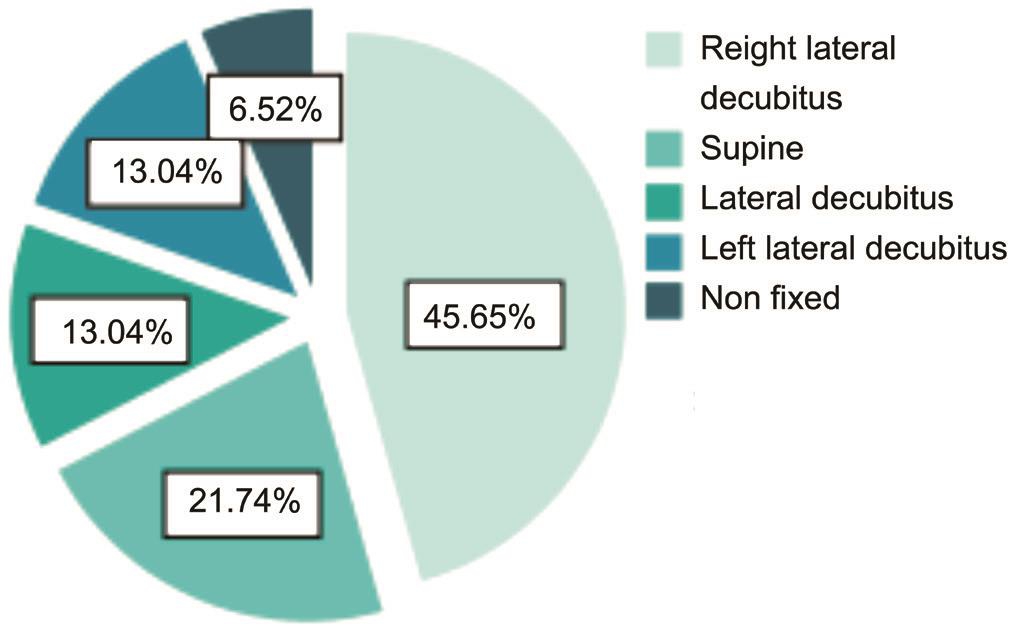
Figure 2 The sleep laterality of normal population.
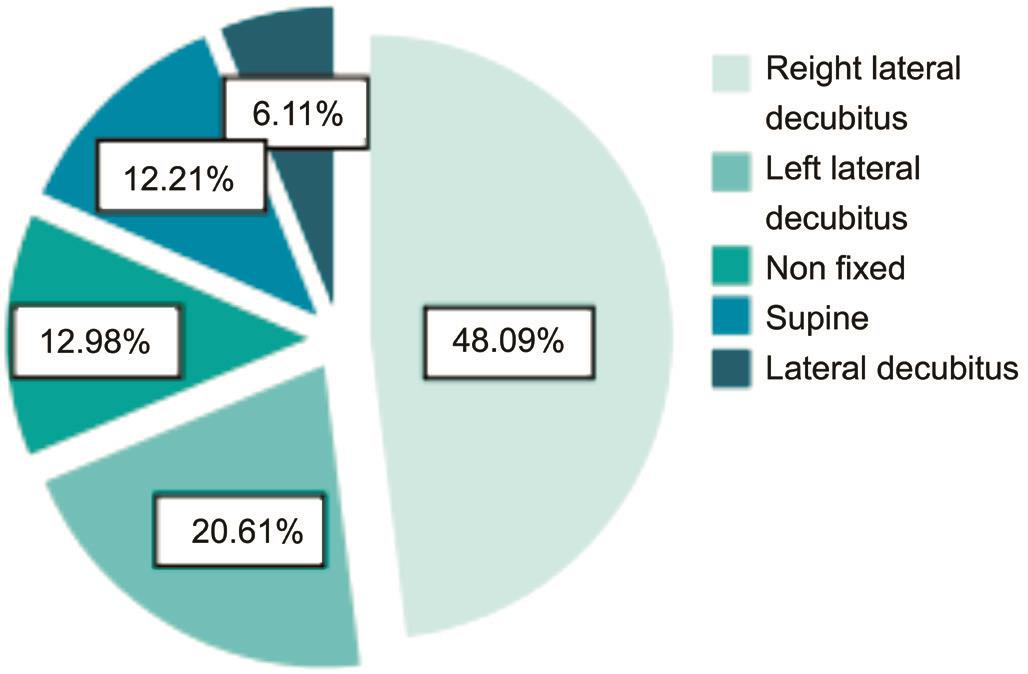
Figure 3 The sleep laterality of POAG patients.
Table 1 Comparison of sleep laterality between normal population and POAG patients

Because small sample size of no fixed and lateral decubitus posture,it was combined into a set of statistics; POAG: Primary open angle glaucoma; LLD: Left lateral decubitus posture; RLD: Right lateral decubitus posture.
When the patients assumed the RLD position, the IOP of the right eye was 1.40±5.70 mm Hg higher than that of the left eye, and in the LLD position, the IOP of the left eye was 1.96±6.58 mm Hg higher than that of the right eye. A significant difference in the IOP between the right and left eyes was observed when patients were lying in the lateral decubitus posture (Figure 4; RLD: P=0.006; LLD: P=0.001). The IOP of the dependent eye always increased to a greater extent than the IOP of the independent eye. The IOP was significantly elevated in the dependent eye when the patients were lying in the lateral decubitus posture.
Table 2 The IOP of right/left eye on different position mean±SD; mm Hg

RLD: Right lateral decubitus posture; LLD: Left lateral decubitus posture.
Table 3 Description of right eye IOP in POAG patients with RLD mean±SD; mm Hg

POAG: Primary open angle glaucoma; LLD: Left lateral decubitus posture; RLD: Right lateral decubitus posture.
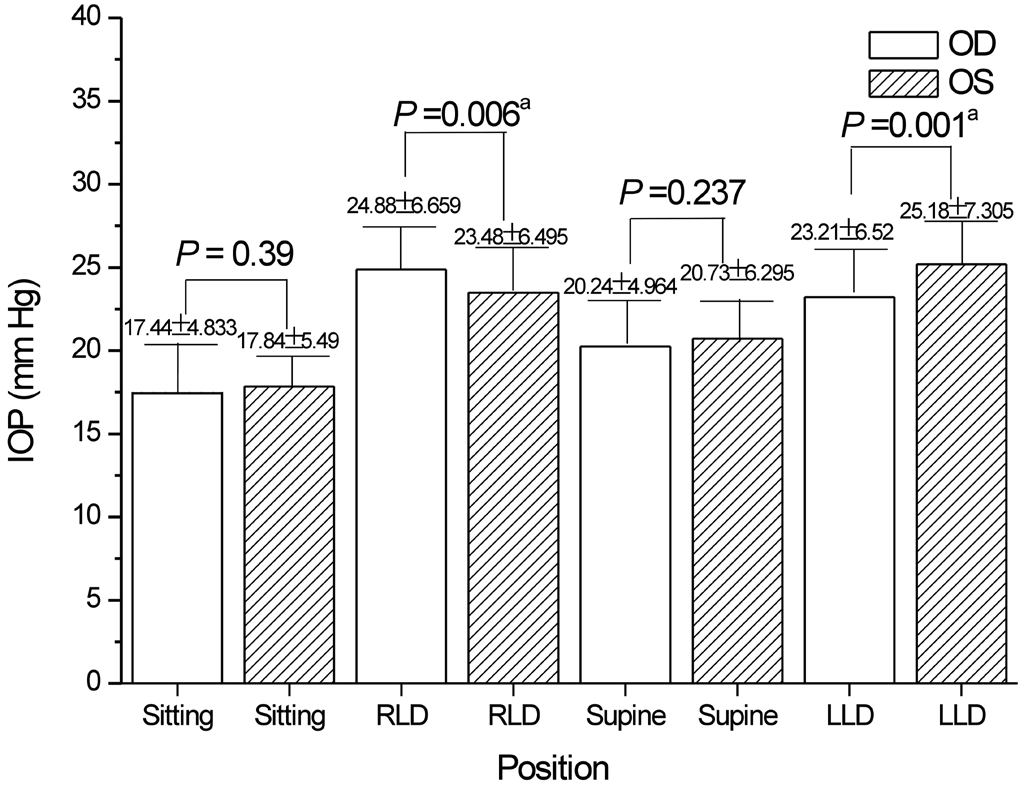
Figure 4 Comparison of the binocular IOP in the POAG patients at different positionsaStatistical significance. POAG: Primary open angle glaucoma; LLD: Left lateral decubitus posture; RLD: Right lateral decubitus posture.

Figure 5 Comparison of binocular IOP in RLD preferences of POAG patientsaStatistical significance. LLD: Left lateral decubitus posture; RLD: Right lateral decubitus posture.
Ipsilateral intraocular pressure of patients with primary open angle glaucoma and a preferred sleep position The IOP of individuals who expressed a preference for the RLD position was not significantly different from individuals who preferred other positions (Table 3). The results for patients who preferred the LLD position were similar (Table 4). Based on these results, it appears that unilateral IOP and preferred sleep position are not significantly associated.
Regarding the RLD preference, a significant difference in the bilateral IOP was observed only when patients slept facing right (Figure 5); similar results were observed for patients lying in their preferred LLD position (Figure 6).The IOP of the right eye is greater than the IOP of the left eye in patients with POAG who prefer the RLD position,and similar results were observed in patients with POAG who preferred the LLD position. This result was primarily observed in the same subject. Hence, the variation in the IOP due to changes in sleeping position mainly existed in the same individual difference between preoperative and postoperative measurement, with no specificity in the overall sample.
Table 4 Description of left eye IOP in POAG patients with LLD mean±SD; mm Hg
LLD: Left lateral decubitus posture; RLD: Right lateral decubitus posture.
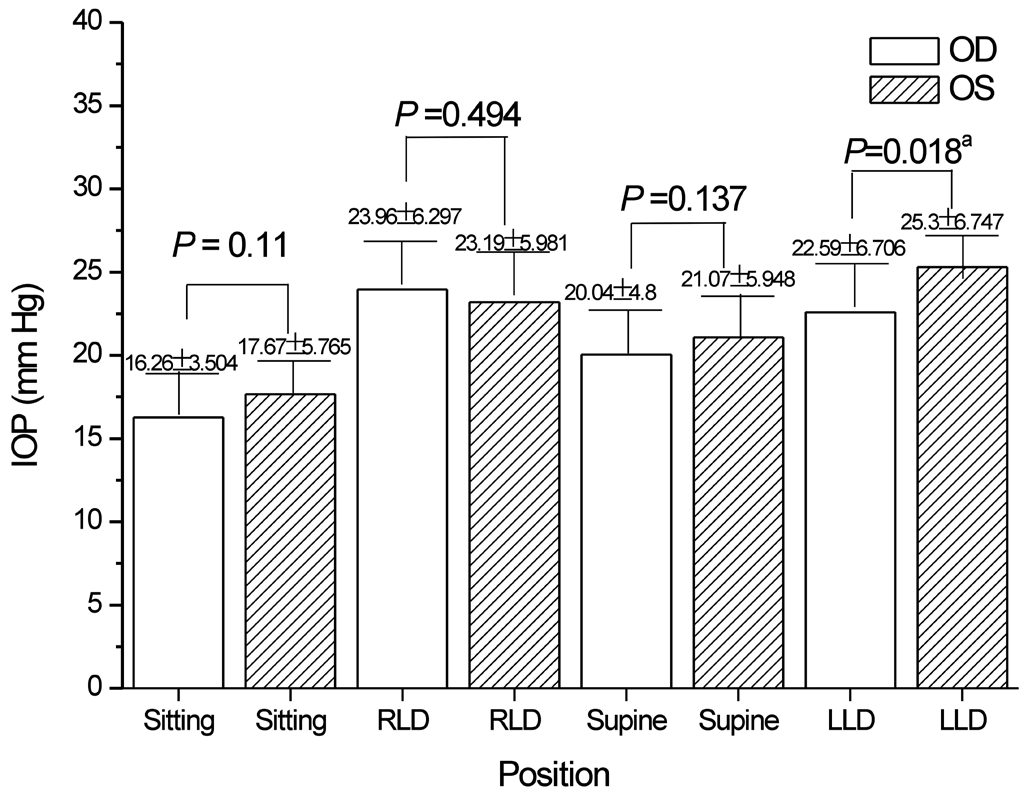
Figure 6 Comparison of binocular IOP in LLD preferences of POAG patientsaStatistical significance. LLD: Left lateral decubitus posture; RLD: Right lateral decubitus posture.
Relationship Between Lateral Sleep Position Tendency and Asymmetric Severity in Primary Open Angle Glaucoma POAG patients with a C/D ratio greater than or equal to 0.2 and with a difference in binocular visual field MD more than 5 dB were classified as the standard for bilateral asymmetric POAG. In these patients, the ipsilateral eye with the larger C/D ratio and greater visual field defect was the worst of both eyes.In the patients with binocular asymmetric POAG (Table 5), the questionnaire clearly showed a lateral sleep position tendency for 42 people, of whom 14 tended to lie on the left side and 28 on the right side. The left eyes of 12 patients with a left side preference (85.7%, 52.2% in overall patients) were found to be the worse eyes. Similarly, the right eyes of 20 patients with a right side preference (71.4%, 62.6% in overall patients) were the more serious. There was no such pattern for the supine or non-fixed position. Hence, when the C/D ratio was greater than or equal to 0.2, the worse eye was significantly associated with sleep laterality (χ2=16.762, P=0.001).
Table 5 Distribution of sleeping laterality in asymmetric group
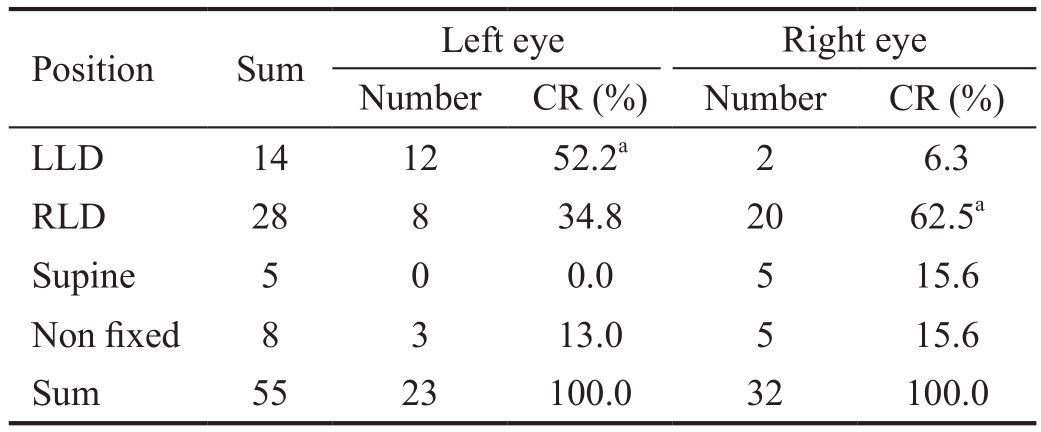
CR: Constituent ratio; LLD: Left lateral decubitus posture; RLD: Right lateral decubitus posture. χ2=16.762, P=0.001.aStatistical significance.
Table 6 Distribution of sleeping laterality in relative symmetric group
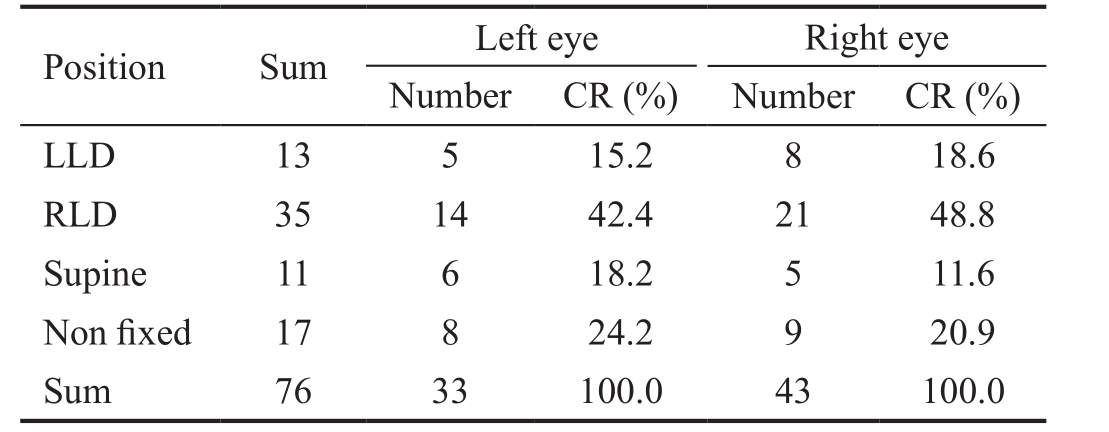
CR: Constituent ratio; LLD: Left lateral decubitus posture; RLD:Right lateral decubitus posture. χ2=0.943, P=0.815.
By contrast, this situation was not found for the POAG patients with a binocular C/D ratio less than 0.2, and the worse eye was not significantly associated with sleeping laterality (χ2=0.943,P=0.815) in these subjects (Table 6).
DISCUSSION
This study confirmed the findings of previous studies showing that the lateral decubitus posture induced POAG progression[14-15], though other investigators concluded this effect was not obvious[16]. However, most of these studies examining risk factors used only visual field loss as an indicator of progression, which may be insufficient to elucidate a relationship between sleep posture and POAG severity, particularly in patients with asymmetric POAG, since changing the sleep position has a proven effect on patients with asymmetric POAG. We analyzed the relationship between differences in sleeping laterality and variations in IOP and further speculated on the effect of the independent eye on patients with asymmetric POAG, which may provide new insights into the pathogenesis of asymmetric glaucoma.
Moreover, the greatest mean IOP variation across different positions was approximately 2 mm Hg in healthy people and more than 7 mm Hg in patients with POAG. The peak value always appeared in the LLD or RLD position. Even when the IOP is controlled, some patients with glaucoma continue to progress[17], possibly because of pathologically significant fluctuations in their IOPs during sleep[18]. Kaplowitz et al[12]used an initial sleep laboratory video to analyze patients’continuous sleep positions and observed a significant association (77%) between the objective primary sleep position and the self-reported sleep position (P=0.03), which led us to believe that sleep data obtained from a questionnaire are reasonable. Based on the study by Kaplowitz et al[12], people tend to prefer the RLD posture compared with other positions,which was also observed in the patients with POAG in our study. When the IOP of the right eye was compared across positions, the peak value (24.88 mm Hg) appeared in the RLD posture, whereas the highest IOP of the left eye (25.18 mm Hg)was measured in the LLD posture. Similarly, adjustments to the pillow height may affect the increase in IOP[16]. The IOP may fluctuate as the elevation of the eye changes, regardless of whether it is measured in the right or left eye. Elevation may play an important role related to Schlemm’s canal area[19],in which case the lateral decubitus posture should have more influence on IOP fluctuations than the supine position because changes in the 24-hour bilateral IOP between diurnal sitting and the nocturnal supine position are similar[20].
According to previous investigations, postural changes induce an increase in IOP. However, in other studies, the difference in the IOP of patients with POAG between the sitting and supine postures was greater in the worse eye than that in the better eye[14-15,21-23]. However, in contrast to previous findings, the sleep laterality of patients with POAG was not different from healthy individuals in the present study. In the analysis of the ipsilateral eye, the IOP of patients with POAG and laterality preferences was not significantly different from patients without such a preference, suggesting that sleep laterality preferences in patients with POAG were not correlated with the overall IOP because an obvious difference between these patients and those with other preferences was not observed.Moreover, the dependent eye was significantly more likely to be the worse eye when the asymmetry of the C/D ratio was ≥0.2.Sleep laterality is likely related to the asymmetry in severity between eyes. We concluded that a laterality preference does not dispose patients to an overall high IOP. The tendency to spend a long time sleeping in a certain position is related only to an increase in baseline IOP in patients with POAG but not in the general population. Therefore, the sleeping position has no long-term effect. With a suitable sleeping position, such as lying supine with an elevated pillow, patients may exhibit a relatively lower IOP during sleep.
The maximum IOP fluctuations always occurred in the dependent eye when the patient was sleeping in a lateral position. We assumed that IOP fluctuations were related to the sleeping position. Thus, the long-term adoption of a lateral sleeping position by patients with POAG may increase the IOP in the dependent eye, which might affect the progression of POAG and asymmetric pathogenesis. Thus, the relationship between the asymmetric IOP and sleeping position may play a role in the pathogenesis of glaucoma.
Although this case-control study utilized a standard protocol for diagnosis and the inclusion criteria, some limitations need to be recognized. First, the age of the participants ranged from 18 to 80. Age, sex and systematic disease are associated with the IOP[24]; the formation of the aqueous humor is sensitive to pressure, and the outflow of aqueous humor decreases with age in patients with glaucoma[25]. The study reflects the total population studied and did not stratify subjects by age. Next,we tested the IOPs in patients lying in different positions at a fixed time of day, which is not the habitual sleep time. This protocol likely resulted in lower measured IOP values, as the IOP is higher at night than during the day[26]. Although some devices for continuous IOP monitoring via an integrated pressure sensor have been developed and are available for commercial or scientific use[26], truly continuous IOP monitoring in individual patients is not yet available.
In conclusion, the IOP of the dependent eye might be higher than that of the independent eye when patients are lying in a lateral sleeping position. The long-term tendency to sleep on the right or left side may cause the dependent eye to be more severely affected than the independent eye. Based on these findings, an unsuitable sleeping position may accelerate the progression of POAG, and a lateral sleeping position might be one cause of asymmetric POAG. Therefore, we conclude that the adoption of the supine position may benefit patients with glaucoma.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Major Scientific Equipment Program (NO.2012YQ12008005); the Health Research Funding of Sichuan Province in 2017 (No.17PJ539).
Conflicts of Interest: Tang J, None; Li N, None; Deng YP,None; Qiu LM, None; Chen XM, None.
REFERENCES
1 Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY.Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121(11):2081-2090.
2 Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90(3):262-267.
3 McMonnies CW. The importance of and potential for continuous monitoring of intraocular pressure. Clin Exp Optom 2017;100(3):203-207.
4 Ocular Hypertension Treatment Study Group; European Glaucoma Prevention Study Group, Gordon MO, Torri V, Miglior S, Beiser JA,Floriani I, Miller JP, Gao F, Adamsons I, Poli D, D'Agostino RB, Kass MA. Validated prediction model for the development of primary openangle glaucoma in individuals with ocular hypertension. Ophthalmology 2007;114(1):10-19.
5 Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K.Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000;9(2):134-142.
6 Varma R, Hwang LJ, Grunden JW, Bean GW. Inter-visit intraocular pressure range: an alternative parameter for assessing intraoc-ular pressure control in clinical trials. Am J Ophthalmol 2008;145(2):336-342.
7 McMonnies CW. The interaction between intracranial pressure,intraocular pressure and lamina cribrosal compression in glaucoma. Clin Exp Optom 2016;99(3):219-226.
8 Liu JH, Weinreb RN. Monitoring intraocular pressure for 24h. Br J Ophthalmol 2011;95(5):599-600.
9 Fujino Y, Asaoka R, Murata H, Miki A, Tanito M, Mizoue S, Mori K, Suzuki K, Yamashita T, Kashiwagi K, Shoji N; Japanese Archive of Multicentral Databases in Glaucoma (JAMDIG) Construction Group.Evaluation of glaucoma progression in large-scale clinical data: the Japanese archive of multicentral databases in glaucoma (JAMDIG). Invest Ophthalmol Vis Sci 2016;57(4):2012-2020.
10 Broman AT, Quigley HA, West SK, Katz J, Munoz B, Bandeen-Roche K, Tielsch JM, Friedman DS, Crowston J, Taylor HR, Varma R,Leske MC, Bengtsson B, Heijl A, He M, Foster PJ. Estimating the rate of progressive visual field damage in those with open-angle glaucoma from cross-sectional data. Invest Ophthalmol Vis Sci 2008;49(1):66-76.
11 Lee TE, Yoo C, Kim YY. Effects of different sleeping postures on intraocular pressure and ocular perfusion pressure in healthy young subjects. Ophthalmology 2013;120(8):1565-1570.
12 Kaplowitz K, Blizzard S, Blizzard DJ, Nwogu E, Hamill CE, Weinreb RN, Mohsenin V, Loewen NA. Time spent in lateral sleep position and asymmetry in glaucoma. Invest Ophthalmol Vis Sci 2015;56(6):3869-3874.
13 Hecht I, Achiron A, Man V, Burgansky-Eliash Z. Modifiable factors in the management of glaucoma: a systematic review of current evidence.Graefes Arch Clin Exp Ophthalmol 2017;255(4):789-796.
14 Kim KN, Jeoung JW, Park KH, Lee DS, Kim DM. Effect of lateral decubitus position on intraocular pressure in glaucoma patients with asymmetric visual field loss. Ophthalmology 2013;120(4):731-735.
15 Sawada A, Yamamoto T. Comparison of posture-induced intraocular pressure changes in medically treated and surgically treated eyes with open-angle glaucoma. Invest Ophthalmol Vis Sci 2014;55(1):446-450.
16 Lee TE, Yoo C, Lin SC, Kim YY. Effect of different head positions in lateral decubitus posture on intraocular pressure in treated patients with open-angle glaucoma. Am J Ophthalmol 2015;160(5):929-936.
17 Hong S, Seong GJ, Hong YJ. Long-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedure. Arch Ophthalmol 2007;125(8):1010-1013.
18 McMonnies CW. The significance of intraocular pressure elevation during sleep-related postures. Clin Exp Optom 2014;97(3):221-224.
19 Hong J, Xu J, Wei A, Wen W, Chen J, Yu X, Sun X. Spectral-domain optical coherence tomographic assessment of Schlemm's canal in Chinese subjects with primary open-angle glaucoma. Ophthalmology 2013;120(4):709-715.
20 Liu JH, Sit AJ, Weinreb RN.Variation of 24-hour intraocular pressure in healthy individuals: right eye versus left eye. Ophthalmology 2005;112(10):1670-1675.
21 Kiuchi T, Motoyama Y, Oshika T. Postural response of intraocular pressure and visual field damage in patients with untreated normal-tension glaucoma. J Glaucoma 2010;19(3):191-193.
22 Liu JH, Kripke DF, Twa MD, Gokhale PA, Jones EI, Park EH, Meehan JE, Weinreb RN. Twenty-four-hour pattern of intraocular pressure in young adults with moderate to severe myopia. Invest Ophthalmol Vis Sci 2002;43(7):2351-2355.
23 Kiuchi T, Motoyama Y, Oshika T. Relationship of progression of visual field damage to postural changes in intraocular pressure in patients with normal-tension glaucoma. Ophthalmology 2006;113(12):2150-2155.
24 Chan MP, Grossi CM, Khawaja AP, Yip JL, Khaw KT, Patel PJ, Khaw PT, Morgan JE, Vernon SA, Foster PJ; UK Biobank Eye and Vision Consortium. Associations with intraocular pressure in a large cohort:results from the UK Biobank. Ophthalmology 2016;123(4):771-782.
25 Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res 2005;24(5):612-637.
26 Aptel F, Weinreb RN, Chiquet C, Mansouri K. 24-h monitoring devices and nyctohemeral rhythms of intraocular pressure. Prog Retin Eye Res 2016;55:108-148.