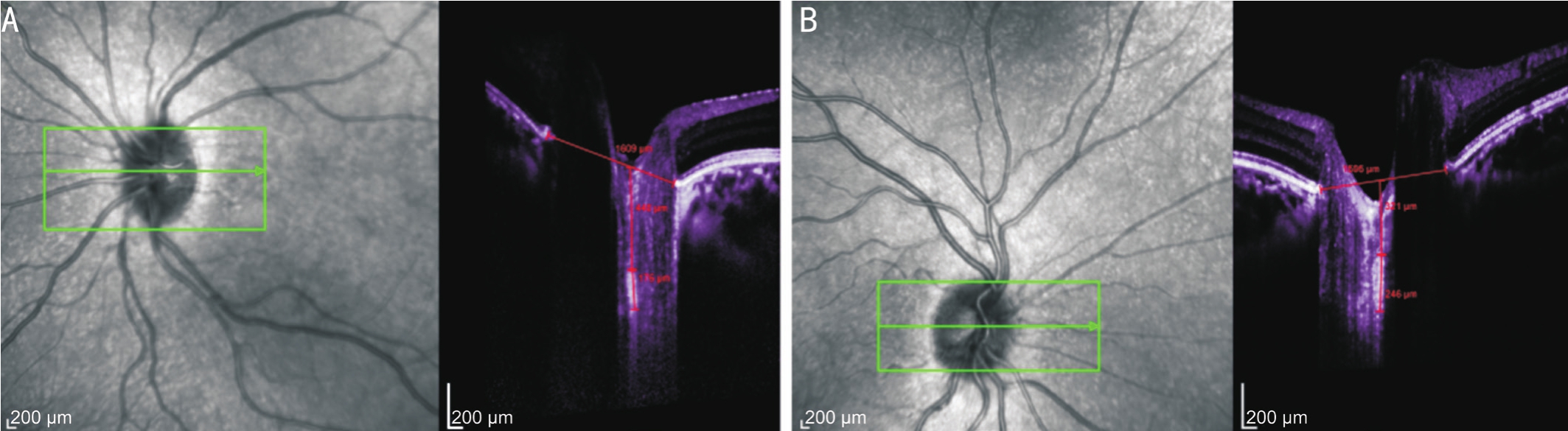
Figure 1 Images (OCT) from an amblyopic patient (9-year-old)
Horizontal scans. A: Amblyopic eye with a BCVA of 1.0 logMAR units and the LCT of 176 µm; B: Patient’s fellow eye with a BCVA of 0 logMAR units and the LCT of 246 µm.
Serkan Akkaya
Department of Ophthalmology, Kayseri Training and Research Hospital, Kayseri 38010, Turkey
Abstract
●AlM:To determine lamina cribrosa thickness (LCT) in the optic nerve head region of the eyes in children with hyperopic anisometropic amblyopia and to compare this thickness with that of fellow eyes, hyperopic nonamblyopia, and age-matched controls.
●METHODS:Thirty-two patients (12.0±1.8y, mean±standard deviation) with hyperopic anisometropic amblyopia,31 subjects with age- and refractive error- matched hyperopic non-amblyopia (10.7±2.2y), and 32 age-matched controls(11.2±2.0y) were included in this prospective, crosssectional study. LCT was measured using an enhanced depth-imaging program of a spectral domain optical coherence tomographic instrument in all participants, and the correlation between LCT and axial length was calculated.
●RESULTS:The mean LCT was 180.9±29.4 µm in amblyopic eyes, 247.7±19.0 µm in fellow eyes, 251.6±27.3 µm in hyperopic non-amblyopic eyes, and 240.2±15.8 µm in control eyes. Lamina cribrosa in amblyopic eyes was significantly thinner than fellow, hyperopic non-amblyopic, and control eyes (P<0.05). There was no significant correlation in LCT and axial length between amblyopic (P=0.16) and control(P=0.31) group.
●CONCLUSlON:Lamina cribrosa of eyes with hyperopic anisometropic amblyopia is significantly thinner than that of fellow eyes, hyperopic non-amblyopia, and age-matched controls. The LCT profile in amblyopic eyes is different from that observed in fellow, hyperopic non-amblyopic, and control eyes.
●KEYWORDS:anisometropia; amblyopia; hyperopia; lamina cribrosa; optical coherence tomography.
Amblyopia is a visual system developmental disorder that is accompanied by reductions in unilateral or bilateral visual acuity (VA) even in cases with structurally normal eyes with no pathology that are receiving optimal refractive correction[1]. The visual deficit is associated with the presence in early life of strabismus, anisometropia, or, less often, a visual axis obstruction (e.g.congenital cataract)[2-3].
Among these, the most important risk factor for amblyopia is hyperopic anisometropia[4].
Several animal and human studies have shown that visual deprivation after birth effects growth of cells in the lateral geniculate body[5-6]and visual cortex[7]; whether or not the choroid and/or retina are altered in amblyopic eyes is still being debated. Amblyopic eyes can be examined with optical coherence tomography (OCT), which determines the retinal morphology of the human eye and confirm whether or not the patient has a structurally normal retina. Recently, there have been several reports regarding the thickness of the retinal nerve fiber layer[8-9], macular volume[10], thickness of the retina[11-13],and choroidal thickness[14-15]in eyes with amblyopia. However,in human children, the relationship between lamina cribrosa thickness (LCT) and amblyopia has not yet to be determined.
The present study aimed to evaluate the LCT and lamina cribrosa depth (LCD) of eyes with hyperopic anisometropic amblyopia and to determine the differences between these parameters with fellow eyes, eyes with hyperopic nonamblyopia, and eyes of age-matched controls.
PatientsThis cross-sectional, comparative, non-interventional study conducted at the Kayseri Training and Research Hospital between July 2016 and February 2017. The study’s protocol was written in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of University of Erciyes, Turkey. All participants (or parents/legal guardians, as applicable) consented for original measurements and review of their medical records.
Subjects were diagnosed as being amblyopic in cases where the best-corrected visual acuity (BCVA) of one eye was ≤20/30 or was worse than the fellow eye by at least two Snellen VA lines. A difference of ≥2.00 diopters (D) in both eyes was classified as anisometropia.

Figure 1 Images (OCT) from an amblyopic patient (9-year-old)
Horizontal scans. A: Amblyopic eye with a BCVA of 1.0 logMAR units and the LCT of 176 µm; B: Patient’s fellow eye with a BCVA of 0 logMAR units and the LCT of 246 µm.
In total, 32 eyes with hyperopic anisometropic amblyopia and their fellow eyes and 31 hyperopic non-amblyopic eyes were included the study. The mean patient age was 12.0±1.8y(range: 9-14y). All participants underwent dilated funduscopic examinations. Patients were not included in the study who had a history of undergoing an intraocular surgery, strabismus,laser treatment, organic eye diseases, cataract, glaucoma, or any other retinal disorders. I also had to exclude any patient who could not cooperate for an OCT examination.
Thirty two right eyes of 32 control subjects (11.2±2.0y) who had VA that was either normal or corrected-to-normal[0 logarithm of the minimal angle of resolution (logMAR)units or better] in both eyes were included the study.
These age-matched controls were all between 8 and 13 years old and were visiting Kayseri Training and Research Hospital for regular visual screening. All of the controls had good VA with a spherical equivalent (SE) ranging from -0.50 square diopters (DS) to 0.87 DS and did not have any retinal diseases.The parents of the controls also provided informed consent after being told about the study and its procedures.
Ophthalmic ExaminationEach patient was given 3 drops of 1% cyclopentolate (Cyclogyl; Alcon Couvreur, Purrs, Belgium)at 5min intervals, and underwent cycloplegic refraction 45min later. I measured refraction using a TonorefII autorefractor/tonometer (Nidek Co., Ltd., Japan). Every study participant underwent 5 consecutive autorefractor readings, and each of these had to be within 0.25 D of one other. The following formula was used to calculate SE: sum of the spherical + 1/2 of the cylindrical error.
All of the patients also underwent other clinical examinations including BCVA, refractive error (SE), slit lamp examination,extraocular movements, ophthalmoscopic examination, intraocular pressure, central corneal thickness (viaa Scheimpflug camera(Pentacam HR; Oculus GmbH, Wetzlar, Germany), and the eye’s axial lengthviathe IOL Master (Carl Zeiss Meditec, Dublin,California, USA). I used a standard Snellen chart to measure the VA of each patient, and VA decimal values were converted to logMAR units in order to undergo statistical analysis.
Optical Coherence TomographyA Heidelberg Spectralis spectral domain OCT (SD-OCT; Heidelberg Engineering,Heidelberg, Germany) with an enhanced depth imaging program determined the LCT of each patient from their images following pupillary dilation. I excluded all scans having a quality score <20 as well as those of inadequate quality (e.g.unclear fundus images, unclear LCT border).
Enhanced Depth Imaging SD-OCT of the Optic Nerve HeadI used a previously described method for SD-OCT(EDI-OCT) enhanced depth imaging of optic nerve head(ONH)[16]. In general, the instrument was set to image a 15°×10°rectangle centered on the optic disk. This rectangle was divided into approximately 65 sections, each of which had 100 OCT frames on average. From these horizontal B scans, three frames(center, mid-superior, and mid-inferior) that passed through the ONH were selected, and parameters were measured in each of these frames. The center of the lamina cribrosa plate was utilized to determine thickness during the measurements.The borders of the lamina cribrosa and the Bruch’s membrane opening (BMO) are shown in an ONH OCT image in Figure 1.The BMO was defined as the line connecting both ends of Bruch’s membrane. Every distance was determined on a line at a right angle to the reference line, and all parameters were measured as close to the perpendicular ONH center as possible.In cases where the measurements could not be made due to a vessel trunk, measurements were recorded at the temporal side.Lamina cribrosa borders were defined as the posterior and anterior borders of the highly reflective area at the ONH’s perpendicular center in the horizontal SD-OCT cross-section.The thickness of the lamina cribrosa was defined as the distance between these two borders. I used image adjustments(e.g.contrast) in order to provide the clearest images of the lamina cribrosa. The distance between the BMO and the anterior border of the lamina cribrosa was defined as LCD.
HEYEX software 6.0 (Heidelberg Engineering Inc., Heidelberg,Germany) was used to obtain all measurements. Two examiners independently analyzed every image in this study. Both examiners independently determined both LCT and LCDtwice. Thus, each LCT and LCD value was obtained four times. The mean of these four values was used in the primary analysis. Before the primary analysis, intraexaminer, intraclass correlation coefficients were calculated using 15 randomly selected images to test the reproducibility of the LCT and LCD measurements.
Table 1 Demographic and ocular characteristics of patients, hyperopic non-amblyopic eyes, and controls
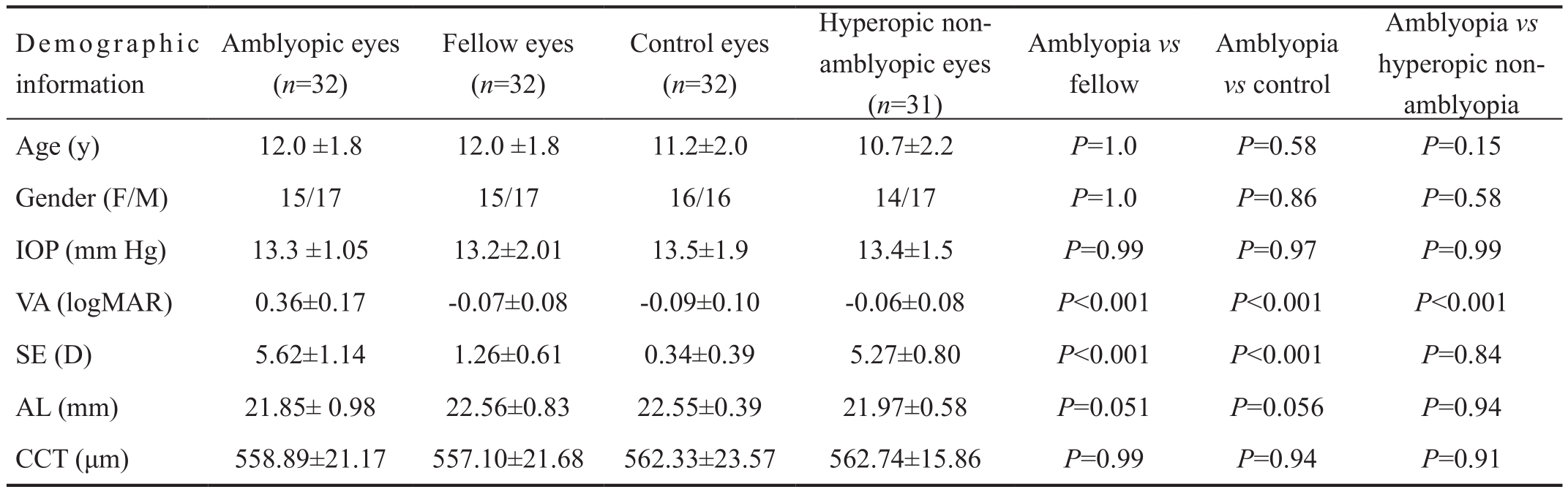
IOP: Intraocular pressure; logMAR: Logarithm of the minimal angle of resolution; AL: Axial length; CCT: Central corneal thickness.
Statistical AnalysesAll of the statistical analyses in this study were executedviastatistical software (SPSS version 21.0; SPSS, Inc., Chicago, IL, USA). Pretest statistical power was 90%. Descriptive statistics for continuous variables were calculated as means±SD. SE was determined as follows:spherical power + 1/2 of the minus cylinder power. I used logMAR VA (converted from Snellen VA) for statistical analysis. Distributions of SE, VA of the amblyopic eye, VA of the fellow eye, axial length, LCT, and LCD were confirmed as normally distributed. One-way analysis of variance (ANOVA)was used to determine the significance of differences in values among amblyopic eyes, fellow eyes, hyperopic non-amblyopic eyes, and control eyes. LCT in the amblyopic eyes was compared with that in the fellow, hyperopic non-amblyopic eyes, and control eyes by using Tukey tests. Pearson’s correlation coefficient was used to determine the significance of correlations among LCT and axial length, age, and SE.Significance was set at aPvalue of less than 0.05.
Table 1 presents the demographic and ocular characteristics of patients, hyperopic non-amblyopia, and controls. No significant differences were observed for age, sex, and intraocular pressure between groups (P>0.05). The mean BCVA was 0.36±0.17 logMAR units in amblyopic eyes, -0.07±0.08 logMAR units in fellow eyes, -0.06±0.08 logMAR units in hyperopic non-amblyopic eyes, and -0.09±0.10 logMAR units in control eyes. The amblyopic eyes had significantly worse mean BCVA than fellow eyes, hyperopic non-amblyopic eyes, and control eyes (allP<0.001).
The mean SE was 5.62±1.14 (range, 4.12-7.50) D in amblyopic eyes, 1.26±0.61 (range, 0.50-3.12) D in fellow eyes, 5.27±0.80(range, 4.00-6.87) D in hyperopic non-amblyopic eyes, and 0.34±0.39 (range, -0.50-0.87) D in control eyes (P<0.001,ANOVA). There was no significant difference in SE value between amblyopic and hyperopic non-amblyopic eyes(P=0.84) however, there was significantly more hyperopia in amblyopic and hyperopic non-amblyopic eyes than fellow eyes (P<0.001) and control eyes (P<0.001). Fellow eyes had significantly more hyperopia than control eyes (P<0.001).
The mean axial lengths were as follows: 21.85±0.98 mm in amblyopic eyes, 22.56±0.83 mm in fellow eyes, 21.97±0.58 mm in hyperopic non-amblyopic eyes, and 22.55±0.39 mm in agematched control eyes. There was no significant difference in this length among all groups (P=0.051, ANOVA).
The mean central corneal thickness was 558.89±21.17 µm in amblyopic eyes, 557.10±21.68 µm in fellow eyes, 562.74±15.86 mm in hyperopic non-amblyopic eyes, and 562.33±23.57 µm in age-matched control eyes. There was no significant difference in central corneal thickness among all groups(P=0.74, ANOVA).
The intra-examiner, intraclass correlation coefficient values[95% confidence interval (CI)] were as follows: LCT; 0.895(0.751-0.969) and LCD; 0.974 (0.916-0.992). The interexaminer, intraclass correlation coefficient values were as follows: LCT 0.849 (0.761-0.979) and LCD 0.943 (0.816-0.972).LCT and LCD of patients, hyperopic non-amblyopia, and controls are shown in Table 2.
The mean LCT was 180.9±29.4 μm in amblyopic eyes,247.7±19.0 μm in fellow eyes, 251.6±27.3 μm in hyperopic non-amblyopic eyes, and 240.2±15.8 μm in age-matched control eyes. The lamina cribrosa was significantly thinner in amblyopic eyes than in fellow, hyperopic non-amblyopic,and age-matched control eyes [P<0.001, ANOVA;P<0.001(95%CI: 86.8-46.7),P<0.001 (95%CI: 89.2-52.1),P<0.001(95%CI: 78.2-40.2), respectively, Tukey test].
The mean LCD was 371.7±85.8 μm in amblyopic eyes,272.1±51.6 μm in fellow eyes, 397.4±75.7 μm in hyperopic non-amblyopic eyes, and 391.1±87.7 μm in control eyes. Thelamina cribrosa was located significantly posteriorly in the amblyopic eyes than in the fellow eyes but not the hyperopic non-amblyopic eyes, and the control eyes (P<0.001, ANOVA;P<0.001,P=0.68,P=0.84, respectively, Tukey test).
Table 2 LCT and LCD of amblyopic, fellow, hyperopic non-amblyopic, and control eyes

LCD: Lamina cribrosa depth; LCT: Lamina cribrosa thickness.
A significant negative correlation was noted between LCT and axial length in fellow eyes (r=-0.50,P=0.02) but not in amblyopic, hyperopic non-amblyopic, and control eyes(r=-0.33,P=0.16;r=0.18,P=0.36 andr=0.21,P=0.31,respectively; Pearson’s correlation coefficient).
No significant correlations were observed among LCT, age,and SE in amblyopic, fellow, hyperopic non-amblyopic, and control eyes (P>0.05; Pearson’s correlation coefficient).
However, a significant positive correlation was noted between LCD and age in amblyopic eyes (r=0.51,P=0.02) but not in fellow, hyperopic non-amblyopic, and control eyes (r=0.25,P=0.29;r=0.20,P=0.31 andr=0.37,P=0.07, respectively;Pearson’s correlation coefficient).
Moreover, a significant negative correlation was observed between LCD and axial length in control eyes (r=-0.28,P=0.04) but not in amblyopic, hyperopic non-amblyopic, and fellow eyes (r=-0.20,P=0.39;r=0.05,P=0.77 andr=0.26,P=0.28, respectively).
Furthermore, no significant correlations were noted between LCD and SE among amblyopic, fellow, hyperopic nonamblyopic, and control eyes (P>0.05; Pearson’s correlation coefficient).
Differences in Lamina Cribrosa Thickness of Amblyopic EyesAnalysis of this study shows that the lamina cribrosa was significantly thinner in amblyopic eyes than in fellow,hyperopic non-amblyopic, and age-matched control eyes.Numerous studies have examined the topography of amblyopic eyes, but these have examined only retinal and choroidal topography and not laminar topography. To the best of my knowledge, this study is the first to evaluate LCT of amblyopic eyes and compare the same with fellow, hyperopic nonamblyopic, and age-matched control eyes.
According to a previous study, in monkeys with advanced glaucomatous optic nerve damage, the lamina cribrosa was significantly thinner than in those with non-glaucomatous vascular optic nerve damage[17]. Similarly, human studies have revealed that eyes with secondary angle closure glaucoma and advanced glaucomatous optic nerve damage have thinner lamina cribrosa than normal eyes[18-20].
The present study revealed that the lamina cribrosa of amblyopic eyes was significantly thinner than that of fellow,hyperopic non-amblyopic, and age-matched control eyes; this implies that amblyopic eyes may have an altered optic nerve,although a structurally normal appearance.
Histological studies performed in amblyopic animals have reported atrophy in ganglion cells, optic nerve fibers, and thinning of the inner plexiform layer[21-22].
In a similar study, amacrine synapses were significantly increased in the inner plexiform layer of unilaterally visually deprived rats[23]. In addition, atrophy of ganglion cells was demonstrated in the lateral geniculate nucleus of animals with unilateral lid suture[24]. Histological studies have revealed changes in areas ranging from retina to visceral cortex in amblyopic animals[6,21-24], suggesting that lamina cribrosa may be affected as well.
Guoet al[25]aimed to demonstrate retinal and choroidal microstructural abnormalities in amblyopic eyes by using digital subtraction autofluorescence and split-spectrum amplitude-decorrelation angiography. In this study, choroidal thickness was increased in amblyopic eyes, but choroidal capillaries revealed atrophy.
The lamina cribrosa receives its primary blood supply from the choroidal circulation[26]. Despite there is no study about choroidal circulation and LCT in the ophthalmology literature,as an inference of anatomical blood supply, choroidal circulation might play a role in LCT. It can be suggested that choroidal circulation might be a provocative factor in pathogenesis thinner lamina cribrosa in hyperopic amblyopic eyes. Either blood flow increase or decrease might affect LCT.The relation between choroidal blood flow changes and LCT may be an interesting subject of a study.
The factors LCD and LCT attracted the attention of glaucoma experts, given the known significant role of the lamina cribrosa in the development of optic neuropathy. My study assessed IOP in all subjects and its effect was eliminated. In this study, I also evaluated hyperopic non-amblyopic eyes and compare them with amblyopic eyes. The analysis demonstrated that LCT was decreased in amblyopic eyes independent of hypermetropia.
The present study has certain limitations. I studied only anisometropic amblyopic patients and did not evaluate LCT in other types of amblyopia. Moreover, the study sample wasrelatively small. Furthermore, LCT was determined manually as there is a lack of accessible automated software.
Therefore, additional studies including larger samples and the ability to conduct deeper tissue imaging are warranted to confirm my findings. In conclusion, the lamina cribrosa was thinner in amblyopic eyes than in normal fellow, hyperopic non-amblyopic, and age-matched control eyes. The LCT profile in amblyopic eyes was different from that observed in fellow, hyperopic non-amblyopic, and control eyes.
Conflicts of Interest:Akkaya S, None.
REFERENCES
1 Holmes JM, Clarke MP. Amblyopia.Lancet2006;367(9519):1343-1351.
2 Helveston EM, Saunders RA, Ellis FD. Unilateral cataracts in children.Ophthalmic Surg1980;11(2):102-108.
3 Weisberg OL, Sprunger DT, Plager DA, Neely DE, Sondhi N. Strabismus in pediatric pseudophakia.Ophthalmology2005;112(9):1625-1628.
4 von Noorden GK, Crawford ML,Levacy RA. The lateral geniculate nucleus in human anisometropic amblyopia.Invest Ophthalmol Vis Sci1983;24(6):788-790.
5 Headon MP, Powell TP. Cellular changes in the lateral geniculate nucleus of infant monkeys after suture of the eyelids.J Anat1973;116(1):135-145.
6 von Noorden GK. Histological studies of the visual system in monkeys with experimental amblyopia.Invest Ophthalmol Vis Sci1973;12(10):727-738.
7 Crawford ML, Von Noorden GK. Optically induced concomitant strabismus in monkeys.Invest Ophthalmol Vis Sci1980;19(9):1105-1109.
8 Singh N, Rohatgi J, Gupta VP, Kumar V. Measurement of peripapillary retinal nerve fiber layer thickness and macular thickness in anisometropia using spectral domain optical coherence tomography: a prospective study.Clin Ophthalmol2017;11:429-434.
9 Yassin SA, Al-Tamimi ER, Al-Hassan S. Macular and retinal nerve fiber thickness in recovered and persistent amblyopia.Int Ophthalmol2015;35(6):833-842.
10 Kasem MA, Badawi AE. Changes in macular parameters in different types of amblyopia: optical coherence tomography study.Clin Ophthalmol2017;11:1407-1416.
11 Araki S, Miki A, Goto K, Yamashita T, Takizawa G, Haruishi K, Ieki Y,Kiryu J, Yaoeda K. Macular retinal and choroidal thickness in unilateral amblyopia using swept-source optical coherence tomography.BMC Ophthalmol2017;17(1):167.
12 Bruce A, Pacey IE, Bradbury JA, Scally AJ, Barrett BT. Bilateral changes in foveal structure in individuals with amblyopia.Ophthalmology2013;120(2):395-403.
13 Chen W, Xu J, Zhou J, Gu Z, Huang S, Li H, Qin Z, Yu X. Thickness of retinal layers in the foveas of children with anisometropic amblyopia.PLoS One2017;12(3):e0174537.
14 Nishi T, Ueda T, Hasegawa T, Miyata K, Ogata N. Choroidal thickness in children with hyperopic anisometropic amblyopia.Br J Ophthalmol2014;98(2):228-232.
15 Xu J, Zheng J, Yu S, Sun Z, Zheng W, Qu P, Chen Y, Chen W, Yu X. Macular choroidal thickness in unilateral amblyopic children.Invest Ophthalmol Vis Sci2014;55(11):7361-7368.
16 Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography.Am J Ophthalmol2008;146(4):496-500.
17 Jonas JB, Hayreh SS, Yong T. Thickness of the lamina cribrosa and peripapillary sclera in Rhesus monkeys with nonglaucomatous or glaucomatous optic neuropathy.Acta Ophthalmol2011;89(5):e423-e427.
18 Jonas JB, Königsreuther KA, Naumann GO. Optic disk histomorphometry in normal eyes and eyes with secondary angle-closure glaucoma.Graefes Arch Clin Exp Ophthalmol1992;230(2):134-139.
19 Jonas JB, Berenshtein E, Holbach L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space.Invest Ophthalmol Vis Sci2003;44(12):5189-5195.
20 Ren R, Wang N, Li B, Li L, Gao F, Xu X, Jonas JB. Lamina cribrosa and peripapillary sclera histomorphometry in normal and advanced glaucomatous Chinese eyes with various axial length.Invest Ophthalmol Vis Sci2009;50(5):2175-2184.
21 Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cat's lateral geniculate body.J Neurophysiol1963;26:978-993.
22 Goodman L. Effect of total absence of function on the optic system of rabbits.Am J Physiol1932;100(1):46-63.
23 Sosula L, Glow PH. Increase in number of synapses in the inner plexiform layer of light deprived rat retinae: quantitative electron microscopy.J Comp Neurol1971;141(4):427-451.
24 Goldby F. A note on transneuronal atrophy in the human lateral geniculate body.J Neurol Neurosurg Psychiatry1957;20(3):202-207.
25 Guo L, Tao J, Xia F, Yang Z, Ma X, Hua R. In vivo optical imaging of amblyopia: digital subtraction autofluorescence and split-spectrum amplitude-decorrelation angiography.Lasers Surg Med2016;48(7):660-667.
26 Sugiyama K, Gu ZB, Kawase C, Yamamoto T, Kitazawa Y. Optic nerve and peripapillary choroidal microvasculature of the rat eye.Invest Ophthalmol Vis Sci1999;40(13):3084-3090.
Citation:Akkaya S. Lamina cribrosa thickness in children with hyperopic anisometropic amblyopia.Int J Ophthalmol2018;11(10):1663-1667
DOl:10.18240/ijo.2018.10.14
Accepted:2018-08-07
Received:2018-03-09
Correspondence to:Serkan Akkaya. Department of Ophthalmology, Kayseri Training and Research Hospital, Kayseri 38010, Turkey. drsakkaya@gmail.com