INTRODUCTION
Glaucoma is the leading cause of irreversible blindness worldwide, and the vision loss often occurs gradually over a long period of time[1]. The major pathological features of glaucoma are loss of retinal ganglion cells (RGCs) and degeneration of their axons.
The World Health Organization reported that glaucoma affects over 60 million people worldwide[2]. And it is expected that approximately 80 million people would suffer from glaucoma by the year of 2020, leading to 11.2 million cases of bilateral blindness[3-4].
Although many studies and investigations have shown some effect factors which related to glaucoma, the specific etiology and pathogenesis still remains unclear. Among these factors,elevated intraocular pressure (IOP) is one of the primary and important one, while other factors have also been identified that associated with the disease, including low diastolic ocular perfusion pressure, other ocular hemodynamic parameters,and systemic diseases such as sustained high blood pressure or diabetes, ethnic group, age, myopia, migraine, as well as the nutritional state of RGCs[5].
Besides IOP, other factors which related to immunological mechanisms also play key roles in the pathogenesis of glaucoma[6].Microglia are immune cells that normally residing in the central nervous system (CNS). Recent studies showed that microglia play essential roles in the interactions between the CNS and the immune system[7]. By scavenging, phagocytosis,extracellular signaling and other functions, microglia might be an important mediator of immune response and maintain homeostasis within the CNS.
Acute glial hyperplasia promotes the survival of neurons by reconstructing the protection and recruitment of nerve tissue in the extracellular medium. However, the uncontrolled response occurs in most neurodegenerative diseases like glaucoma,would have a negative impact on the tissue[8].
Therefore, the role of microglia in the pathogenesis of glaucoma as well as its possible mechanism(s) are discussed in this review.
INTERACTION OF MICROGLIA AND GLAUCOMA
In the eyes of patients with glaucoma, microglial cells with a variety of morphology, gathered at the lamina cribrosa and its surrounding blood vessels, suggesting that microglial cells on the blood retina barrier (BRB) protective effect[9].Furthermore, microglia also have an important function to continuously serve the microenvironment and respond rapidly to neuronal injury by the phagocytosis of potentially harmful neuronal debris to limit damage, the secretion of local inflammatory mediators and the signal transmission with other potential immune effector cells[10]. However, microglial cells may have beneficial effects in the presence of acute inflammation, whereas in chronic inflammation the activation of microglia is often detrimental, leading to the pathogenesis of neurodegenerative disorders[11].
In animal models of ocular hypertension[12]and chronic glaucoma[13], microglia become reactive and redistribute in the retina, optic nerve, and optic tract as early alterations,which may contribute to the disease onset or progression.Nevertheless, it was observed that microglia proliferated near the RGCs[14], and recruitment as well as activation of microglia occurred before RGC death[13]. This provides direct evidence for the involvement of microglia in RGC death in glaucoma. In addition, in glaucomatous animal models, the CD200, which is closely associated with microglial activation,was early detected and increased in the retina, indicating this process accompanies ongoing axonal degeneration[15].Microglia reactivity in glaucoma is not limited in the retina.In glaucomatous monkeys, activated microglia in the lateral geniculate nucleus (LGN), the primary processing center for visual information received from the retina, can be observed though positron emission tomography[16]. Other studies of neurodegeneration showed that glaucoma occured in the LGN,perhaps with microglial-related activations[17-18].
In addition, a tetracycline derivative, minocycline, known to inhibit the activation of microglia, also inhibited RGCs neurodegeneration in ischemia and glaucoma models[19-21]and improved the integrity of the optic nerve, supporting further evidence of the role of microglia in neuropathy in glaucoma.In the early stage of glaucomatous model, minocycline can also inhibit the axonal transport deficit contribute to microglial reactivity[22]. Furthermore, high doses of irradiation can reduce microglia activation and proliferation in the central retina, the optic nerve head (ONH) region in glaucoma animal models, leading to a decrease of degeneration in RGC and an improvement of the structural and functional integrity of RGC axons[23].
In an experimental autoimmune glaucoma animal model, IgG autoantibody is accompanied with the loss of RGCs and could be a useful glaucoma biomarker, found in co-localization with activated microglia cells[24].
Microglia and Neuroinflammation in Glaucoma As with many neurodegenerative diseases, there are similar inflammatory responses in glaucoma. Microglia activation not only occurs in the eyes with high IOP, but also occurs in the contralateral healthy eye[25], indicating complex mechanisms are involved in the pathogenesis of glaucoma.
Microglia perceive the signal from microenvironment and protect neurons from disturbances. Accompanied with changes in signaling and gene expression, activated microglial cells with morphous altered would proliferate and migrate to the site of injury[26]. However, in order to limit the damage, the persistent impairment may activate the neurotoxic phenotype of microglia[27]. The prolonged and excessive activation of retinal microglia is related to the degeneration of neurons, especially the loss of RGC, which is a characteristic of glaucoma[21,28].
The activations of microglial cells is closely regulated by several inhibitory pathways[29-30]. Fractalkine, also known as chemokine (C-X3-C motif) ligand 1 (Cx3cl1), is a membranebound chemokine primarily expressed by neurons, while microglia is the mainly expression of its anti-inflammatory receptor Cx3cr1. These molecules are important to maintain microglial function in physiological and pathological conditions. In mouse Parkinson and amyotrophic lateral sclerosis (ALS) models, the lack of Cx3cr1 leads to microglia neurotoxicity and neuronal vulnerability[31]. Following the loss of Cx3cr1 in microglia, a selective worsening of axon transport dysfunction in RGCs can be caused in glaucoma mouse model[32]. Similarly, Cx3cr1 deficiency evokes subretinal microglia accumulation and leads to age-related macular degeneration (AMD)[33]. On the other hand, Cx3cr1 deficiency showed a prevention of neuron loss in Alzheimer’s disease (AD), a neurodegenerative condition[34]. Therefore,under different conditions of neuroinflammatory, inhibition of the receptor have different effects on proinflammatory role of microglia. Thus, suppression of neuroinflammatory responses could be a potential treatment for glaucoma.
Microglia and Cytokines in Glaucoma Microglia are thought to play important roles in the inflammatory response of glaucoma. Over activation of microglia would result in the production of proinflammatory cytokines and increase the oxidation and nitrification reactions, thereby endangering the retinal neurons[35]. Indeed, stimulating by these cytokines,fibroblasts, endothelial cells and macrophages could produce chemokines, recruit neutrophils and macrophages to the retina, which leads to more severe tissue damage and chronic inflammatory response.
Tumor necrosis factor-alpha (TNF-α), which is produced by macrophage and microglia in the optic nerve and ONH[36], is related with innate immune respondence[37-39].TNF-α was considered to be an important mediator of RGC death in glaucoma, and the up-regulation of TNF-α and its receptor were involved in the process of glaucomatous neurodegeneration[40]. Indeed, further in vitro studies have shown that anti-TNF-α attenuates ischemia or ocular hypertension-induced RGC apoptosis[41]. Moreover, while TNF-α antagonized by etanercept, inflammation and RGC loss in a glaucoma animal model was attenuated[42].
Other investigations supported that TNF-α is beneficial and protective to neurons. TNF-α appears to protect RGCs in the early stage of optic nerve crushed in mice, perhaps with some indirect mechanisms[43-46].
Interleukin-1β (IL-1β) has been considered to be an essential pro-inflammatory cytokine which produced by activated microglia in glaucoma patients, and are thought to promote the progression of glaucoma[47-48]. IL-1β has also been reported to increase the generation of ROS[49]and nitric oxide synthesis(NOS)[50]and is involved in RGC damage which leads to neurodegeneration[51-52]. Indeed, tetrandrine can effectively suppress the activity of microglia[53]and inhibit the production of IL-1β and TNF-α, suggesting that it may be effectively suppressing over activated microglia and protecting RGCs in glaucoma[49,54].
Interleukin-6 (IL-6) is a key component of pressure-induced retinal microglia response[55-57]. In animal models of glaucoma and aging retina, stressor-dependent of IL-6 and IL-6 receptors have been detected[58]. Similarly, in the iris specimens from patients of neovascular glaucoma, mRNA level of IL-6 was significantly increased[59]. Nevertheless, it has been demonstrated that IL-6 increased the survival of RGCs challenged with pressure, and the stimulus for IL-6 synthesis arose from axonal injury rather than ocular hypertension[57].
Microglia and Adenosine Receptors Adenosine is a neuromodulator, which also exerts important functions in the immune-inflammatory system[60]. Microglial cells express all subtypes of adenosine receptors, A1, A2A, A2B,and A3 receptors and particular attention has been paid to adenosine A2A receptors (A2AR).
A2AR is associated with neurodegeneration as the blockade of A2AR providing protection against a variety of deleterious conditions[61-65]. It is speculated that the neuroprotective effect of A2AR antibodies is thought to control microglia-mediated neurodegeneration[66-67].
A2AR blockade was provided to prevented retinal microglia reactivity and neuroinflammation[67-68]. This might be concerned with the ability of A2AR controlling the formation and release of cytokines such as IL-1β and/or TNF, as previously observed in different brain preparations[69-73].
Microglia and Oxidative Stress Nitric oxide (NO) is known to be secreted by microglia[74]and inflammation upregulated inducible nitric oxide synthase (iNOS) can raise the production of NO[75]. Upregulated iNOS and increased NO levels were found in the ONH of glaucomatous patients[76]and in the retina and ONH of glaucoma animal models[77-79]. Inhibition of iNOS with aminoguanidine confers neuroprotection to RGCs in an animal model of glaucoma[80], supporting the existence of a role of NO in the pathophysiology of glaucoma.
Nicotinamide-adenine dinucleotide phosphate (NADPH)oxidase, which is closely related to microglial cells, is capable of generating reactive oxygen species ROS that also associated with microglia-mediated neurotoxicity in photoreceptor cells[81].
Microglia and Complement The complement system is part of innate immune defense, consists of a number of small proteins that can eliminate alien cells and debris[82]. Complement proteins are expressed in the normal physiological processes of the retina[83], and complement activation would increase in pathological conditions like inflammation[84]and ageing[85].
The classical pathway is triggered by activation of the C1-complex and the upregulation of complement protein C1q was detected in some neurodegenerative diseases including glaucoma[86-87]. Most of the secreted C1q is released by microglia,which express C1q mRNA strongly. The complement cascade is thought to present a target for subsequent elimination by microglial engulfment[87]. Besides, dendritic and synaptic architecture can be protected in genetic knockout of C1qa[D2.C1qa (-/-) mouse] or pharmacological inhibition of C1[88].In complement depleted rats model with increased IOP, the apoptosis of RGCs in retina was decreased and the activation of both caspase-8 and caspase-9 was inhibited[89]. These findings suggested that complement mediated apoptosis plays a pivotal role in glaucomatous neurodegeneration.
Microglia and Fas Ligand Fas ligand (FasL) is associated with activation of microglia-induced RGCs. FasL could be divided into two types, the truncated soluble product (sFasL)and membrane-bound FasL (mFasL). Considerable data proved that in animal models the mFasL is proinflammatory and proapoptotic, while sFasL is anti-inflammatory and non-apoptotic[90-91]. In the animal model of chronic glaucoma, ectogenic sFasL provided complete and sustained neuroprotection, reduced production of TNF-α, and decreased apoptosis of RGCs and loss of axons[92]. The opposing activities of mFasL and sFasL further suggest that FasL cleavage, mediated primarily by matrix metalloproteinases(MMPs) and their inhibitors (TIMPs), is a major mechanism for limiting the neurotoxic activity of FasL in the eye[93].In addition, MMPs and TIMPs were expressed by retinal microglia, RGCs, and their axons[94], indicated that microglia participates in the division of FasL.
M1/M2 Polarization of Microglia Similar to macrophages,microglia can be categorized into at least two phonotypes: M1 and M2. The activation of different phenotypes depending on the disease stages and severity can produce either cytotoxic or neuroprotective effects[95]. Microglia conduct similar functions in CNS compared with peripheral macrophages. Therefore,different phenotypes of microglia are considered to participate in the degeneration of the CNS. In AD models, the convertion of microglia from M1 to M2 lead to decreased toxicity, while the ability to swallow β-amyloid increased[96]. Furthermore,M1 activation may have association with dopaminergic cell death in Parkinson's disease[97]. Aiming at the transformation of different subtypes of microglia could also provide new therapeutic targets. Further investigations should be made in the roles played by M1/M2 microglia in glaucoma.
However, recently some opinions claimed that the evidence of microglial M1/M2 polarization is inadequate[98]. There are other transcriptional profiles failed to fit with these two phenotypes[99]. New markers to distinguish microglia from macrophages will be a significant task in future[100].
Microglia and MicroRNAs MicroRNAs (miRNAs),as important epigenetic regulators, are small noncoding single-stranded RNA molecules regulating gene expression post transcriptionally[101]. In an animal model of acute ocular hypertension (AOH), the loss of RGCs was associated with an activation of retinal microglial cells and thirty-one miRNAs significantly changed. For instance, miR-350/MAPK14, miR-539/MAP3K8 and miR-93/MAPK9 altered in AOH eyes could regulate the mitogen-activated protein kinases (MAPKs)signaling pathways, which could lead to inflammation and RGCs death[102].
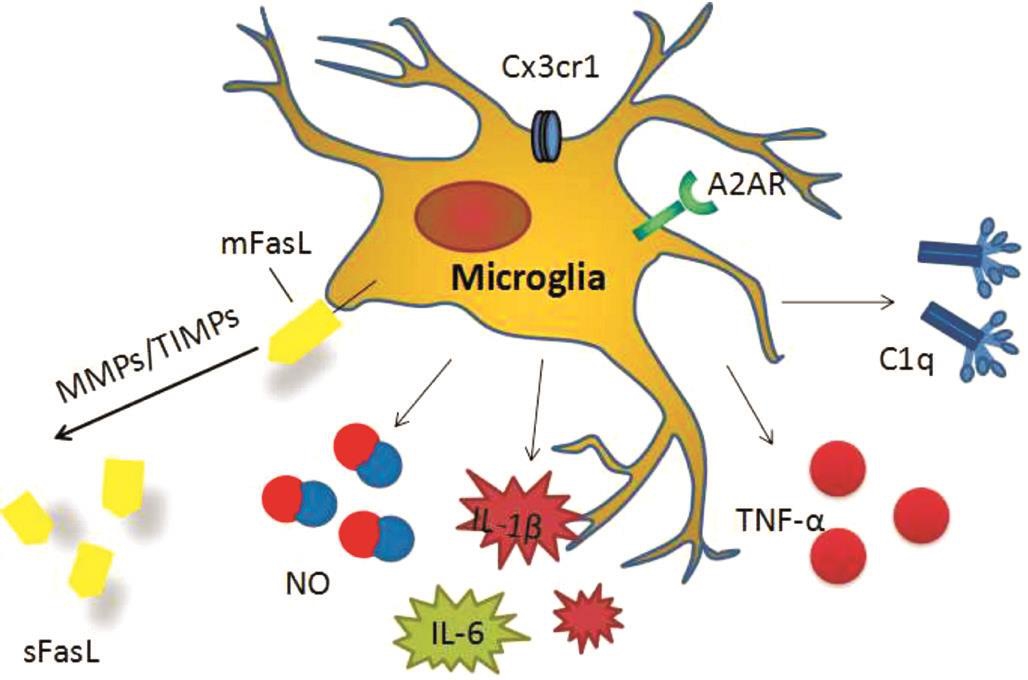
Figure 1 Factors and cytokines related to microglia in glaucoma.
CONCLUSION
Microglia, involved in inflammatory factors, cytokine activation, complement cascade as well as FasL cleavage, are closely related to glaucoma neurodegeneration (Figure 1). In the early stages of the disease, microglia-mediated inflammatory response may have protective effects on glaucoma in patients with RCG injury. While in the process of chronic disease,inhibition of microglia activity and its metabolites, can reduce glaucoma progress. Although its subtype classification is still controversial, microglial cells could be potential targets in treating glaucoma, especially normal-tension glaucoma.
ACKNOWLEDGEMENTS
Conflicts of Interest: Zeng HL, None; Shi JM, None.
REFERENCES
1 Rizzo MI, Greco A, De Virgilio A, Gallo A, Taverniti L, Fusconi M, Conte M, Pagliuca G, Turchetta R, de Vincentiis M. Glaucoma: recent advances in the involvement of autoimmunity. Immunol Res 2017;65(1):207-217.
2 Mantravadi AV, Vadhar N. Glaucoma. Prim Care 2015;42(3):437-449.
3 Kingman S. Glaucoma is second leading cause of blindness globally.Bull World Health Organ 2004;82(11):887-888.
4 Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90(3):262-267.
5 Hernandez M, Rodriguez FD, Sharma SC, Vecino E. Immunohistochemical changes in rat retinas at various time periods of elevated intraocular pressure. Mol Vis 2009;15(11):2696-2709.
6 Soto I, Howell GR. The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect Med 2014;4(8):pii:a017269.
7 Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev 2006;213:48-65.
8 Chen SD, Wang L, Zhang XL. Neuroprotection in glaucoma: present and future. Chin Med J 2013;126(8):1567-1577.
9 Neufeld AH. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch Ophthalmol 1999;117(8):1050-1056.
10 Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 2007;10(11):1387-1394.
11 Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol 2010;92(3):293-315.
12 Ebneter A, Casson RJ, Wood JP, Chidlow G. Microglial activation in the visual pathway in experimental glaucoma: spatiotemporal characterization and correlation with axonal injury. Invest Ophthalmol Vis Sci 2010;51(12):6448-6460.
13 Bosco A, Steele MR, Vetter ML. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol 2011;519(4):599-620.
14 Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia 2007;55(9):942-953.
15 Taylor S, Calder CJ, Albon J, Erichsen JT, Boulton ME, Morgan JE.Involvement of the CD200 receptor complex in microglia activation in experimental glaucoma. Exp Eye Res 2011;92(5):338-343.
16 Shimazawa M, Ito Y, Inokuchi Y, Yamanaka H, Nakanishi T, Hayashi T, Ji B, Higuchi M, Suhara T, Imamura K, Araie M, Watanabe Y, Onoe H, Hara H. An alteration in the lateral geniculate nucleus of experimental glaucoma monkeys: in vivo positron emission tomography imaging of glial activation. PLoS One 2012;7(1):e30526.
17 Ito Y, Shimazawa M, Chen YN, Tsuruma K, Yamashima T, Araie M, Hara H. Morphological changes in the visual pathway induced by experimental glaucoma in Japanese monkeys. Exp Eye Res 2009;89(2):246-255.
18 Gupta N, Greenberg G, de Tilly LN, Gray B, Polemidiotis M, Yücel YH. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br J Ophthalmol 2009;93(1):56-60.
19 Abcouwer SF, Lin CM, Shanmugam S, Muthusamy A, Barber AJ,Antonetti DA. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J Neuroinflammation 2013;10:149.
20 Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci 2007;27(12):3057-3063.
21 Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci 2008;49(4):1437-1446.
22 Bordone MP, González Fleitas MF, Pasquini LA, Bosco A, Sande PH,Rosenstein RE, Dorfman D. Involvement of microglia in early axoglial alterations of the optic nerve induced by experimental glaucoma. J Neurochem 2017;142(2):323-337.
23 Bosco A, Crish SD, Steele MR, Romero CO, Inman DM, Horner PJ, Calkins DJ, Vetter ML. Early reduction of microglia activation by irradiation in a model of chronic glaucoma. PLoS One 2012;7(8):e43602.
24 Von Thun Und Hohenstein-Blaul N, Kunst S, Pfeiffer N, Grus FH. Biomarkers for glaucoma: from the lab to the clinic. Eye (Lond)2017;31(2):225-231.
25 Rojas B, Gallego BI, Ramírez AI, Salazar JJ, de Hoz R, Valiente-Soriano FJ, Avilés-Trigueros M, Villegas-Perez MP, Vidal-Sanz M,Triviño A, Ramírez JM. Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. J Neuroinflammation 2014;11:133.
26 Beynon SB, Walker FR. Microglial activation in the injured and healthy brain: what are we really talking about? Practical and theoretical issues associated with the measurement of changes in microglial morphology. Neuroscience 2012;225:162-171.
27 Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol 2007;81(6):1345-1351.
28 Fischer AJ, Zelinka C, Milani-Nejad N. Reactive retinal microglia,neuronal survival, and the formation of retinal folds and detachments.Glia 2015;63(2):313-327.
29 Chen M, Zhao J, Luo C, Pandi SP, Penalva RG, Fitzgerald DC, Xu H.Para-inflammation-mediated retinal recruitment of bone marrow-derived myeloid cells following whole-body irradiation is CCL2 dependent. Glia 2012;60(5):833-842.
30 Copland DA, Hussain K, Baalasubramanian S, Hughes TR, Morgan BP, Xu H, Dick AD, Nicholson LB. Systemic and local anti-C5 therapy reduces the disease severity in experimental autoimmune uveoretinitis.Clin Exp Immunol 2010;159(3):303-314.
31 Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN,Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 2006;9(7):917-924.
32 Breen KT, Anderson SR, Steele MR, Calkins DJ, Bosco A, Vetter ML.Loss of fractalkine signaling exacerbates axon transport dysfunction in a chronic model of glaucoma. Front Neurosci 2016;10:526.
33 Combadière C, Feumi C, Raoul W, Keller N, Rodéro M, Pézard A,Lavalette S, Houssier M, Jonet L, Picard E, Debré P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest 2007;117(10):2920-2928.
34 Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease.Nat Neurosci 2010;13(4):411-413.
35 Karlstetter M, Ebert S, Langmann T. Microglia in the healthy and degenerating retina: insights from novel mouse models. Immunobiology 2010;215(9-10):685-691.
36 Tezel G, Yang X, Yang J, Wax MB. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res 2004;996(2):202-212.
37 Yuan L, Neufeld AH. Tumor necrosis factor-alpha: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia 2000;32(1):42-50.
38 Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci 2001;42(8):1787-1794.
39 Bajic D, Commons KG. Acute noxious stimulation modifies morphine effect in serotonergic but not dopaminergic midbrain areas. Neuroscience 2010;166(2):720-729.
40 Al-Gayyar MM, Elsherbiny NM. Contribution of TNF-α to the development of retinal neurodegenerative disorders. Eur Cytokine Netw 2013;24(1):27-36.
41 Tezel G, Wax MB. Increased production of tumor necrosis factoralpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci 2000;20(23):8693-8700.
42 Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG,Benowitz LI, Miller JW. Etanercept, a widely used inhibitor of tumor necrosis factor-α (TNF-α), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS One 2012;7(7):e40065.
43 Veroni C, Gabriele L, Canini I, Castiello L, Coccia E, Remoli ME, Columba-Cabezas S, Aricò E, Aloisi F, Agresti C. Activation of TNF receptor 2 in microglia promotes induction of anti-inflammatory pathways. Mol Cell Neurosci 2010;45(3):234-244.
44 Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K,Eisel U. Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci 2002;22(7):RC216.
45 Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamateinduced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem 2004;279(31):32869-32881.
46 Bartsch JW, Wildeboer D, Koller G, Naus S, Rittger A, Moss ML, Minai Y, Jockusch H. Tumor necrosis factor-alpha (TNF-alpha)regulates shedding of TNF-alpha receptor 1 by the metalloproteasedisintegrin ADAM8: evidence for a protease-regulated feedback loop in neuroprotection. J Neurosci 2010;30(36):12210-12218.
47 Mookherjee S, Banerjee D, Chakraborty S, Banerjee A, Mukhopadhyay I, Sen A, Ray K. Association of IL1A and IL1B loci with primary open angle glaucoma. BMC Med Genet 2010;11:99.
48 Balaiya S, Edwards J, Tillis T, Khetpal V, Chalam KV. Tumor necrosis factor-alpha (TNF-α) levels in aqueous humor of primary open angle glaucoma. Clin Ophthalmol 2011;5:553-556.
49 Huang P, Xu Y, Wei R, Li H, Tang Y, Liu J, Zhang SS, Zhang C.Efficacy of tetrandrine on lowering intraocular pressure in animal model with ocular hypertension. J Glaucoma 2011;20(3):183-188.
50 Okada M, Yamawaki H. Levosimendan inhibits interleukin-1β-induced apoptosis through activation of Akt and inhibition of inducible nitric oxide synthase in rat cardiac fibroblasts. Eur J Pharmacol 2015;769:86-92.
51 Chen C, Xu Y, Zhang J, Zhu J, Zhang J, Hu N, Guan H. Altered expression of nNOS/NIDD in the retina of a glaucoma model of DBA/2J mice and the intervention by nNOS inhibition. J Mol Neurosci 2013;51(1):47-56.
52 Rokicki W, Żaba M, Wyględowska-Promieńska D, Kabiesz A,Reichman-Warmusz E, Brzozowa M, Majewski W, Wojnicz R.Inducible and endothelial nitric synthetase expression and nitrotyrosine accumulation in iris vasculature of patients with primary open-angle glaucoma: a pilot study. Med Sci Monit 2015;21:76-81.
53 Dang Y, Xu Y, Wu W, Li W, Sun Y, Yang J, Zhu Y, Zhang C.Tetrandrine suppresses lipopolysaccharide-induced microglial activation by inhibiting NF-κB and ERK signaling pathways in BV2 cells. PLoS One 2014;9(8):e102522.
54 Li W, Yang C, Lu J, Huang P, Barnstable CJ, Zhang C, Lu J, Zhang S.Tetrandrine protects mouse retinal ganglion cells from ischemic injury.Drug Des Devel Ther 2014;8:327-339.
55 Sappington RM, Calkins DJ. Pressure-induced regulation of IL-6 in retinal glial cells: involvement of the ubiquitin/proteasome pathway and NFkappaB. Invest Ophthalmol Vis Sci 2006;47(9):3860-3869.
56 Sappington RM, Chan M, Calkins DJ. Interleukin-6 protects retinal ganglion cells from pressure-induced death. Invest Ophthalmol Vis Sci 2006;47(7):2932-2942.
57 Chidlow G, Wood JP, Ebneter A, Casson RJ. Interleukin-6 is an efficacious marker of axonal transport disruption during experimental glaucoma and stimulates neuritogenesis in cultured retinal ganglion cells.Neurobiol Dis 2012;48(3):568-581.
58 Sims SM, Holmgren L, Cathcart HM, Sappington RM. Spatial regulation of interleukin-6 signaling in response to neurodegenerative stressors in the retina. Am J Neurodegener Dis 2012;1(2):168-179.
59 Hou XR, Miao H, Tao Y, Li XX, Wong IY. Expression of cytokines on the iris of patients with neovascular glaucoma. Acta Ophthalmol 2015;93(2):e100-e104.
60 Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage.Nature 2001;414(6866):916-920.
61 Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 1999;19(21):9192-9200.
62 Gonçalves N, Simões AT, Cunha RA, de Almeida LP. Caffeine and adenosine A(2A) receptor inactivation decrease striatal neuropathology in a lentiviral-based model of Machado-Joseph disease. Ann Neurol 2013;73(5):655-666.
63 Anuradha S, Gopalakrishna V. Selective IgA deficiency-a case report.Indian J Pathol Microbiol 1989;32(4):310-311.
64 Cerri S, Levandis G, Ambrosi G, Montepeloso E, Antoninetti GF, Franco R, Lanciego JL, Baqi Y, Müller CE, Pinna A, Blandini F, Armentero MT. Neuroprotective potential of adenosine A2A and cannabinoid CB1 receptor antagonists in an animal model of Parkinson disease. J Neuropathol Exp Neurol 2014;73(5):414-424.
65 Canas PM, Porciúncula LO, Cunha GM, Silva CG, Machado NJ,Oliveira JM, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci 2009;29(47):14741-14751.
66 Blackburn MR, Vance CO, Morschl E, Wilson CN. Adenosine receptors and inflammation. Handb Exp Pharmacol 2009;(193):215-269.
67 Santiago AR, Baptista FI, Santos PF, Cristóvão G, Ambrósio AF, Cunha RA, Gomes CA. Role of microglia adenosine A(2A) receptors in retinal and brain neurodegenerative diseases. Mediators Inflamm 2014;2014:465694.
68 Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration.Biochim Biophys Acta 2011;1808(5):1380-1399.
69 Rebola N, Simões AP, Canas PM, Tomé AR, Andrade GM, Barry CE,Agostinho PM, Lynch MA, Cunha RA. Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J Neurochem 2011;117(1):100-111.
70 Yu L, Shen HY, Coelho JE, Araújo IM, Huang QY, Day YJ, Rebola N, Canas PM, Rapp EK, Ferrara J, Taylor D, Müller CE, Linden J,Cunha RA, Chen JF. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol 2008;63(3):338-346.
71 Dai SS, Zhou YG, Li W, An JH, Li P, Yang N, Chen XY, Xiong RP,Liu P, Zhao Y, Shen HY, Zhu PF, Chen JF. Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J Neurosci 2010;30(16):5802-5810.
72 Orr AG, Orr AL, Li XJ, Gross RE, Traynelis SF. Adenosine A(2A)receptor mediates microglial process retraction. Nat Neurosci 2009;12(7):872-878.
73 Simões AP, Duarte JA, Agasse F, Canas PM, Tomé AR, Agostinho P,Cunha RA. Blockade of adenosine A2A receptors prevents interleukin-1β-induced exacerbation of neuronal toxicity through a p38 mitogenactivated protein kinase pathway. J Neuroinflammation 2012;9:204.
74 Jha MK, Seo M, Kim JH, Kim BG, Cho JY, Suk K. The secretome signature of reactive glial cells and its pathological implications. Biochim Biophys Acta 2013;1834(11):2418-2428.
75 Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 2012;33(7):829-837.
76 Neufeld AH, Hernandez MR, Gonzalez M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch Ophthalmol 1997;115(4):497-503.
77 Cho KJ, Kim JH, Park HY, Park CK. Glial cell response and iNOS expression in the optic nerve head and retina of the rat following acute high IOP ischemia-reperfusion. Brain Res 2011;1403:67-77.
78 Vidal L, Díaz F, Villena A, Moreno M, Campos JG, de Vargas IP.Nitric oxide synthase in retina and optic nerve head of rat with increased intraocular pressure and effect of timolol. Brain Res Bull 2006;70(4-6):406-413.
79 Shibata M, Sugiyama T, Hoshiga M, Hotchi J, Okuno T, Oku H,Hanafusa T, Ikeda T. Changes in optic nerve head blood flow, visual function, and retinal histology in hypercholesterolemic rabbits. Exp Eye Res 2011;93(6):818-824.
80 Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci U S A 1999;96(17):9944-9948.
81 Zeng H, Ding M, Chen XX, Lu Q. Microglial NADPH oxidase activation mediates rod cell death in the retinal degeneration in rd mice.Neuroscience 2014;275:54-61.
82 Trouw LA, Daha MR. Role of complement in innate immunity and host defense. Immunol Lett 2011;138(1):35-37.
83 Luo C, Chen M, Xu H. Complement gene expression and regulation in mouse retina and retinal pigment epithelium/choroid. Mol Vis 2011;17:1588-1597.
84 Chen M, Muckersie E, Luo C, Forrester JV, Xu H. Inhibition of the alternative pathway of complement activation reduces inflammation in experimental autoimmune uveoretinitis. Eur J Immunol 2010;40(10):2870-2881.
85 Chen M, Forrester JV, Xu H. Synthesis of complement factor H by retinal pigment epithelial cells is down-regulated by oxidized photoreceptor outer segments. Exp Eye Res 2007;84(4):635-645.
86 Stasi K, Nagel D, Yang X, Wang RF, Ren L, Podos SM, Mittag T,Danias J. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci 2006;47(3):1024-1029.
87 Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS,Nouri N, Micheva K D, Mehalow AK, Huberman AD, Stafford B,Sher A, Litke A, Lambris JD, Smith SJ, John SWM, Barres BA. The classical complement cascade mediates CNS synapse Elimination. Cell 2007;131(6):1164-1178.
88 Williams PA, Tribble JR, Pepper KW, Cross SD, Morgan BP, Morgan JE, John SWM, Howell GR. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol Neurodegener 2016;11(1):26.
89 Jha P, Banda H, Tytarenko R, Bora PS, Bora NS. Complement mediated apoptosis leads to the loss of retinal ganglion cells in animal model of glaucoma. Mol Immunol 2011;48(15-16):2151-2158.
90 Matsumoto H, Murakami Y, Kataoka K, Notomi S, Mantopoulos D,Trichonas G, Miller J W, Gregory M S, Ksander B R, Marshak-Rothstein A, Vavvas D G. Membrane-bound and soluble Fas ligands have opposite functions in photoreceptor cell death following separation from the retinal pigment epithelium. Cell Death Dis 2015;6(11):e1986.
91 LA OR, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, Haynes N M, Tarlinton D M, Zhang J G, Belz G T, Smyth M J, Bouillet P, Robb L,Strasser A. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature 2009;461(7264):659-663.
92 Krishnan A, Fei F, Jones A, Busto P, Marshak-Rothstein A, Ksander BR, Gregory-Ksander M. Overexpression of soluble fas ligand following adeno-associated virus gene therapy prevents retinal ganglion cell death in chronic and acute murine models of glaucoma. J Immunol 2016;197(12):4626-4638.
93 Gregory MS, Hackett CG, Abernathy EF, Lee KS, Saff RR, Hohlbaum AM, Moody KS, Hobson MW, Jones A, Kolovou P, Karray S, Giani A,John SW, Chen DF, Marshak-Rothstein A, Ksander BR. Opposing roles for membrane bound and soluble Fas ligand in glaucoma-associated retinal ganglion cell death. PLoS One 2011;6(3):e17659.
94 Agapova OA, Ricard CS, Salvador-Silva M, Hernandez MR.Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia 2001;33(3):205-216.
95 Tang Y, Le W. Differential Roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 2016;53(2):1181-1194.
96 McGeer PL, McGeer EG. Targeting microglia for the treatment of Alzheimer's disease. Expert Opin Ther Targets 2015;19(4):497-506.
97 Moehle MS, West AB. M1 and M2 immune activation in Parkinson's Disease: Foe and ally? Neuroscience 2015;302:59-73.
98 Ransohoff RM. A polarizing question: do M1 and M2 microglia exist?Nat Neurosci 2016;19(8):987-991.
99 Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E,Freeman TC, Ulas T, Schultze JL. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014;40(2):274-288.
100 Williams PA, Marsh-Armstrong N, Howell GR, Lasker/IRRF initiative on astrocytes and glaucomatous neurodegeneration participants.neuroinflammation in glaucoma: a new opportunity. Exp Eye Res 2017;157:20-27.
101 Karthikeyan A, Patnala R, Jadhav SP, Eng-Ang L, Dheen ST.MicroRNAs: Key Players in microglia and astrocyte mediated inflammation in CNS pathologies. Curr Med Chem 2016;23(30):3528-3546.
102 Wang J, Valiente-Soriano FJ, Nadal-Nicolás FM, Rovere G, Chen S, Huang W, Agudo-Barriuso M, Jonas JB, Vidal-Sanz M, Zhang X.MicroRNA regulation in an animal model of acute ocular hypertension.Acta Ophthalmol 2017;95(1):e10-e21.