Table 1 Participants’ clinical and morphological characteristics in this study
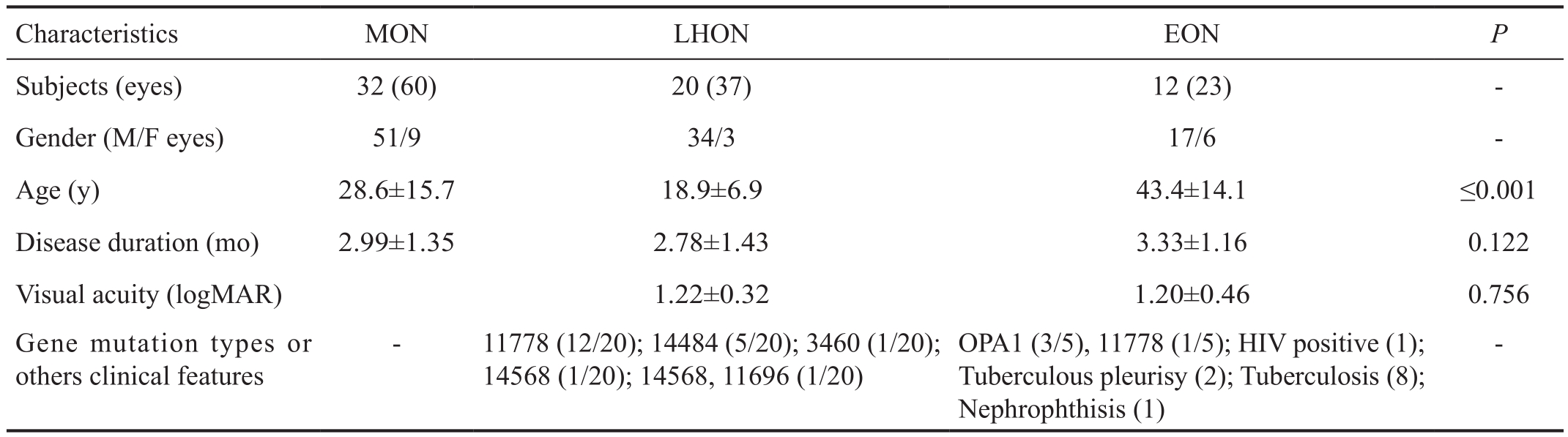
MON: Mitochondrial optic neuropathy; LHON: Leber hereditary optic neuropathy; EON: Ethambutol-induced optic neuropathy.
Da Teng1, Chun-Xia Peng2, Hai-Yan Qian1, Li Li2, Wei Wang3, Jun-Qing Wang1, Bing Chen1, Huan-Fen Zhou1,Shi-Hui Wei1
1Ophthalmology Department, Chinese PLA General Hospital,Beijing 100853, China
2Department of Ophthalmology, Beijing Children’s Hospital,Capital Medical University, Beijing 100045, China
3Zhongshan Ophthalmic Center, Sun Yat-sen University,Guangzhou 510060, Guangdong Province, China
Abstract●AlM:To evaluate the structural injure patterns in peripapillary retinal fiber layer (pRNFL), retinal ganglion cell layer (RGCL) and their correlations to visual function in various mitochondrial optic neuropathies (MON) to offerhelp to their differential diagnosis.
●METHODS:Totally 32 MON patients (60 eyes) were recruited within 6mo after clinical onsets, including 20 Leber hereditary optic neuropathy (LHON) patients (37 eyes), 12 ethambutol-induced optic neuropathy (EON)patients (23 eyes), and 41 age-gender matched healthy controls (HC, 82 eyes). All subjects had pRNFL and RGCL examinations with optic coherence tomography (OCT) and visual function tests.
●RESULTS:ln the early stages of MON, the temporal pRNFL thickness decreased (66.09±22.57 μm), but increased in other quadrants, compared to HC (76.95±14.81 μm). The other quadrants remaining stable for LHON and EON patients besides the second hour sector of pRNFL thickness reduced and the temporal pRNFL decreased (56.78±15.87 μm) for EON. Total macular thickness in MON reduced remarkably(279.25±18.90 μm;P=0.015), which mainly occurring in the inner circle (3 mm diameter of circle) and the nasal temporal sectors in the outer circle (5.5 mm diameter of circle), in contrast to those in HC. RGCL thickness reduced in each sector of the macula (61.90±8.73 μm;P≤0.001). lt strongly showed the correlationship of best corrected visual acuity (R=0.50,P=0.0003) and visual field injury (R=0.54,P=0.0002) in MON patients.
●CONCLUSlON:OCT is a potential tool for detecting structural alterations in the optic nerves of various MON. Different types of MON may have different damage patterns.
●KEYWORDS:mitochondrial optic neuropathies; peripapillary retinal fiber layer; retinal ganglion cell layer; visual function;Leber hereditary optic neuropathy; ethambutol-induced optic neuropathy
In 1988, Leber hereditary optic neuropathy (LHON), the maternally inherited optic atrophy, was firstly identified as a mitochondrial DNA point mutations disease. It helps mitochondrial optic neuropathies (MON) being recognized progressively as a major spectrum of optic neuropathy rooted in different genetic and acquired etiologies[1-3]. Among genetic MON, one of the most common disease in ophthalmological clinical rooms in China was LHON, which causes mitochondrial dysfunction due to mtDNA mutation in the respiratory complex I[1]. For acquired MON, ethambutol-induced optic neuropathy(EON) resulting also from the dysfunction of respiratory complex is the most common drug toxicity worldwide[2]. Energy depletion and active oxidative stress caused by mitochondrial dysfunction triggers apoptosis of retinal ganglion cells (RGCs),which are the common pathways for genetic and acquired MON[4]. As the papillomacular-bundle (PMB), characterized by high-energy demands and low-energy production, is the preferred neural axons to be damaged, various causes of MON have similar clinical features[5-11].
However, even though there are different types of MON and different individuals with the same gene mutations or taking the same dose of ethambutol, not all individuals present with optic nerve impairment. The study of the structural alterations of RGCs and their axons in LHON and EON patients couldpotentially offer help in elucidating the pathogenesis of MON,as well as differential diagnosis of MON diseases from other optic nerve diseases. Spectral-domain optical coherence tomography (SD-OCT) with 2-3 µm of axial resolution can visualize structural alterations of RGCs and their axons inpatients with MON. The OCT technique was used during studies of MON[12-13]. In addition, our previous studies showed LHON had thinner temporal peripapillary nerve fiber layer(pRNFL) in about 6mo, and had their macular thickness reduced within 6mo. However, these alterations do not have significant correlations to the best corrected visual acuity(BCVA)[5-6]. Furthermore, no relationships between RGC layer(RGCL) injury patterns and damage of visual field (VF) has been reported.
Therefore, the aim of our study is to observe structural alterations of pRNFL, total macular, and RGCL in patients with various MON, and then evaluate the relationships between these alterations to VF.
SubjectsThis is a retrospective, cross-sectional and observational study. Thirty-two patients with MON and 41 age-gender matched healthy controls were recruited from Chinese PLA General Hospital from November 2013 to November 2015. The study was approved by the ethics committee of the Chinese PLA General Hospital and complied with the Declaration of Helsinki in its currently applicable version.Furthermore, we obtained the written consents from all patients or their families before OCT images of the patients were collected. Among the subjects, one eye in an EON patient with greater than -6.00 diopters refractive error was excluded; and one eye without LHON affected, one eye in a LHON patient with more than 6mo after onset, and one eye in an LHON patient with remarkable papilledema were all excluded. In total, 32 MON patients (60 eyes) whose course of disease were within 6mo after clinical onset for each of both eyes included 20 LHON patients (37 eyes) and 12 EON patients (23 eyes).At the same time, 41 (82 eyes), 32 (64 eyes) and 33(66 eyes)age-gender matched healthy controls (HC1, HC2 and HC3 compared to MON group, LHON and EON subgroup) were recruited from the Department of Ophthalmology, Chinese PLA General Hospital.
LHON patients were diagnosed by screening for mtDNA. The EON patients were diagnosed according to the criteria[14-15]:1) visual symptom onset after taking ethambutol; 2) meeting more than one required criteria or two supportive criteria as follows, the required criteria: color anomalopia without other causes, bilateral centre visual loss or cecocentral scotomas evaluated by Humphrey perimetry; the supportive criteria:papillary pale, visual loss, or other types of VF defects other than centre or cecocentral scotomas. The inclusion criteria for healthy eyes were listed as follows: normal visual acuity,refractive error less than ±6.00 diopters or an astigmatism of 2.00 diopters, intraocular pressure (IOP) lower than 21 mm Hg,and those without systematic diseases and central nervous systematic diseases. The exclusion criteria for LHON and EON subjects were as follows: ocular diseases other than LHON or EON, a history of ocular surgery, refractive error greater than±6.00 diopters or astigmatism of 2.00 diopters, IOP more than 21 mm Hg, and systemic diseases such as diabetes and diseases of the central nervous system. First, we compared pRNFL, total macular thickness and RGCL measurements detected by OCT in MON group to those in HC1 to reveal structural injury pattern of MON patients. Second, we evaluated OCT data alterations in LHON and EON patients,and compare them to their HC (HC2 and HC3), in order to reveal their different injury patterns.
Ophthalmologic ExaminationAll subjects experienced ophthalmologic examinations including BCVA, IOP, slit lamp microscope examination and detailed fundus examinations by ophthalmoscope.
Spectral-domain optical coherence tomography examinationAll the subjects had OCT examinations without pupil dilation by SD-OCT (Carl Zeiss Meditec, Inc., Germany). The pRNFL was detected by a 3.4-mm circular scan around the optic disc,and the following parameters for pRNFL were calculated:average pRNFL thickness, pRNFL thickness in 4 respective quadrants (superior, temporal, nasal and inferior quadrants)and 12h of pRNFL. Total macular, excluding the fovea (1 mm central circle), is divided into 9 sectors according to the Early Treatment Diabetic Retinopathy Study (ETDRS); circle diameters: 1, 3, 5.5 mm to accquire the retinal thickness, and is divided into 6 sectors, including superior, nasal superior,temporal superior, nasal inferior, inferior and temporal inferior,to measured RGCL.
Visual function testingAll patients with MON underwent VF tests and were evaluated by a Humphrey Field Analyzer II(Carl Zeiss Meditec, Inc., Germany) using Goldmann size III stimulus. The relationship of average VFs from the 12 central points and macular measurements was assessed, as previously described in detail by Monteiroet al[16].
The BCVA was assessed by a Snellen Eye Chart (decimal acuity) and converted into a logarithm of the minimal angle of resolution (logMAR) notations as previously reported by Schulze-Bonselet al[17].
Statistical AnalysesCohort difference in age or gender were analyzed by Kruskal-Wallis and Chi-square test respectively.Analysis of the differences in OCT measurements between patients’ groups and their HC groups,t-tests were performed.For correlations between BCVA, VFs and OCT measurements,Pearson linear regression models were used. All statistical analyses were performed using SPSS 17.0 and drawing graphics with Prism 6.0. Statistical significance was achieved atP≤0.001.
Table 1 Participants’ clinical and morphological characteristics in this study

MON: Mitochondrial optic neuropathy; LHON: Leber hereditary optic neuropathy; EON: Ethambutol-induced optic neuropathy.
Table 2 pRNFL thickness in MON patients compared to that in healthy control subjects mean±SD, μm
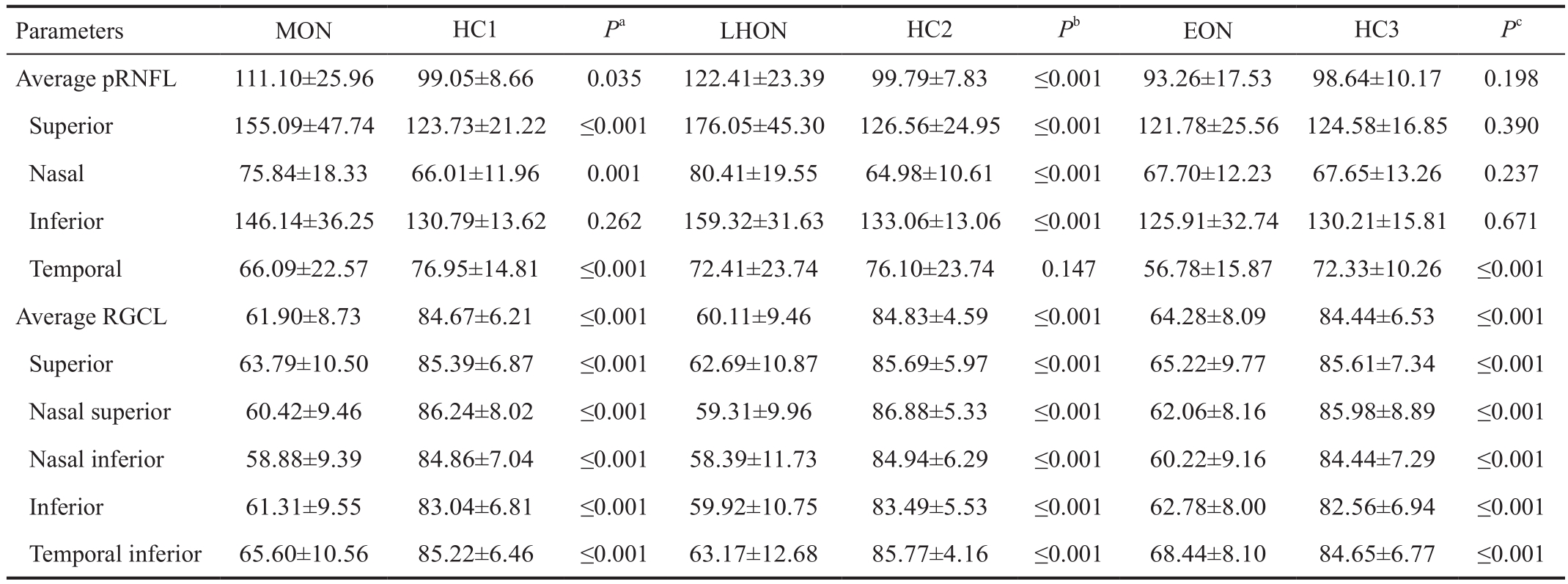
MON: Mitochondrial optic neuropathy; LHON: Leber hereditary optic neuropathy; EON: Ethambutol-induced optic neuropathy. HC1: Age and gender matched of health controls to those of MON group; HC2: Age and gender matched of healthy controls to those of LHON group; HC3:Age and gender matched of healthy controls to those of EON group.Pa:MONvsHC1;Pb:LHONvsHC2;Pc:EONvsHC3.
There were 32 MON patients (60 eyes) including 20 LHON patients (37 eyes) and 12 EON patients (23 eyes) in the present study. The age was 28.6±15.7y with a range of 12-72y in the MON cohort, 18.9±6.9y with a range of 12-36y in the LHON cohort, and 43.4±14.1y with a range of 23-72y in the EON cohort. Among them, LHON patients mainly resulted in 11 778 mtDNA mutations (12/20), with the other patients 14 484 (5/20), 3460, 14 568 (1/20) and 14 568 combined with 11 696 mtDNA mutations (1/20). For the EON cohort,average duration of ethambutol treatment was 6.23±6.20mo with a range of 2 to 24mo, and the average total amount of ethambutol was 127.15±101.70 (18 to 360) g with daily dosages of 0.658±0.201 (0.25-1) g/per day. Among them, 5/12 EON patients underwent mtDNA and OPA1 gene screening for LHON and dominant optic atrophy (DOA)[18]. Consequently,3/5 (60%) of patients presented OPA1 gene mutation, and 1/5 (20%) patients presented 11 778 mtDNA gene mutation.All MON patients were in the early stages within 6mo after clinical onset. The BCVA in the LHON cohort was similar to that of EON (P=0.756; Table 1). Their age-gender matched HC1 group was comprised of 41 subjects (82 eyes) with mean ages of 30.14±14.88y (range 7-72y, male/female: 30/11)matching the MON cohort; HC2 group 24 subjects (48 eyes)with mean ages of 19.88±6.92y (range 7-37y, male/female:19/5) matching the LHON cohort, and 33 HC3 subjects (66 eyes)with mean ages of 40.30±14.33y (range 19-72y, male/female:10/23) matching the EON cohort.
The pRNFL Thickness in MON Patients Compared to ControlsThe average pRNFL thickness in MON patients was significantly thicker (P=0.035), increasing in LHON patients(P≤0.001) and without difference in EON patients (P=0.198),in comparison with their HCs. In contrast to HC, temporal pRNFL thickness in MON patients decreased markedly with an increase in superior, nasal and inferior pRNFL. Further analysis revealed that in LHON patients, only the second hour of pRNFL thickness in temporal pRNFL decreased (P≤0.001)with the other quadrants of pRNFL increasing, compared to that of HC2. For EON patients, as well as MON, the temporal pRNFL thickness decreased (P≤0.001), however other quadrants of pRNFL thickness was not different, compared to its HC3 (Table 2; Figure 1).

Figure 1 The pRNFL thickness in MON patients compared to controls.
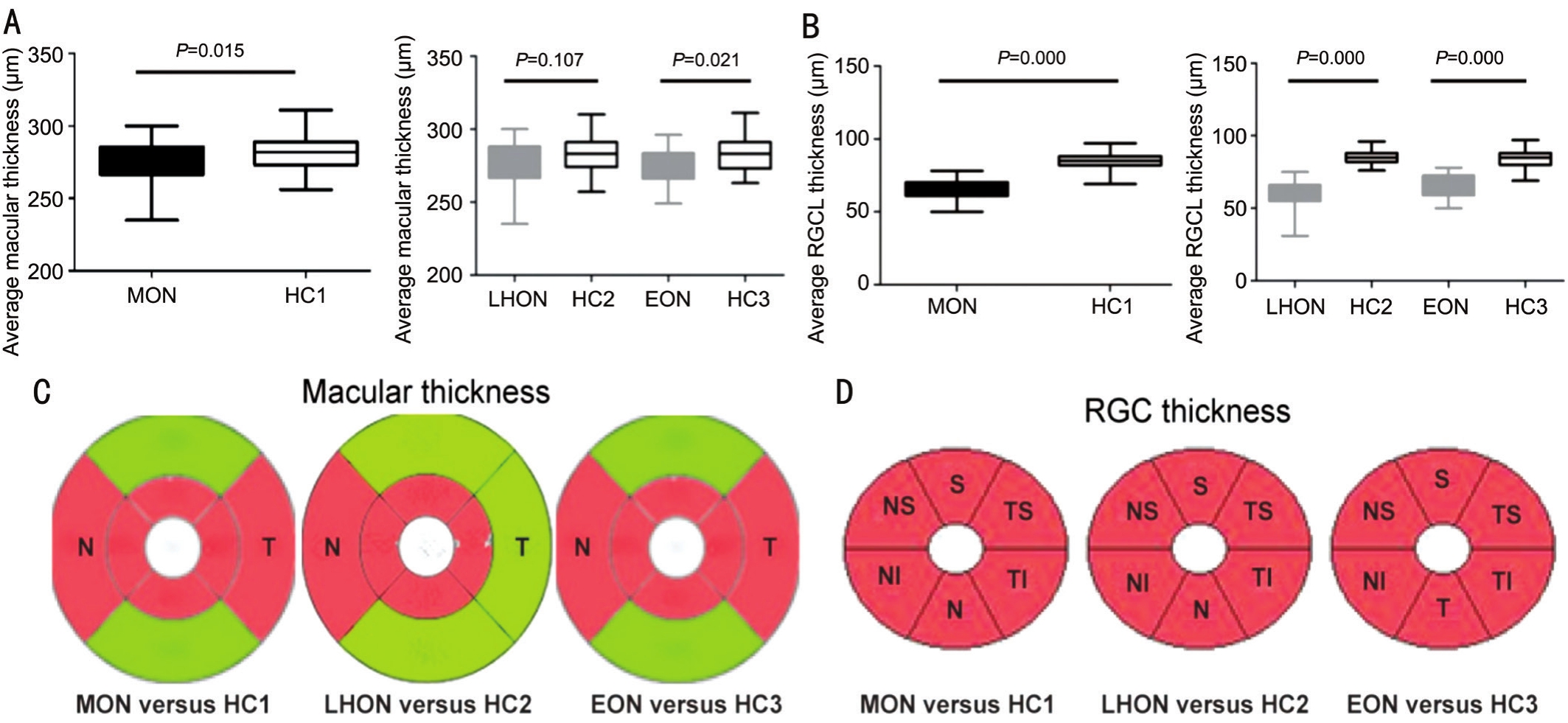
Figure 2 Total macular and retinal ganglion cell layer thickness in MON patients compared to controls.
Total Macular and Retinal Ganglion Cell Layer Thickness in MON Patients Compared to ControlsIn contrast to HCs,the average total macular thickness in MONs remarkably reduced (P=0.015), which mainly occurred in the inner circle,and nasal temporal sectors in the outer circle. Further analysis,the average total macular thickness in LHON and EON patients underwent the same impairment patterns. For RGCL thickness in MON patients, it reduced sharply in the early stages of disease duration, which was then equally distributed in each of the sectors of the macula. Detailed analysis showed that the same impairment patterns occurred in RGCL in LHON and EON patients again (Table 2 and Figure 2).
The Best Corrected Visual Acuity and Visual Field Associations with Structural Injury in MON PatientsThe BCVA and average VFs (dB) in MON patients had no associations with the pRNFL thickness and average RGCL thickness. However, both BCVA (r=0.5;P=0.0003) and VFs (r=0.54;P=0.0002) strongly correlated to average total macular thickness. Further analysis showed BCVA and VFs also had reliable correlations to average total macular thickness in EON and LHON patients (Figure 3).
In our studys, pRNFL thickness in the superior, inferior,and nasal quadrants thickened markedly with the temporal quadrant unchanged, compared with those of healthy eyes within 6mo of disease onset in LHON patients. However,for the second hour of the temporal, which is mainly formed by PMB, it is reduced by a significant amount compared to that of the healthy eyes. For the EON patients within 6mo of the disease onset, the temporal pRNFL thickness decreasedsharply with pRNFL in other quadrants unchanged. These results were consistent with the impairment pattern of DOA that pRNFL thickness gradually decreased with age and the temporal quadrant was preferentially damaged[19]. Furthermore,these outcomes in the present study confirmed the conclusion that the PMB was vulnerable to injury in patients with MON[5].Saviniet al[11]studied 38 LHON patients using across-sectional approach and demonstrated that in 8 LHON patients with a disease duration of less than 6mo, all pRNFL were thickened except for the temporal quadrant of pRNFL, showing no significant changes. In 2010, Barboniet al[20]also observed 4 LHON patients with a longitudinal approach and found the same changes of disease duration. These results were similar to those of the presented study. However, for EON patients in the early stages, there was no pRNFL compensatory swelling, as was the case in the previous study[21]. The pRNFL thickening in LHON patients may be explained by the following reasons:1) PMB has undergone a shortage of energy and the vessels around the optic disc engorge to compensate, resulting in the thickening of the pRNFL in patients with LHON; 2) the lack of energy of optic nerve caused pRNFL swelling within all quadrants although the previous PMB atrophy made it sometimes look normal. For EON, due to the sudden arrival of the causative agent, visual function has been impaired before the protection compensations could take place. Additionally,there may be a nuclear compensatory effect in patients with LHON[22]because the energy defect caused by the mutation of mtDNA results in an increase in mitochondrial mass[23]and an augment in the DNA copy number[24-25]. In the harbor circle of a cell, a mutation in mitochondrial tRNA, the overproduction of ROS can trigger the mitochondrial biogenesis, and in LHON cybrids[26], ß-estradiol can induce the increase of mitochondrial biogenesis and rescue the axons of RGCL from energy depletion[27]. The latter maybe a good explanation for the male prevalence.

Figure 3 The BCVA and VF associations with structural injury in MON patients.
In patients with MON including LHON and EON, the macular thickness in the inner circle which contains most RGCs was markedly reduced. The nasal outer circle sector of the macular thickness (containing the PMB) has a significant reduction in an early stage of MON. The impairment in MON was consistent with the results of our previous study[9].
Further analysis of RGCL demonstrated that its thickness was remarkably reduced in the early stages of diseases in MON patients, as well as LHON and EON patients, without differences among each sector of macula. It implied that whether or not the cause was edema or atrophy of pRNFL,both caused RGC loss in the macula in MON patients.
Both LHON and EON are characterized by sudden bilateral,central visual loss with structural changes. Therefore, we evaluated the average correlations of VFs and BCVA to structural alterations in MON patients. The results showed that the VFs and BCVA had no correlations to pRNFL and RGC loss whether in LHON or in EON patients, they correlated strongly to macular thickness. Their mechanisms were still unclear which may imply that other segmented macular layers also suffer damages in MON patients, except for pRNFL and RGCL.
In addition, in EON patients, 3/5 (60%) carriers with OPA1 gene mutations and 1/5 (20%) LHON carrier which indicated that ethambutol could be a trigger to attack of DOA or LHON gene in carriers. It suggests that ethambutol should be avoided for patients with a family history of DOA or LHON.
In conclusion, in the early stage of LHON, the temporal pRNFL thickness decreased, although pRNFL in other quadrants were edema. For EON, we only observed the thinning of temporal pRNFL without pRNFL edema in other quadrants.For macular thickness and RGCL thickness, there were the same impairment patterns in LHON and EON, and RGCL in early stage of MON had suffered severe damages. VFs in MON strongly correlated to the macular thickness. These results imply that OCT could be a potential tool for detecting structural alterations in the optic nerves, different type of MON may have different damage patterns. Otherwise, due to the small samples and individual differences of optic nerves and retinas in OCT imaging, a large sample and a longitudinal study would be helpful to confirm these results again in the future.
We thank the medical staff from the Department of Ophthalmology at the Chinese PLA General Hospital for their contributions in collecting patient cases.
REFERENCES
1 Maresca A, la Morgia C, Caporali L, Valentino ML, Carelli V. The optic nerve: a “mito-window” on mitochondrial neurodegeneration.Mol Cell Neurosci2013;55:62-76.
2 Wang MY, Sadun AA. Drug-related mitochondrial optic neuropathies.J Neuroophthalmol2013;33(2):172-178.
3 Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ 2nd, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy.Science1988;242(4884):1427-1430.
4 Sadun AA. Mitochondrial optic neuropathies.J Neurol Neurosurg Psychiatry2002;72(4):423-425.
5 Zhang Y, Huang H, Wei S, Qiu H, Gong Y, Li H, Dai Y, Jiang Z,Liu Z. Characterization of retinal nerve fiber layer thickness changes associated with Leber’s hereditary optic neuropathy by optical coherence tomography.Exp Ther Med2014;7(2):483-487.
6 Zhang Y, Huang H, Wei S, Gong Y, Li H, Dai Y, Zhao S, Wang Y, Yan H. Characterization of macular thickness changes in Leber’s hereditary optic neuropathy by optical coherence tomography.BMC Ophthalmol2014;14:105.
7 Cortelli P, Montagna P, Pierangeli G, Lodi R, Barboni P, Liguori R,Carelli V, Iotti S, Zaniol P, Lugaresi E, Barbiroli B. Clinical and brain bioenergetics improvement with idebenone in a patient with Leber’s hereditary optic neuropathy: a clinical and 31P-MRS study.J Neurol Sci1997;148(1):25-31.
8 Sabet-Peyman EJ, Khaderi KR, Sadun AA. Is Leber hereditary optic neuropathy treatable? Encouraging results with idebenone in both prospective and retrospective trials and an illustrative case.J Neuroophthalmol2012;32(1):54-57.
9 Heitz FD, Erb M, Anklin C, Robay D, Pernet V, Gueven N. Idebenone protects against retinal damage and loss of vision in a mouse model of Leber’s hereditary optic neuropathy.PLoS One2012;7(9):e45182.
10 Sadun AA, Chicani CF, Ross-Cisneros FN, Barboni P, Thoolen M,Shrader WD, Kubis K, Carelli V, Miller G. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy.Arch Neurol2012;69(3):331-338.
11 Savini G, Barboni P, Valentino ML, Montagna P, Cortelli P, De Negri AM, Sadun F, Bianchi S, Longanesi L, Zanini M, Carelli V.Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber’s hereditary optic neuropathy mutations.Ophthalmology2005;112(1):127-131.
12 Wojtkowski M, Bajraszewski T, Gorczyńska I, Targowski P, Kowalczyk A, Wasilewski W, Radzewicz C. Ophthalmic imaging by spectral optical coherence tomography.Am J Ophthalmol2004;138(3):412-419.
13 Wojtkowski M, Srinivasan V, Fujimoto JG, Ko T, Schuman JS,Kowalczyk A, Duker JS. Three-dimensional retinal imaging with highspeed ultrahigh-resolution optical coherence tomography.Ophthalmology2005;112(10):1734-1746.
14 Lim SA. Ethambutol-associated optic neuropathy.Ann Acad Med Singap2006;35(4):274-278.
15 Fraunfelder FW, Sadun AA, Wood T. Update on ethambutol optic neuropathy.Expert Opin Drug Saf2006;5(5):615-618.
16 Monteiro ML, Fernandes DB, Apóstolos-Pereira SL, Callegaro D.Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography.Invest Ophthalmol Vis Sci2012;53(7):3959-3966.
17 Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motiong” and “counting fingers” can be quantified with the freibury visual acuity test.Invest Ophthalmol Vis Sci2006;47(3):1236-1240.
18 Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies-disease mechanisms and therapeutic strategies.Prog Retin Eye Res2011;30(2):81-114.
19 Barboni P, Savini G, Parisi V, Carbonelli M, La Morgia C, Maresca A, Sadun F, De Negri AM, Carta A, Sadun AA, Carelli V. Retinal nerve fiber layer thickness in dominant optic atrophy measurements by optical coherence tomography and correlation with age.Ophthalmology2011;118(10):2076-2080.
20 Barboni P, Carbonelli M, Savini G, Ramos Cdo V, Carta A, Berezovsky A, Salomao SR, Carelli V, Sadun AA. Natural history of Leber’s hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography.Ophthalmology2010;117(3):623-627.
21 Zoumalan CI, Agarwal M, Sadun AA.Optical coherence tomography can measure axonal loss in patients with ethambutol-induced optic neuropathy.Graefes Arch Clin Exp Ophthalmol2005;243(5):410-416.
22 Yen MY, Lee HC, Liu JH, Wei YH. Compensatory elevation of complex II activity in Leber’s hereditary optic neuropathy.Br J Ophthalmol1996;80(1):78-81.
23 Carta A, Carelli V, D’Adda T, Ross-Cisneros FN, Sadun AA. Human extraocular muscles in mitochondrial diseases: comparing chronic progressive external ophthalmoplegia with Leber’s hereditary optic neuropathy.Br J Ophthalmol2005;89(7):825-827.
24 Yen MY, Chen CS, Wang AG, Wei YH. Increase of mitochondrial DNA in blood cells of patients with Leber’s hereditary optic neuropathy with 11778 mutation.Br J Ophthalmol2002;86(9):1027-1030.
25 Nishioka T, Soemantri A, Ishida T. mtDNA/nDNA ratio in 14484 LHON mitochondrial mutation carriers.J Hum Genet2004;49(12):701-705.26 Moreno-Loshuertos R, Ferrín G, Acín-Pérez R, Gallardo ME, Viscomi C, Pérez-Martos A, Zeviani M, Fernández-Silva P, Enríquez JA. Evolution meets disease: penetrance and functional epistasis of mitochondrial tRNA mutations.PLoS Genet2011;7(4):e1001379.
27 Giordano C, Montopoli M, Perli E, Orlandi M, Fantin M, Ross-Cisneros FN, Caparrotta L, Martinuzzi A, Ragazzi E, Ghelli A, Sadun AA,d’Amati G, Carelli V. Oestrogens ameliorate mitochondrial dysfunction in Leber’s hereditary optic neuropathy.Brain2011;134(Pt 1):220-234.
Citation:Teng D, Peng CX, Qian HY, Li L, Wang W, Wang JQ, Chen B, Zhou HF, Wei SH. Structural impairment patterns in peripapillary retinal fiber layer and retinal ganglion cell layer in mitochondrial optic neuropathies.Int J Ophthalmol2018;11(10):1643-1648
DOl:10.18240/ijo.2018.10.11
Accepted:2018-07-13
Received:2018-02-04
Correspondence to:Shi-Hui Wei. Ophthalmology Department,Chinese PLA General Hospital, Fuxing Road No.28, Haidian District, Beijing 100853, China. weishihui58@126.com
Foundation:Supported by the National High Technology Research and Development Program of China (863 Program,No.2015AA020511).