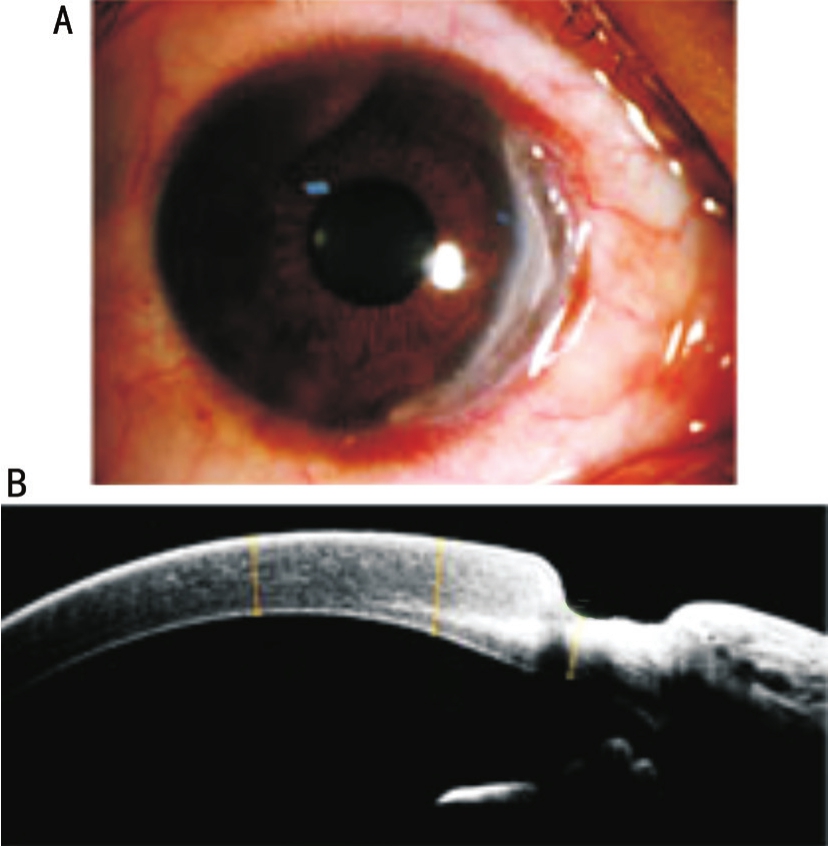
Figure 1 Ocular surface of patients with Mooren’s ulcer A: The representative slit lamp photography; B: The representative RTvue OCT image.
·Basic Research·
Lin Li1,2, Yan-Ling Dong2, Ting Liu2, Dan Luo2, Chao Wei2, Wei-Yun Shi2
1School of Medicine and Life Sciences, University of Jinan-Shandong Academy of Medical Sciences, Jinan 250022,Shandong Province, China
2State Key Laboratory Cultivation Base, Shandong Provincial Key Laboratory of Ophthalmology, Shandong Eye Institute,Shandong Academy of Medical Sciences, Qingdao 266071,Shandong Province, China
Abstract
● AlM:To investigate the expression of succinate receptor GPR91 and its pathogenic roles in Mooren’s ulcer (MU).
● METHODS:Biopsy specimens were obtained from 7 patients with MU and 6 healthy donors.The expression of GPR91 in MU tissues was evaluated using quantitative realtime reverse transcription polymerase chain reaction (qRTPCR) and immunohistochemistry (lHC).Succinate was used to activate GPR91 signaling, and the effect of GPR91 on the expression of interleukin-1β (lL-1β), NLRP3, vascular endothelial growth factor (VEGF) and matrix metalloproteinase-13(MMP-13) in human peripheral blood mononuclear cells(PBMCs) was determined.The influence of GPR91 on the nuclear factor-κB (NF-κB) signaling in PBMCs was investigated by detecting the phosphorylation of p65.Moreover, the expression of lL-1β, VEGF, MMP-13 and phosphorylated p65 (p-p65) in the tissues of MU was examined by qRT-PCR or lHC.
● RESULTS:GPR91 mRNA expression showed a higher level in the MU group than in the healthy control group.lHC analysis also revealed that the expression of GPR91 was elevated in patients with MU compared with healthy controls.Moreover, ligation of GPR91 with succinate promoted the lipopolysaccharide-induced production of NLRP3, lL-1β, VEGF and MMP-13 in PBMCs through increased phosphorylation of p65.Pharmacological inhibition of the NF-κB signaling reversed GPR91 induced production of NLRP3, lL-1β, VEGF and MMP-13.These findings, coupled with the elevated amounts of lL-1β,VEGF, MMP-13 and p-p65 observed in the MU biopsies,constituted a rational basis for the involvement of GPR91 in the pathogenesis of MU.
● CONCLUSlON:This study indicates the increased succinate receptor GPR91 in conjunctival or corneal tissues is involved in the pathogenesis of MU through elevated NF-κB activity, which may provide a new therapeutic target for MU.
● KEYWORDS:succinate receptor; Mooren’s ulcer; nuclear factor-κB; pathogenesis
M ooren’s ulcer (MU) is a chronic, progressive, and painful peripheral ulcerative keratitis, which occurs without any diagnosable systemic disorders or scleritis[1].A typical MU lesion is characterized by the undermined ulcer edge, inflamed adjacent conjunctiva, and stromal melting.Although its underlying etiology remains largely unknown,it is widely accepted that MU is an idiopathic autoimmune disease[2].Accumulative evidence suggests that both cellmediated immunity and humoral immunity are implicated in the pathogenesis of MU[3-4].Histopathologically, aberrant inflammation was observed in the cornea and conjunctiva adjacent to MU, with more infammatory infltration and elevated pro-inflammatory cytokines[5-8].Circulating autoantibodies were also detectable in the cornea and conjunctiva of patients with MU[4,9].Moreover, steroid and/or immunosuppressive therapy was found to be effective for patients with MU[10-11].Importantly, anti-tumor necrosis factor alpha (TNFα) or CD20 monoclonal antibody was proven effective in the management of MU[12-14].However, the pathogenesis of this disease in details needs to be investigated.
Succinate receptor GPR91 is a G protein coupled receptor that functions as a cell-surface receptor of extracellular succinate[15].It is shown to be highly expressed in liver, spleen, intestine, and by dendritic cells[15].As an intermediate of tricarboxylic acid cycle, succinate is released and accumulated under hypoxia and oxidative stress[16].The raised succinate through its receptor GPR91 is involved in the pathogenesis of several physiological and pathological conditions, including obesity[17-18], rheumatoid arthritis[19], renin-induced hypertension[20-21], ischemia/reperfusion injury[20]and infammation[20,22-25], as well as diabetes-induced bone damage[26].Furthermore, GPR91, which plays a key role in retinal angiogenesis, is also expressed in retinal ganglion cells (RGCs) and retinal pigment epithelial cells,and implicated in the pathogenesis of proliferative ischemic retinopathy and diabetic retinopathy[16].
However, the expression profle of GPR91 in corneal/conjunctival tissues is largely unknown, and whether GPR91 is involved in the pathogenesis of MU also remains unclear.Therefore,in the current study, we examined the expression profile of GPR91 in corneal/conjunctival tissues with MU, and further investigated the possible mechanisms of GPR91 signaling in the pathogenesis of MU using human peripheral blood mononuclear cells (PBMCs).
Subjects and Tissue Samples Seven eyes of 7 patients (Table 1),who were diagnosed with MU at Qingdao Eye Hospital,Shandong Eye Institute between October 2015 and September 2017, were included.The diagnosis was based on the medical history, lesion morphology, and laboratory testing.Patients with collagen-vascular and infectious disease were excluded.Representative microscopic images of the ocular surface in these patients are shown in Figure 1.These patients were considered to be in need of surgical treatment.Samples from diseased tissue (cornea and/or conjunctiva) were collected after surgical excision.Necrotic corneal stroma and limbal conjunctival tissues adjacent to the ulcerative lesions were used in this study.The corneoscleral rings of healthy donor corneal grafts, of which the central parts had been used for penetrating keratoplasty, were used as controls.This study was approved by the Review Board of Shandong Eye Institute and strictly adhered to the guidelines of the Declaration of Helsinki.Informed consent was obtained from all subjects.
ImmunohistochemistryTo obtain paraffin sections, the resected MU biopsy specimens were immersed in 4%paraformaldehyde at 4℃ before embedded in paraffn wax.Fourmicrometer-thick sections were deparaffinized, and antigen was retrieved using a steamer in an epitope retrieval solution(Maixin Co., Fuzhou, China).Anti-GPR91 (abs123380, Absin),anti-matrix metalloproteinase-13 (MMP-13; AP13706c,Abgent), anti-vascular endothelial growth factor A (VEGF-A;A5708, Abclonal), anti-phosphorylated p65 (p-p65; ab86299,Abcam) and anti-interleukin-1β (IL-1β; ab9722, Abcam) were used as primary antibodies and subsequently reacted with secondary antibodies for immunofuorescent staining.

Figure 1 Ocular surface of patients with Mooren’s ulcer A: The representative slit lamp photography; B: The representative RTvue OCT image.
Table 1 Demographics of patients with MU
Isolation of Human Peripheral Blood Mononuclear Cells andin vitroStimulationHuman PBMCs were isolated from citrate-anticoagulated bloodviathe Ficoll-Paque Plus(Tianjin Haoyang Biological Manufacture Co., Tianjin, China)density gradient centrifugation method.The blood obtained from donors was layered with Ficoll-Hypaque according to the manufacturer’s instructions, and centrifuged at 500 g for 30min at room temperature.After collected from the interphase and washed 3 times with phosphate-buffered saline, PBMCs(2.5×105cells in 500 μL) were grown in the RPMI-1640 medium(HyClone) supplemented with 5% fetal bovine serum (Gibco),100 μg/mL penicillin (Sigma) and 100 μg/mL streptomycin(Sigma) in a 24-well plate (Corning) at 37℃ in a humidifed atmosphere containing 5% CO2overnight.
Isolated PBMCs were divided into 4 groups: control group,succinic acid-treated group (Succinate, 2000 μmol/L),lipopolysaccharide-stimulated group (LPS, 200 ng/mL) and succinate combined with LPS group (Succinate, 2000 μmol/L;LPS, 200 ng/mL).After stimulation, the cells and supernatants were respectively stored at -80℃ until used.
Quantitative Real-time Polymerase Chain ReactionTotal RNA was extracted from human biopsy specimens and PBMCs using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and was reverse-transcribed using the reverse transcriptase (Toyobo,Osaka, Japan).Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) analysis was performed on a Rotor-GeneQ (Qiagen, Duesseldorf, Germany) usingSYBR G REEN mix (Toyobo).The results were assayed by the comparative threshold method (2-△△Ct).The primers used are listed in Table 2.
Table 2 Nucleotide sequences for RT-PCR

RT-PCR: Reverse transcription polymerase chain reaction; GAPDH: Glyceraldehyde 3 phosphate dehydrogenase; IL-1β:Interleukin-1β; NLRP3: Nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family of protein 3;MMP-13: Matrix metalloproteinase-13; VEGF-A: Vascular endothelial growth factor A; GPR91: G protein coupled receptor 91.
Western Blot AnalysisThe protein lysates were prepared from PBMCs using the Radio-Immunoprecipitation Assay buffer(P0013B, Beyotime) with a protease inhibitor cocktail (Millipore).The protein extracts were subjected to electrophoresis on 10%polyacrylamide gel and then transferred onto polyvinylidene fluoride membranes.The membranes were probed with the following primary antibodies: anti-glyceraldehyde 3 phosphate dehydrogenase (GAPDH; KC-5G5, Kangchen), anti-MMP-13(AP13706c, Abgent), anti-IL-1β (ab9722, Abcam), and anti-p65(ab16502, Abcam), anti-p-p65 (ab86299, Abcam), anti-VEGF-A (A5708, ABclonal), and anti-NLRP3 (AG-20B-0014-c100, AdipoGen).Horseradish peroxidase-conjugated specifc antibodies were used as secondary antibodies (Pierce,1:2000).
Enzyme-linked Immunosorbent AssayThe production of IL-1β in the cell-free supernatants of PBMCs was measured using the enzyme-linked immunosorbent assay (ELISA) kits(Proteintech, Rosemont, IL, USA) according to the manufacturer’s instructions.
Statistical AnalysisGraphPad Prism 5.0 (GraphPad Software)was used for statistical analysis.The Student’st-test (comparison between two groups) and one-way analysis of variance(ANOVA, comparison among more than two groups) were performed to assess the difference in the data of PCR,Western blot and ELISA.P<0.05 was considered statistically signifcant.Data are presented as mean±standard deviation.
Increased Expression of GPR91 in MU SamplesUsing immunohistochemistry (IHC), GPR91 protein levels were found to be much higher both in the cornea and conjunctiva of patients with MU than those in healthy control subjects (Figure 2A).qRT-PCR displayed elevated GPR91 transcriptional levels in the MU samples compared with samples from healthy controls (Figure 2B).Moreover, the IHC staining also showed that the increased GPR91 was mainly expressed in infltrated infammatory cells (Figure 2A).These data clearly demonstrated the upregulated amounts of GPR91 in human eyes with MU.
Ligation of GPR91 with Succinate Augmented LPS-induced Production of IL-1β, NLRP3, VEGF-A and MMP-13Succinate is a pro-inflammatory metabolite that activates GPR91 signaling and favors the expression of proinfammatory genes in macrophages and dendritic cells[19-20,22].However, whether GPR91 signaling is involved in initiating or exacerbating inflammatory responses in MU remains unclear.We observed an increased expression of GPR91 in the infiltrated inflammatory cells.Therefore, we used PBMCs as a model to examine the pro-infammatory effect of GPR91 signaling on MU.As shown in Figure 3A, activation of PBMCs with LPS induced 7- to 8-fold increase of IL-1β mRNA, 3-fold increase of NLRP3 mRNA, 2-fold increase of MMP-13 mRNA, and 2-fold increase of VEGF-A mRNA,while succinate pronouncedly boosted the transcriptional expression of IL-1β, NLRP3, MMP-13 and VEGF-A induced by LPS, which indicated the pro-inflammatory role of GPR91 signaling.We also performed the immunoblotting,and found that ligation of GPR91 with succinate augmented the expression of IL-1β, NLRP3, MMP-13 and VEGF-A induced by LPS, which was consistent with the results from qRT-PCR (Figure 3B).Moreover, ligation GPR91 with succinate contributed to the elevated concentrations of IL-1β in supernatants of PBMCs stimulated with LPS, which further confirmed the pro-inflammatory effect of GPR91 signaling pathway (Figure 3C).Collectively, the GPR91 signaling pathway may be involved in the pathogenesis of MU through its pro-infammatory effect.

Figure 2 Increased expression of succinate receptor GPR91 in the biopsies of MU patients A: IHC staining showed the elevated expression of GPR91 in the cornea and conjunctiva (magnification ×400); B: Quantitative analysis of transcriptional expression of GPR91 showed signifcantly elevated expression in the MU samples (aP<0.05vscontrol,n=3).

Figure 3 Succinate promoted the expression of inflammatory cytokines and MMP-13 through GPR91 in PBMCs A: Quantitative analysis of mRNA expression of IL-1β, NLRP3, VEGF-A and MMP-13 in PBMCs treated with LPS, succinate and JSH-23 for 12h; B: Western blot analysis of IL-1β, NLRP3, VEGF-A and MMP-13 in lysates of PBMCs treated with LPS, succinate and JSH-23 for 12h; C: The concentrations of IL-1β in supernatants of PBMCs for 12h.aP<0.05;bP<0.01.
GPR91 signaling, Depending on NF-κB Activity, Triggered the Production of IL-1β, NLRP3, MMP-13 and VEGF-ANuclear factor-κB (NF-κB) is a master transcriptional factor to regulate the inflammatory responses[27].Therefore, we subsequently investigated whether the pro-inflammatory effect of GPR91 signaling depended on NF-κB activity.We found that the transcriptional levels of IL-1β, NLRP3,MMP-13 and VEGF-A in PBMCs stimulated with LPS and succinate were significantly decreased after treatment with JSH-23 (an inhibitor of NF-κB; Figure 3A).The results from Western blot also indicated that co-treatment with JSH-23 could pronouncedly downregulated the expression of IL-1β,NLRP3, MMP-13 and VEGF-A in PBMCs stimulated with LPS and succinate (Figure 3B).Moreover, we also found the decreased concentrations of IL-1β in supernatants of PBMCs co-stimulated with LPS, succinate and JSH-23.These results revealed the important roles of NF-κB in GPR91-induced infammatory responses.
Phosphorylation of p65 is a key biological event for NF-κB signaling,and elevated p-p65 indicates the increased NF-κB activity[27-28].To evaluate NF-κB activity, we detected the level of p-p65 through immunoblotting.The results showed that compared with the control group, ligation of GPR91 with succinate significantly augmented the level of p-p65, an indicative of increased NF-κB activity, while decreased p-p65 was observed after co-treatment with LPS, succinate and JSH-23 (Figure 4).Overall, these data demonstrated that GPR91 signaling triggered the upregulation of IL-1β, NLRP3, MMP-13 and VEGF-A in an NF-κB dependent manner.
Increased Expression of IL-1β, MMP-13, VEGF-A and Phosphorylated p65 in the MU BiopsiesWe examined whether human eyes with MU displayed an increased expression of IL-1β, VEGF-A, and MMP-13, as well as an elevated activity of NF-κB.IHC showed the protein levels of IL-1β, MMP-13 and VEGF-A in the cornea and conjunctiva of MU patients significantly increased compared with the healthy controls (Figure 5A-5C).We also found that IL-1β,MMP-13 and VEGF-A were mainly located in the infltrated inflammatory cells (Figure 5A-5C).qRT-PCR revealed the increased expression of IL-1β and VEGF-A (Figure 5E), which was in correspondence with the results of IHC.Moreover, the elevated p-p65 in the MU samples was also observed by IHC(Figure 5D), which suggested the increased NF-κB activity.These findings provide evidence of the increased expression of GPR91, IL-1β, VEGF-A and MMP-13, as well as elevated NF-κB activityin situin human eyes with MU, mirroring the functional data in PBMCs culture.
Immunosuppression and surgical intervention are two common approaches for MU management.However, resistance to such treatment and recurrence are major obstacles affecting the prognosis[5,29].The autoimmune factor has been reported as one of the mechanisms of MU, but the exact pathogenesis remains unknown.

Figure 4 Ligation of GPR91 with succinate significantly augmented NF-κB activity in LPS-stimulated PBMCs A: Western blot analysis of p-p65 and p65 in lysates of PBMCs after treatment with LPS, succinate and JSH-23 for 1h; B: Quantifcation of p-p65 in stimulated cells relative to that in unstimulated cells as in A.bP<0.01.
Recently, the crucial role of succinate in autoimmune and auto-infammatory diseases has been well documented[19,30-33].Intracellular succinate regulates infammatory response mainly through stabilizing hypoxia-inducible factor-1α and posttranslational modification of proteins by succinylation[34].Unlike intracellular succinate, the function of extracellular succinate in inflammation is mediated by ligation with its receptor GPR91, which is also renamed succinate receptor 1[15,22,34].Immune-associated inflammation has been reported to play a key role in the pathological damage caused by MU.However, the exact mechanism of infammation development in the MU is not clear.We speculated that succinate receptor GPR91 signaling may be involved in the MU pathogenesis.In this study, we found that the level of succinate receptor GPR91 in the MU subjects was much higher than that in controls.IHC revealed that GPR91 was mainly located in the infltrated infammatory cells.These fndings suggested the involvement of GPR91 in the pathogenesis of MU through sensing extracellular succinate.
Regarding the possible roles of GPR91 in the pathomechanism of MU, we found that succinate promoted the expression of NLRP3 and IL-1β in LPS-stimulated PBMCs through its receptor GPR91, which may partially explain the increased expression of NLRP3 inflammasome components in the MU samples previously reported[7].We also observed the pronounced expression of VEGF-A and MMP-13 in PBMCs after succinate stimulation.Mechanistically, we found that succinate enhanced LPS-induced NF-κB activity through GPR91, and pharmacologically blocked NF-κB activity reversed the effect of GPR91 signaling.Combined with our fndings of the increased expression of NLRP3, IL-1β, VEGF-A and MMP-13 in the MU samplesin situ, as well as increased p-p65, we addressed that GPR91 signaling could contribute to the pathogenesis of MU through increased NF-κB activity.
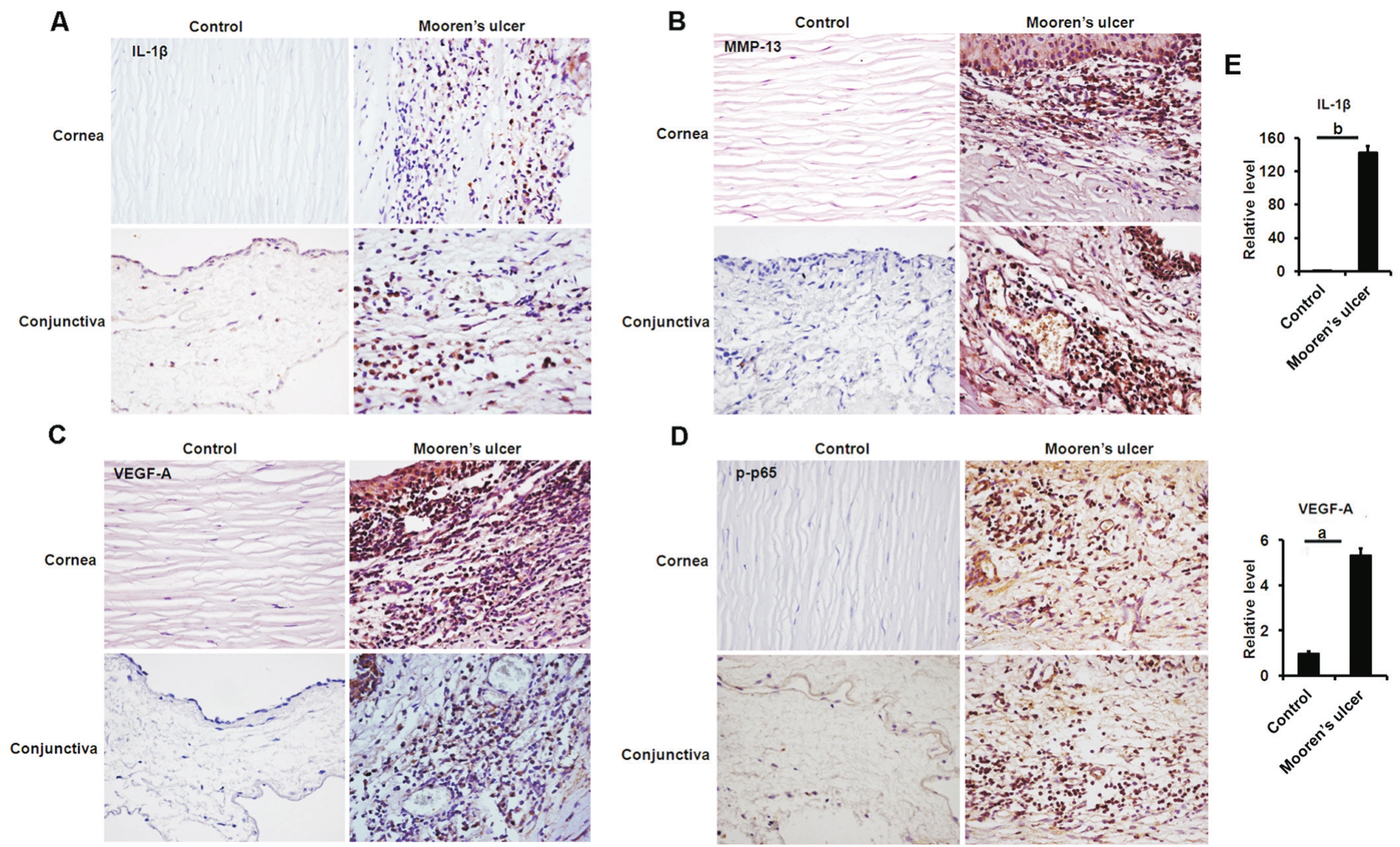
Figure 5 Analyses of IL-1β, MMP-13, VEGF-A and p65 expressions in the MU samples Analysis of IL-1β (A), MMP-13 (B), VEGF-A (C)and p65 (D) expressions in the cornea and conjunctiva by IHC (magnifcation ×400); E: Analyzing the transcriptional expression of IL-1β and VEGF-A in the samples of patients with MU (n=3).aP<0.05,bP<0.01.
MMP-13 is one of the collagenases that degrade native collagen fbrilsin vivo, which is essential for morphogenesis and tissue remodeling[35-36].The pathogenic roles of the overexpressed or activated MMP-13 have been reported in osteoarthritis[37], rheumatoid arthritis[38], and chronic cutaneous ulcers[39].In the cornea, MMP-13 has been reported to play a role in corneal wound healing[40], corneal vascularization[41]and corneal ulceration caused by Pseudomonas aeruginosa infection[36].In this study, we not only found the increased expression of MMP-13 in MU, but also showed that GPR91 signaling upregulated the MMP-13 expression through increased NF-κB activity.These findings suggested the potential involvement of GPR91 signaling in the pathological process of corneal stromal melting in MU patients.Moreover,there are many MMP-13 specific inhibitors designed to treat osteoarthritis and rheumatoid arthritis[42].These inhibitors may also have a potential to ameliorate corneal ulceration caused by MU.
Succinate receptor GPR91 was reported to promote retinal angiogenesis through upregulation of proangiogenic factors,including VEGF[16].VEGF-A plays an important role in the corneal neovascularization[43].In the current study, we showed the increased expression of VEGF-A in biopsy specimens with MU, and also found that ligation of GPR91 with succinate promoted the production of VEGF-A.Therefore, we proposed that corneal vascularization induced by GPR91 signaling could be another contributor to the pathogenesis of MU.
The limitations of this study should also be acknowledged.Firstly, the pathogenic roles of GPR91 cannot be effectively verifiedin vivobecause of shortage of MU animal models.Secondly, although we found the elevated expression of GPR91 in the MU samples and its ligation with succinate promoted inflammatory responses though NF-κB signaling using PBMCs, further investigations are needed to determine the extracellular concentration of succinate and elucidate the mechanisms of succinate in the pathogenesis of MU in details.Thirdly, we mainly used PBMCs to investigate the roles of GPR91 signaling pathway, but cells isolated from MU samples may be better for the functional examination.
To our knowledge, this is the first study to investigate the changes and roles of GPR91 in MU patients.The fndings of increased succinate receptor GPR91 and its pro-infammatory effect provide novel clues for the pathogenesis of MU, thereby broadening therapeutic targets for MU patients.
Foundations:Supported by National Natural Science Foundation of China (No.81530027; No.81500767; No.81570821);Natural Science Foundation of Shandong Province (No.ZR2018PH020; No.ZR2015YL037); the Innovation Project of Shandong Academy of Medical Sciences.
Conflicts of Interest:Li L, None; Dong YL, None; Liu T,None; Luo D, None; Wei C, None; Shi WY, None.
REFERENCES
1 Foster CS, Kenyon KR, Greiner J, Greineder DK, Friedland B, Allansmith MR.The immunopathology of Mooren’s ulcer.Am J Ophthalmol1979;88(2):149-159.
2 Alhassan MB, Rabiu M, Agbabiaka IO.Interventions for Mooren’s ulcer.Cochrane Database Syst Rev2014;(1):CD006131.
3 Brown SI, Mondino BJ, Rabin BS.Autoimmune phenomenon in Mooren’s ulcer.Am J Ophthalmol1976;82(6):835-840.
4 Gottsch JD, Liu SH, Minkovitz JB, Goodman DF, Srinivasan M, Stark WJ.Autoimmunity to a cornea-associated stromal antigen in patients with Mooren’s ulcer.Invest Ophthalmol Vis Sci1995;36(8):1541-1547.
5 Shinomiya K, Ueta M, Sotozono C, Inatomi T, Yokoi N, Koizumi N,Kinoshita S.Immunohistochemical analysis of inflammatory limbal conjunctiva adjacent to Mooren’s ulcer.Br J Ophthalmol2013;97(3):362-366.
6 Lee HJ, Kim MK, Wee WR, Oh JY.Interplay of immune cells in Mooren ulcer.Cornea2015;34(9):1164-1167.
7 Li Z, Wei C, Wang S, Liu T, Zhai H, Shi W.Upregulation of NLRP3 inflammasome components in Mooren’s ulcer.Graefes Arch Clin Exp Ophthalmol2017;255(3):607-612.
8 Kafkala C, Choi J, Zafirakis P, Baltatzis S, Livir-Rallatos C, Rojas B, Foster CS.Mooren ulcer: an immunopathologic study.Cornea2006;25(6):667-673.
9 Schallenberg M, Westekemper H, Steuhl KP, Meller D.Amniotic membrane transplantation ineffective as additional therapy in patients with aggressive Mooren’s ulcer.BMC Ophthalmol2013;13:81.
10 Liu J, Shi W, Li S, Gao H, Wang T.Modifed lamellar keratoplasty and immunosuppressive therapy guided by in vivo confocal microscopy for perforated Mooren’s ulcer.Br J Ophthalmol2015;99(6):778-783.
11 Ashar JN, Mathur A, Sangwan VS.Immunosuppression for Mooren’s ulcer: evaluation of the stepladder approach-topical, oral and intravenous immunosuppressive agents.Br J Ophthalmol2013;97(11):1391-1394.
12 Cordero-Coma M, Benito MF, Fuertes CL, Antolín SC, García Ruíz JM.Adalimumab for Mooren’s ulcer.Ophthalmology2009;116(8):1589,1589.e1.
13 Fontana L, Parente G, Neri P, Reta M, Tassinari G.Favourable response to infliximab in a case of bilateral refractory Mooren’s ulcer.Clin Exp Ophthalmol2007;35(9):871-873.
14 Guindolet D, Reynaud C, Clavel G, Belangé G, Benmahmed M,Doan S, Hayem G, Cochereau I, Gabison EE.Management of severe and refractory Mooren’s ulcers with rituximab.Br J Ophthalmol2017;101(4):418-422.
15 He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL,Tian H, Ling L.Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors.Nature2004;429(6988):188-193.
16 Sapieha P, Sirinyan M, Hamel D,et al.The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis.Nat Med2008;14(10):1067-1076.
17 McCreath KJ, Espada S, Gálvez BG, Benito M, de Molina A,Sepúlveda P, Cervera AM.Targeted disruption of the SUCNR1 metabolic receptor leads to dichotomous effects on obesity.Diabetes2015;64(4):1154-1167.
18 van Diepen JA, Robben JH, Hooiveld GJ, Carmone C, Alsady M,Boutens L, Bekkenkamp-Grovenstein M, Hijmans A, Engelke UFH,Wevers RA, Netea MG, Tack CJ, Stienstra R, Deen PMT.SUCNR1-mediated chemotaxis of macrophages aggravates obesity-induced infammation and diabetes.Diabetologia2017;60(7):1304-1313.
19 Littlewood-Evans A, Sarret S, Apfel V, Loesle P, Dawson J, Zhang J, Muller A, Tigani B, Kneuer R, Patel S, Valeaux S, Gommermann N,Rubic-Schneider T, Junt T, Carballido JM.GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis.J Exp Med2016;213(9):1655-1662.
20 Gilissen J, Jouret F, Pirotte B, Hanson J.Insight into SUCNR1 (GPR91)structure and function.Pharmacol Ther2016;159:56-65.
21 Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, Meer E, Peti-Peterdi J.Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney.J Clin Invest2008;118(7):2526-2534.
22 Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J,Carballido-Perrig N, Schwärzler C, Junt T, Voshol H, Meingassner JG, Mao X, Werner G, Rot A, Carballido JM.Triggering the succinate receptor GPR91 on dendritic cells enhances immunity.Nat Immunol2008;9(11):1261-1269.
23 Schneider C, O’Leary CE, von Moltke J, Liang HE, Ang QY,Turnbaugh PJ, Radhakrishnan S, Pellizzon M, Ma A, Locksley RM.A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling.Cell2018;174(2):271-284.e14.
24 Lei W, Ren W, Ohmoto M, Urban JF Jr, Matsumoto I, Margolskee RF, Jiang P.Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine.Proc Natl Acad Sci U S A2018;115(21):5552-5557.
25 Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, Miller CN, Pollack JL, Nagana Gowda GA, Fontana MF,Erle DJ, Anderson MS, Locksley RM, Raftery D, von Moltke J.Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit.Immunity2018;49(1):33-41.e7.
26 Guo Y, Xie C, Li X, Yang J, Yu T, Zhang R, Zhang T, Saxena D,Snyder M, Wu Y, Li X.Succinate and its G-protein-coupled receptor stimulates osteoclastogenesis.Nat Commun2017;8:15621.
27 Afonina IS, Zhong Z, Karin M, Beyaert R.Limiting inflammationthe negative regulation of NF-κB and the NLRP3 inflammasome.Nat Immunol2017;18(8):861-869.
28 Vallabhapurapu S, Karin M.Regulation and function of NF-kappaB transcription factors in the immune system.Annu Rev Immunol2009;27:693-733.
29 Dong Y, Zhang Y, Xie L, Ren J.Risk factors, clinical features,and treatment outcomes of recurrent Mooren ulcers in China.Cornea2017;36(2):202-209.
30 Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S,Vidoni S, Yoneshiro T, Spinelli JB, Lu GZ, Kazak L, Banks AS,Haigis MC, Kajimura S, Murphy MP, Gygi SP, Clish CB, Chouchani ET.Accumulation of succinate controls activation of adipose tissue thermogenesis.Nature2018;560(7716):102-106.
31 Li Y, Liu Y, Wang C, Xia WR, Zheng JY, Yang J, Liu B, Liu JQ, Liu LF.Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1α/VEGF axis.Free Radic Biol Med2018;126:1-14.
32 Tannahill GM, Curtis AM, Adamik J,et al.Succinate is an inflammatory signal that induces IL-1β through HIF-1α.Nature2013;496(7444):238-242.
33 Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE,Tourlomousis P, Däbritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G,Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP,O’Neill LA.Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive infammatory macrophages.Cell2016;167(2):457-470.e13.
34 Mills E, O’Neill LA.Succinate: a metabolic signal in infammation.Trends Cell Biol2014;24(5):313-320.
35 Leeman MF, Curran S, Murray GI.The structure, regulation, and function of human matrix metalloproteinase-13.Crit Rev Biochem Mol Biol2002;37(3):149-166.
36 Gao N, Kumar A, Yu FS.Matrix metalloproteinase-13 as a target for suppressing corneal ulceration caused by pseudomonas aeruginosa infection.J Infect Dis2015;212(1):116-127.
37 Li H, Wang D, Yuan Y, Min J.New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis.Arthritis Res Ther2017;19(1):248.
38 Wernicke D, Seyfert C, Gromnica-Ihle E, Stiehl P.The expression of collagenase 3 (MMP-13) mRNA in the synovial tissue is associated with histopathologic type II synovitis in rheumatoid arthritis.Autoimmunity2006;39(4):307-313.
39 Toriseva MJ, Ala-aho R, Karvinen J, Baker AH, Marjomäki VS, Heino J, Kähäri VM.Collagenase-3 (MMP-13) enhances remodeling of threedimensional collagen and promotes survival of human skin fbroblasts.J Invest Dermatol2007;127(1):49-59.
40 Ye HQ, Maeda M, Yu FS, Azar DT.Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas.Invest Ophthalmol Vis Sci2000;41(10):2894-2899.
41 Gao N, Liu X, Wu J, Li J, Dong C, Wu X, Xiao X, Yu FX.CXCL10 suppression of hem- and lymph-angiogenesis in infamed corneas through MMP13.Angiogenesis2017;20(4):505-518.
42 Fields GB.New strategies for targeting matrix metalloproteinases.Matrix Biol2015;44-46:239-246.
43 Ambati BK, Nozaki M, Singh N,et al.Corneal avascularity is due to soluble VEGF receptor-1.Nature2006;443(7114):993-997.
Citation:Li L, Dong YL, Liu T, Luo D, Wei C, Shi WY.Increased succinate receptor GPR91 involved in the pathogenesis of Mooren’s ulcer.Int J Ophthalmol2018;11(11):1733-1740
DOl:10.18240/ijo.2018.11.01
Accepted:2018-09-07
Received:2018-08-12
Correspondence to:Wei-Yun Shi.Shandong Provincial Key Laboratory of Ophthalmology, Shandong Eye Institute,Shandong Academy of Medical Sciences, Qingdao 266071,Shandong Province, China.weiyunshi@163.com
Co-first authors:: Lin Li and Yan-Ling Dong