Comparison of intravitreal ganciclovir monotherapy and combination with foscarnet as initial therapy for cytomegalovirus retinitis
Jing-Jing Fan1,2, Yong Tao1,3, De-Kuang Hwang4,5
1Department of Ophthalmology, People’s Hospital, Peking University, Beijing 100044, China
2Department of Ophthalmology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province,China
3Department of Ophthalmology, Beijing Chaoyang Hospital,Capital Medical University, Beijing 100020, China
4Department of Ophthalmology, Taipei Veterans General Hospital, Taipei 11217, Taiwan, China
5Department of Ophthalmology, School of Medicine, National Yang-Ming University, Taipei 11217, Taiwan, China
Abstract
●AlM:To compare the effectiveness between multiple intravitreal injections of ganciclovir alone and combined with foscarnet as initial treatment for patients with newlyonset cytomegalovirus retinitis (CMVR).
●METHODS:The retrospective study observed 37 patients(58 eyes) who suffered from CMVR onset between 2013 and 2015. Among them, 35 eyes underwent 4 weekly intravitreal injections of 3.0 mg ganciclovir, and 23 eyes underwent 4 weekly injections of 3.0 mg ganciclovir combined with 2.4 mg foscarnet. Visual acuity, intraocular pressure and viral load of cytomegalovirus (CMV) in aqueous humor measured by real-time polymerase chain reaction were compared before and after each injection.
●RESULTS:CMV-DNA copies in aqueous humor decreased remarkably in both groups. The average of CMV-DNA copies in patients’ aqueous decreased from 38.3×104copies/mL at baseline to 2.2×104copies/mL after the 4thinjection in patients who were treated with ganciclovir monotherapy,and decreased from 76.9×104copies/mL to 11.3×104copies/mL after 4 continuous injections of ganciclovir combined with foscarnet. No significant difference was found in reduction of viral load, change of visual acuities or intraocular pressures between monotherapy or combined therapy.
●CONCLUSlON:Results of this study show that the initial effectiveness of treating CMVR after 4 weekly intravitreal injections is not significantly different from ganciclovir alone or combined with foscarnet. Continuous injection of ganciclovir alone is sufficient in treating immunosuppressive patients with newly-onset CMVR.
●KEYWORDS:ganciclovir; foscarnet; cytomegalovirus retinitis; intravitreal; visual acuity
INTRODUCTION
Cytomegalovirus retinitis (CMVR) is one of the major causes of visual blindness in patients with immunocompromised disorders such as acquired immunodeficiency syndrome(AIDS) and hematopoietic stem cell transplantation[1-3]. Recent studies showed that poor visual outcome with visual acuity less than 20/200 will be found in 13% to 60% of affected eyes[4-7]. Intravitreal injection of anti-viral agents against cytomegalovirus (CMV) has been widely used as a crucial treatment for CMVR[8-9]. As compared to systemic use,administration through intravitreal injections results in higher retinal tissue concentrations but lower systemic exposure[10].
Ganciclovir has been used to treat human CMV infection since 1983. However, ganciclovir-resistant CMV infections have been found in some patients with end-organ CMV diseases[11],and the vast majority of infections caused by drug-resistant CMV have been found in patients with AIDS[12-13]. Severe ocular complications such as retinal detachment and irreversible visual loss more commonly happens if the infection cannot be controlled promptly or occurs recurrently. In such cases,foscarnet is usually used to treat the ganciclovir-resistant CMV infections[14-16].
Both ganciclovir or foscarnet have been proven to be effective in treating CMVR through intravitreal administration[17-19].
Moreover, combined therapy by these two drugs has been shown to be effective in treating recurrent CMVR or whenthe infection is clinically resistant to either drug alone[20-21].Theoretically, using a combination of these two drugs as initial therapy might enhance the anti-viral effect and reduce the nonresponse rate of treatments, followed by a better outcome.However, few studies have used the combination therapy as initial treatment, or even compared the effectiveness and safety between monotherapy and combination therapy as initial treatment for CMVR. We therefore performed a comparative study to evaluate the difference in laboratory and clinical outcomes of CMVR by following these two strategies.
SUBJECTS AND METHODS
The study was designed as a retrospective observational study. Patients who were diagnosed and treated with CMVR at People’s Hospital of Peking University between July 2013 and April 2015 were reviewed and enrolled. This study was approved by the Institutional Review Board of the People’s Hospital of Peking University, and adhered to the tenets of the Declaration of Helsinki. During this period, all newly diagnosed patients were treated with two different strategies:weekly intravitreal injection of 3.0 mg ganciclovir (Likewei;Hubei Keyi Pharmaceutical Co., Ltd., Wuhan, China) for 1mo[intravitreal injection of ganciclovir (IVG) group], or injection of 3.0 mg ganciclovir combined with 2.4 mg foscarnet for 1mo (IVG+F group). The decisions of treatment strategy were made almost randomly without considering any patient’s or disease’s condition. The injection method is described in detail in previous reports[19]. Patients’ best corrected visual acuity,viral DNA load of CMV and detail clinical findings at baseline and 1wk after each injection were collected and reviewed.
Real-time polymerase chain reaction (PCR) was applied to detect the DNA load of CMV in patient’s aqueous humor.CMV real-time PCR kit mix contains reagents and enzymes for the specific amplification of immediate early gene region of the human CMV-DNA. A standard curve was obtained from the quantitation standard CMV-DNA positive controls provided by the manufacturer.
Statistical analysis was done using a commercially available statistical software package (SPSS for Windows, version 21.0;IBM-SPSS, Chicago, IL, USA). The normality of data was tested by the Shapiro-Wilk test. Visual acuity was converted into the logarithm of the minimum angle of resolution(logMAR) for statistical calculation[24]. By using boxplots,the mean virus loads of CMV, visual acuity and intraocular pressures were compared against the times of injection, and the trend of which changed with time were judged from it.Chi-square andt-test were used for univariate nonparametric analyses. AllP-values were 2-sided and were considered statistically significant if <0.05.
RESULTS
A total of 58 eyes with CMVR in 37 patients fulfilled the inclusion criteria and did not meet any exclusion criteria withinthe study period. Of these patients, 2 had AIDS, 34 received hematopoietic stem cell transplantation and 1 received thymectomy. Twenty-four patients (35 eyes) with a mean age of 37.2y underwent 4 weekly injections of ganciclovir and were categorized in the IVG group. On the other hand, 23 eyes in 13 patients with a mean age of 41.1y underwent 4 weekly injections of ganciclovir combined with foscarnet and were categorized in the IVG+F group (Table 1). The mean duration from onset of visual symptoms to treatment was 2.6±1.2wk.There is no difference between patients’ characteristics and ocular conditions among these two groups (Tables 1, 2).
Table 1 Characteristics of 37 patients with CMVRn(%)
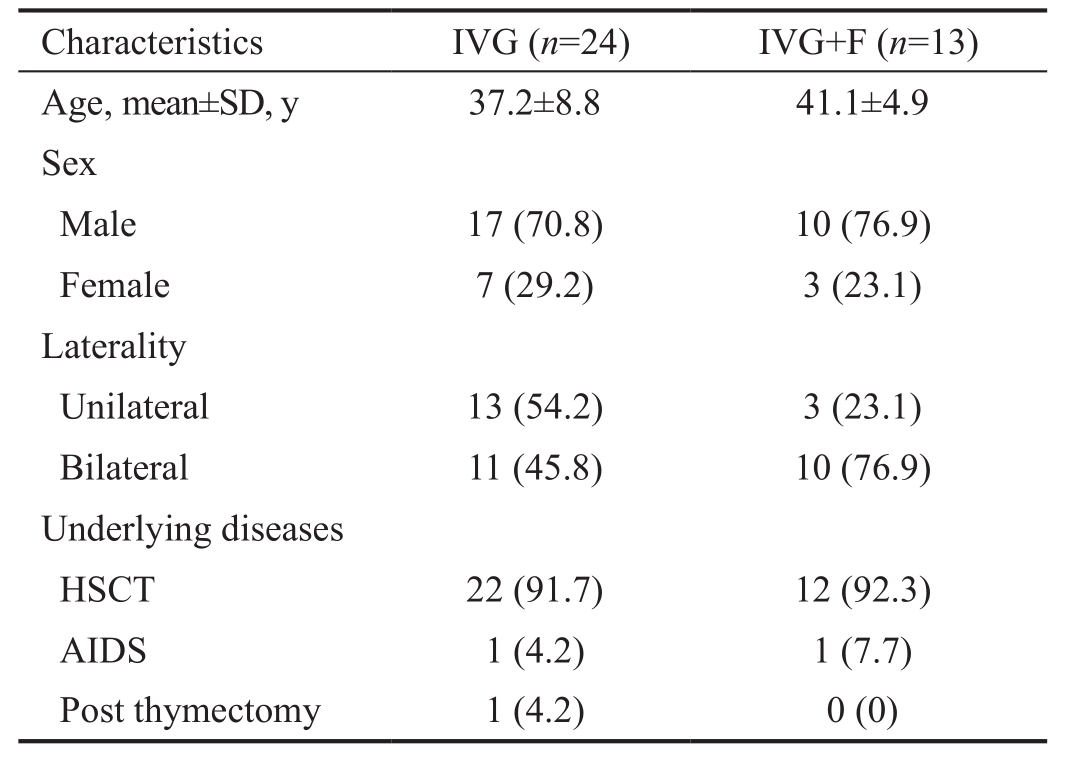
IVG: Weekly intravitreal injection of 3.0 mg ganciclovir 4 times for initial treatment; IVG+F: Weekly intravitreal injection of 3.0 mg ganciclovir plus 2.4 mg foscarnet 4 times for initial treatment;
HSCT: Hematopoietic stem cell transplantation; AIDS: Acquired immunodeficiency syndrome.
Characteristics IVG (n=24) IVG+F (n=13)Age, mean±SD, y 37.2±8.8 41.1±4.9 Sex Male 17 (70.8) 10 (76.9)Female 7 (29.2) 3 (23.1)Laterality Unilateral 13 (54.2) 3 (23.1)Bilateral 11 (45.8) 10 (76.9)Underlying diseases HSCT 22 (91.7) 12 (92.3)AIDS 1 (4.2) 1 (7.7)Post thymectomy 1 (4.2) 0 (0)
The CMV-DNA copies in affected eyes decreased remarkably with the time of injections in both groups. In the IVG group,the mean value of CMV-DNA copies decreased from 38.3×104copies/mL at baseline to 2.2×104copies/mL at the 4thinjection. Whereas in the IVG+F group, the mean value of CMV-DNA copies decreased from 76.9×104copies/mL at baseline to 11.3×104copies/mL at the 4thinjection. Although there were significant reductions of DNA load in both IVG and IVG+F groups (P=0.02 and <0.01, respectively), no statistically significant difference in the amount of reduction between each group were found (P=0.12; Table 2, Figure 1).
No significant worsening or change of patients’ visual acuity from baseline to the 4thinjection was found in both two groups(P=0.37 and 0.71; Figures 2, 3). Among all affected eyes in the IVG group, visual acuity remained the same in 16.7% of eyes,improved in 66.7% of eyes and decreased in 16.7% of eyes.On the other hand, the vision remained the same in 17.4% of eyes in the IVG+F group, 52.2% of eyes had improved vision,and 30.4% of eyes had worse vision. No significant differences were found in change of visual acuity and intraocular pressures between the two groups (P=0.21 and 0.17, respectively).
Table 2 Outcomes of patients with CMVR who underwent different treatments mean±SD
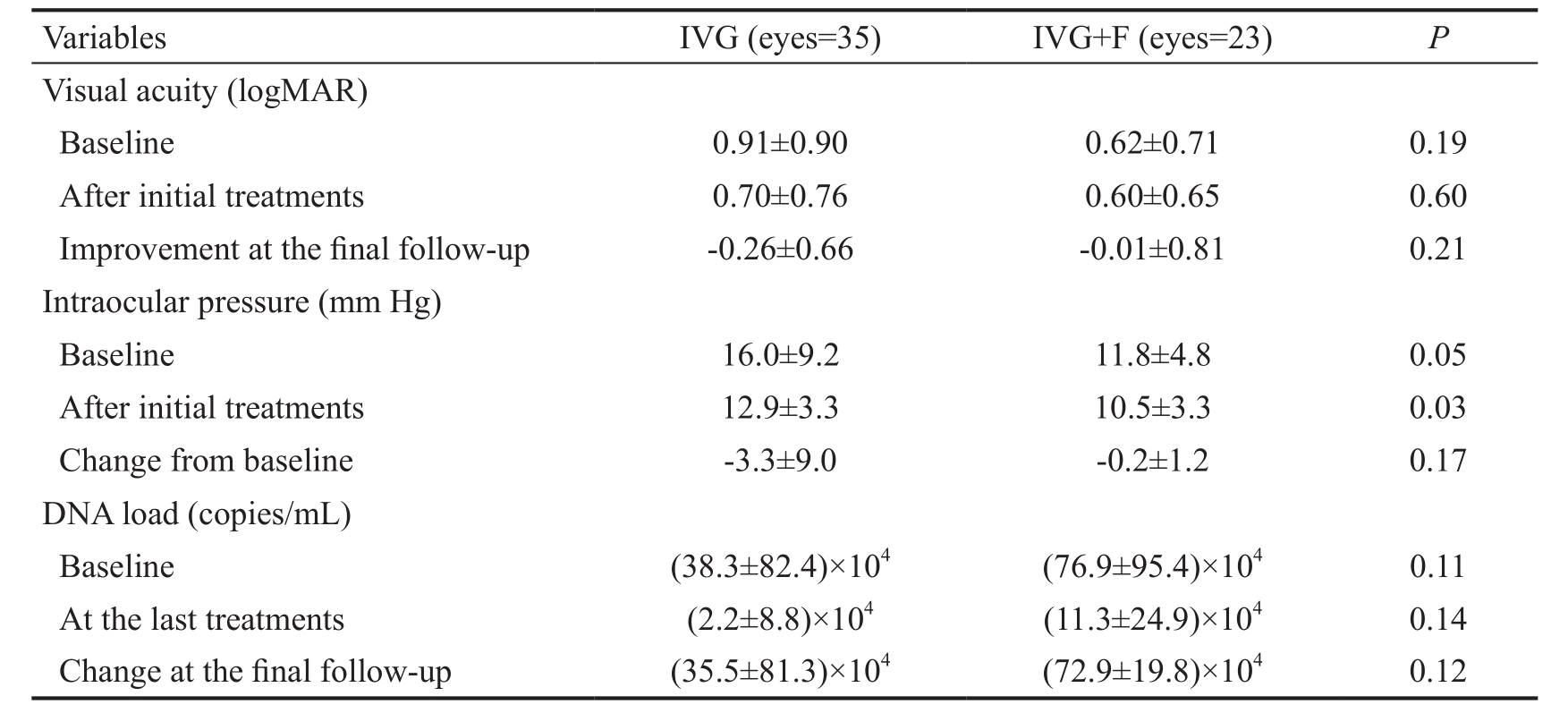
IVG: Weekly intravitreal injection of 3.0 mg ganciclovir 4 times for initial treatment; IVG+F: Weekly intravitreal injection of 3.0 mg ganciclovir plus 2.4 mg foscarnet 4 times for initial treatment.
Variables IVG (eyes=35) IVG+F (eyes=23)PVisual acuity (logMAR)Baseline 0.91±0.90 0.62±0.71 0.19 After initial treatments 0.70±0.76 0.60±0.65 0.60 Improvement at the final follow-up -0.26±0.66 -0.01±0.81 0.21 Intraocular pressure (mm Hg)Baseline 16.0±9.2 11.8±4.8 0.05 After initial treatments 12.9±3.3 10.5±3.3 0.03 Change from baseline -3.3±9.0 -0.2±1.2 0.17 DNA load (copies/mL)Baseline (38.3±82.4)×104(76.9±95.4)×1040.11 At the last treatments (2.2±8.8)×104(11.3±24.9)×1040.14 Change at the final follow-up (35.5±81.3)×104(72.9±19.8)×1040.12
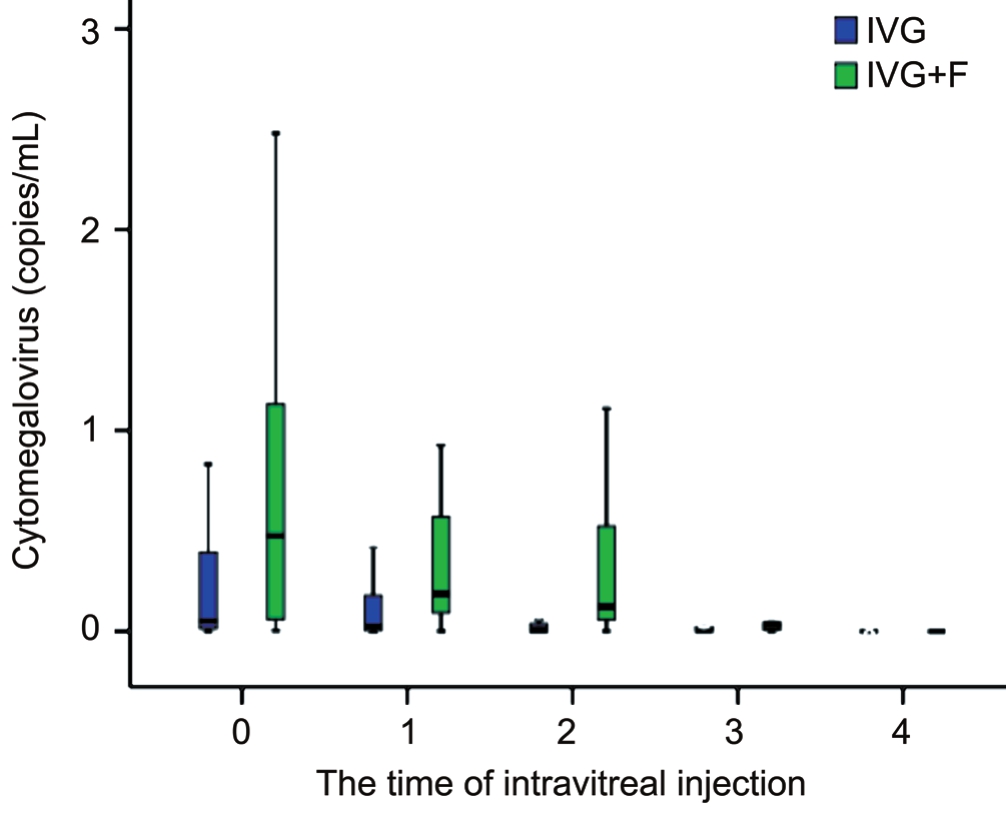
Figure 1 Viral loads of DNA in patients’ aqueous with newly onset CMVR who were treated with IVG and IVG+F.
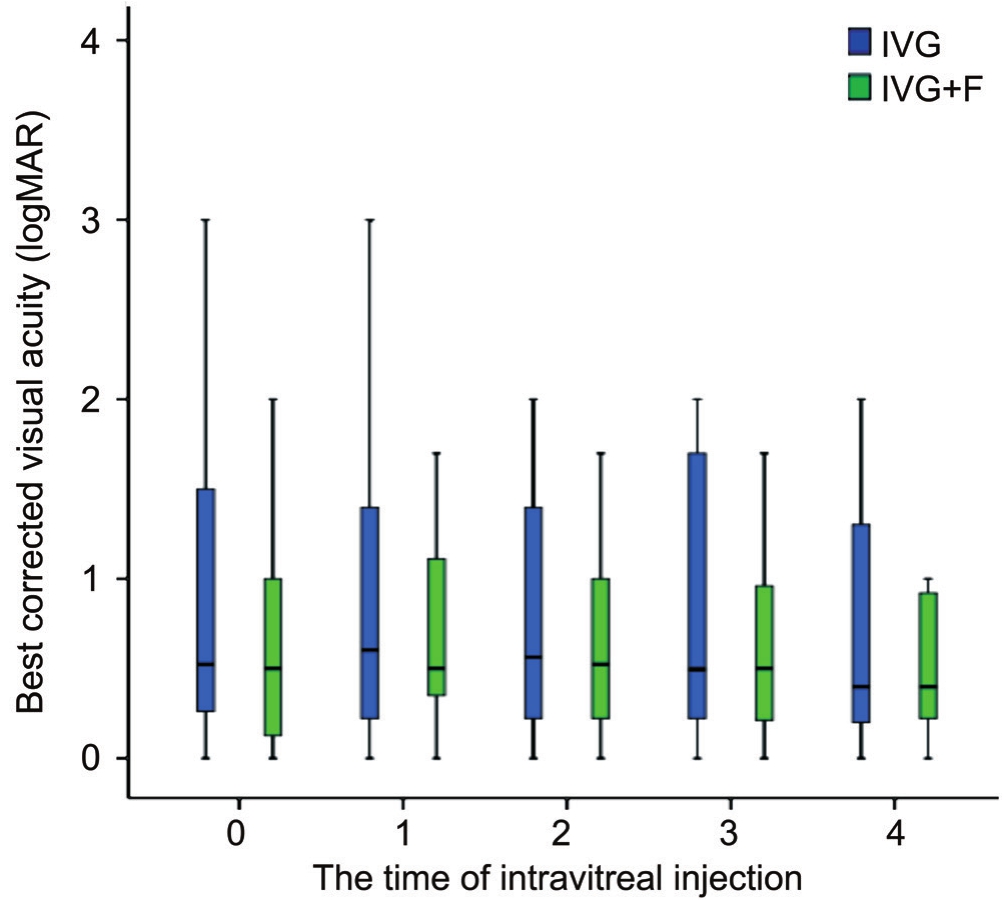
Figure 2 Best corrected visual acuities in patients with newly onset CMVR at each time before and after intravitreal injection.
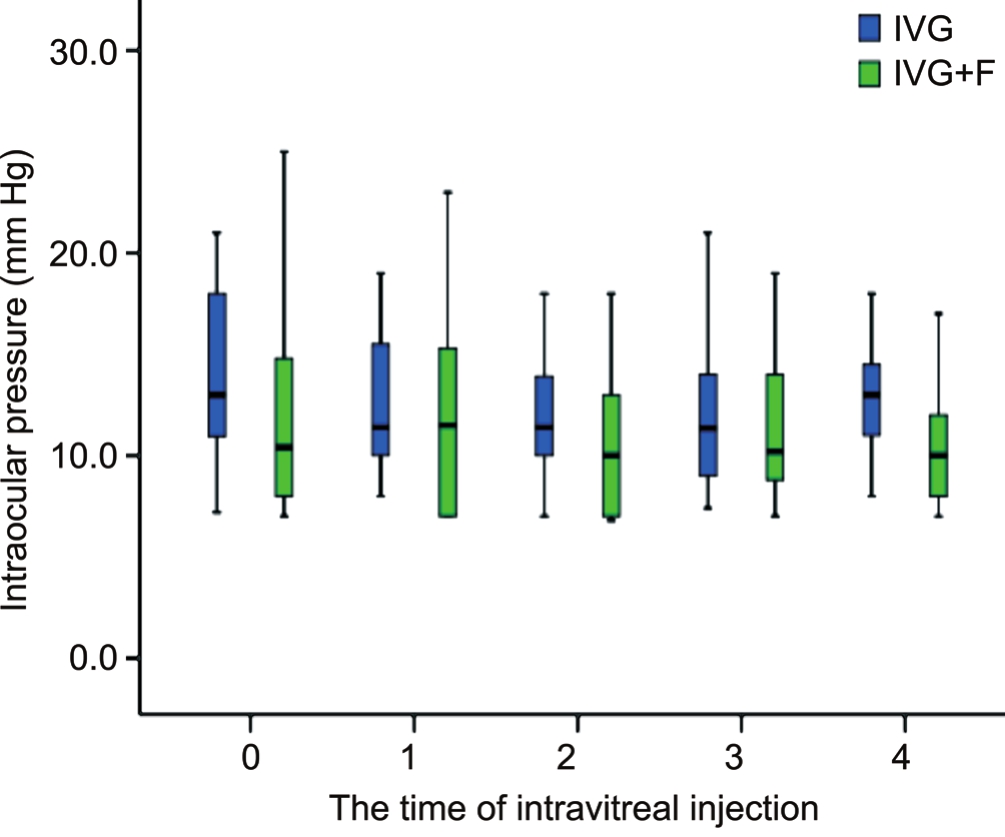
Figure 3 Intraocular pressures in patients with newly onset CMVR at each time before and after intravitreal injection.
DISCUSSION
CMVR is an important sight-threatening disease in immunocompromised patients. Previous studies have clearly showed that intravitreal therapy offers a significant advantage over systemic therapy in the treatment of CMVR by substantially reducing the risk of CMVR-complicated retinal detachment[22]. Ganciclovir is a nucleoside analogue which can be used against viruses of the herpetic family, including CMV.CMV phosphotransferase (UL97) gene and DNA polymerase(UL54) gene play a crucial rule in its anti-viral mechanism[10].CMVs that have mutations in UL97 and UL54 can easily resist ganciclovir. On the other hand, foscarnet is another pyrophosphate analogue which inhibits viral polymerases but does not depend on any phosphorylation or activation secondary to viral infection[16-17,23].
Intravitreal injection of ganciclovir with a dosage of 2.0 to 5.0 mg is effective and has been approved for treating CMVR[24].
Pharmacokinetic studies have demonstrated that the concentrations in vitreous after one shoot can be maintained above the ID50 (0.25 to 1.22 mg/mL) for up to 7d. Based on the clinical response and disease course of CMVR, weekly injections of anti-viral agents were usually required at the acute infectious stage. Monotherapy with intravitreal ganciclovir or foscarnet is usually used when the patient first suffered from CMVR, and a combination of these two drugs is used when the infection resists either one of the drugs or recurs during treatment[20-21]. However, the prolonging or recurrence of disease course may lead to severe ocular complications such as macular involvement or retinal detachment.
The viral DNA load of CMV and visual acuity are two important indicators to evaluate the virological and clinical treatment effect in CMVR. In this study, we recorded the DNA load in aqueous humor and patients’ visual acuity each time before and after the intravitreal injection. The results indicated that the CMV-DNA copies reduced significantly in both groups. However, the reduction of mean CMV-DNA copies after 4 weekly injections for patients with CMVR were not significantly different whether we used monotherapy or combination therapy as initial treatments.
On the other hand, the average duration from onset to treatments was 2.6wk in our patients. The visual acuity in 17%of patients’ eyes remained the same after 4 weekly injections and improved in 52.2% to 66.7% of eyes. Although the mean visual acuity did not change significantly in both groups, our results suggested that treating with intravitreal anti-viral agents promptly was indeed effective in preserving vision in affected eyes. The changes of visual acuities or intraocular pressures between both groups were not statistically significant. This implies that monotherapy with ganciclovir might be enough and sufficient for control of CMVR initially.
There were two limitations in this study. First, the study was designed as a retrospective study, rather than perspective.The possibility of clinical selection bias cannot be completely excluded. However, not only patients’ general characteristics,but also baseline viral DNA load and visual acuity were similar in both groups (P>0.05). We assumed that there was no obvious selection bias that would affect the findings in this study. Second, since the duration of follow-up period in this study was short, the long-term recurrent rate of CMVR or other outcome cannot be compared. However, due to the difficulty of judgement of complete cure of CMVR in a short period of time, it might be better to compare the results of patients between each group after 4 consensus injections.
In summary, weekly intravitreal injections of both ganciclovir monotherapy and combination of gancivlovir with foscarnet can effectively reduce intraocular viral load of CMV. The reduction of mean CMV-DNA copies and the change of visual acuity after 4 continuous injections were not statistically different between monotherapy or combine therapy. To our knowledge, this is the first study to compare the effect of intravitreal ganciclovir monotherapy and that combined with foscarnet as initial treatment for newly developed CMVR.
ACKNOWLEDGEMENTS
Authors’ contributions:Literature search: Dr. Fan and Prof.Tao; Data interpretation: Dr. Hwang and Prof. Tao; Writing:Dr. Fan and Dr. Hwang.
Conflicts of Interest:Fan JJ, None; Tao Y, None; Hwang DK, None.
REFERENCES
1 Wong JX, Wong EP, Teoh SC. Outcomes of cytomegalovirus retinitisrelated retinal detachment surgery in acquired immunodeficiency syndrome patients in an Asian population.BMC Ophthalmol2014;14:150.
2 Radwan A, Metzinger JL, Hinkle DM, Foster CS. Cytomegalovirus retinitis in immunocompetent patients: case reports and literature review.Ocul Immunol Inflamm2013;21(4):324-328.
3 Martens G, Celum C, Lewin SR. HIV infection: epidemiology,pathogenesis, treatment, and prevention.Lancet2014;384(9939):258-271.
4 Kim DY, Jo J, Joe SG, Kim JG, Yoon YH, Lee JY. Comparison of visual prognosis and clinical features of cytomegalovirus retinitis in HIV and non-HIV patients.Retina2017;37(2):376-381.
5 Jabs DA, Ahuja A, Van Natta ML, LyonAT, Yeh S, Danis R. Longterm outcomes of cytomegalovirus retinitis in the era of modern antiretroviral therapy: results from a United States cohort.Ophthalmology2015;122(7):1452-1463.
6 Iu LP, Fan MC, Lau JK, Chan TS, Kwong YL, Wong IY. Long-term followup of cytomegalovirus retinitis in non-HIV immunocompromised patients:clinical features and visual prognosis.Am J Ophthalmol2016;165:145-153.
7 Agarwal A, Kumari N, Trehan A, Khadwal A, Dogra MR, Gupta V,Sharma A, Gupta A, Singh R. Outcome of cytomegalovirus retinitis in immunocompromised patients without human immunodeficiency virus treated with intravitreal ganciclovir injection.Graefes Arch Clin Exp Ophthalmol2014;252(9):1393-1401.
8 Stewart MW. Optimal management of cytomegalovirus retinitis in patients with AIDS.Clin Ophthalmol2010;4:285-299.
9 Langner-Wegscheider BJ, ten Dam-van Loon N, Mura M, Faridpooya K, de Smet MD. Intravitreal ganciclovir in the management of non-AIDS-related human cytomegalovirus retinitis.Can J Ophthalmol2010;45(2):157-160.
10 Jabs DA, Ahuja A, Van Natta M, Dunn JP, Yeh S. Comparison of treatment regimens for cytomegalovirus retinitis in patients with AIDS in the era of highly active antiretroviral therapy.Ophthalmology2013;120(6):1262-1270.
11 Komatsu TE, Pikis A, Naeger LK, Harrington PR. Resistance of human cytomegalovirus to ganciclovir/valganciclovir: a comprehensive review of putative resistance pathways.Antiviral Res2014;101:12-25.
12 Minces LR, Nguyen MH, Mitsani D,et al. Ganciclovir-resistant cytomegalovirus infections among lung transplant recipients areassociated with poor outcomes despite treatment with foscarnet-containing regimens.Antimicrob Agents Chemother2014;58(1):128-135.
13 Fisher CE, Knudsen JL, Lease ED, Jerome KR, Rakita RM, Boeckh M, Limaye AP. Risk factors and outcomes of ganciclovir resistant cytomegalovirus infection in solid organ transplant recipients.Clin Infect Dis2017;65(1):57-63.
14 Bacigalupo A, Boyd A, Slipper J, Curtis J, Clissold S. Foscarnet in the management of cytomegalovirus infections in hematopoietic stem cell transplant patients.Expert Rev Anti Infect Ther2012;10(11):1249-1264.
15 Avery RK, Arav-Boger R, Marr KA,et al. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection.Transplantation2016;100(10):e74-e80.
16 James SH, Prichard MN. Current and future therapies for herpes simplex virus infections: mechanism of action and drug resistance.Curr Opin Virol2014;8:54-61.
17 Huynh N, Daniels AB, Kohanim S, Young LH. Medical treatment for cytomegalovirus retinitis.Int Ophthalmol Clin2011;51(4):93-103.
18 Tawse KL, Baumal CR. Intravitreal foscarnet for recurring CMV retinitis in a congenitally infected premature infant.J AAPOS2014;18(1):78-80.
19 Miao H, Tao Y, Jiang YR, Li XX. Multiple intravitreal injections of ganciclovir for cytomegalovirus retinitis after stem-cell transplantation.Graefes Arch Clin Exp Ophthalmol2013;251(7):1829-1833.
20 Boss JD, Rosenberg K, Shah R. Dual Intravitreal injections with foscarnet and ganciclovir for ganciclovir-resistant recurrent cytomegalovirus retinitis in a congenitally infected infant.J Pediatr Ophthalmol Strabismus2016;53:e58-e60.
21 Cai H, Kapoor A, He R, Venkatadri R, Forman M, Posner GH, Arav-Boger R. In vitro combination of anti-cytomegalovirus compounds acting through different targets: role of the slope parameter and insights into mechanisms of action.Antimicrob Agents Chemother2014;58(2):986-994.
22 Young S, McCluskey P, Minassian DC, Joblin P, Jones C, Coroneo MT,Lightman S. Retinal detachment in cytomegalovirus retinitis: intravenous versus intravitreal therapy.Clin Exp Ophthalmol2003;31(2):96-102.
23 Iwami D, Ogawa Y, Fujita H, Morita K, Sasaki H, Oishi Y, Higuchi H, Hatanaka K, Shinohara N. Successful treatment with foscarnet for ganciclovir-resistant cytomegalovirus infection in a kidney transplant recipient: a case report.Nephrology (Carlton)2016;21 Suppl 1:63-66.
24 Arevalo JF, Garcia RA, Mendoza AJ. High-dose (5000-microg)intravitreal ganciclovir combined with highly active antiretroviral therapy for cytomegalovirus retinitis in HIV-infected patients in Venezuela.Eur J Ophthalmol2005;15(5):610-618.
Citation:Fan JJ, Tao Y, Hwang DK. Comparison of intravitreal ganciclovir monotherapy and combination with foscarnet as initial therapy for cytomegalovirus retinitis.Int J Ophthalmol2018;11(10):1638-1642
DOl:10.18240/ijo.2018.10.10
Accepted:2018-05-06
Received:2017-09-07
Correspondence to:De-Kuang Hwang. Department of Ophthalmology, Taipei Veterans General Hospital, No.201, Sec.2, Shih-Pai Road, Taipei 11217, Taiwan, China. m95gbk@gmail.com
Co-first authors:Jing-Jing Fan and Yong Tao
Foundation:Supported by the 1351 Beijing Chaoyang Talent Training Program (No.CYXX-2017-21).




