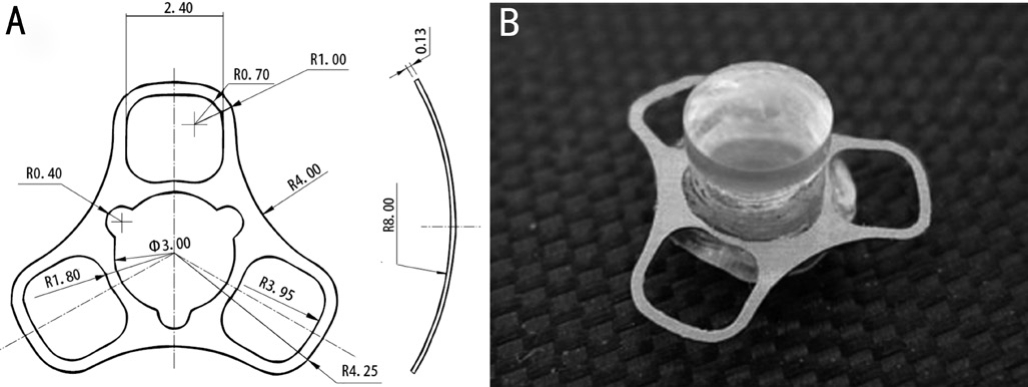
Figure 1 The structure of keratoprosthesis A: Top view diagram and cross-section diagram of titanium bracket; B: Artificial keratoplasty (including lens column and implant).
Li Li1, Hua Jiang2, Li-Qiang Wang3, Yi-Fei Huang3
1Department of Ophthalmology, the 88thHospital of Chinese People’s Liberation Army, Taian 271000, Shandong Province,China
2Department of Ophthalmology, Jinan Military General Hospital, Jinan 250031, Shandong Province, China
3Department of Ophthalmology, Chinese People’s Liberation Army General Hospital, Beijing 100853, China
Abstract
● AlM:To investigate whether hydroxyapatite (HAp)coating can improve keratoprosthesis (KPro) implant biointegration, ultimately to decrease the risk of implantassociated complications.
● METHODS:The modifed titanium implant was designed and prepared for artificial cornea.The titanium implant was treated with sandblasting and hydroxyapatite coating by acid-base two-step method.Surface was analyzed by scanning electron microscopy (SEM), KPro implants coated with HAp and KPro implant sandblasted were implanted in rabbits.Tissue adhesion to the implant was assessed and compared to an unmodifed implant by histopathology (HE),transmission electron microscopy (TEM) and SEM.
● RESULTS:SEM demonstrated successful deposition of HAp on titanium implant sandblasted (HA/SB-Ti).The hydroxyapatite coatings caused enhancement of keratocyte proliferation compared with unmodifed implant surfaces.HAp coating signifcantly increased adhesion forces.HAp coating of implants reduced the inflammatory response around the KPro implantsin vivo.
● CONCLUSlON:HAp-coated surfaces for use in titanium KPro implant greatly enhanced adherence of the titanium KPro implant in the rabbit cornea.
● KEYWORDS:keratoprosthesis; titanium; hydroxyapatite;surface modifed
W ith repeated allograft failure, few treatments are available, and tissue-engineered corneas are not yet suitable for clinical use.Corneal prostheses [keratoprosthesis(KPro)] are the only viable option for restoring sight.We selected titanium as a KPro implant, due to its long history of successful use in bone or dental prostheses[1-3], and the fact that it is biologically very well tolerated, as it induces relatively little infammation and foreign body reaction[4-5].Furthermore,by modifying their topography, titanium surfaces can induce proliferation or differentiation of osteocytes, promoting better apposition and adhesion between the material and bone[3,6].Hydroxyapatite (HAp) is a main component of bone and teeth and has been widely used for surface modification of bone implants[7], because it can bind electrostatically with charged biological molecules[8-9].However, the poor mechanical properties of HAp ceramics make them challenging to work with.In this study, the titanium implant with sandblasting and HAp coating by acid-base two-step (HAp/SB-Ti) was implanted into rabbit cornea, we assessed the effectiveness of titanium-based materials in improving adherence to corneal tissueex vivoandin vivo.
Equipment and ReagentCommercially pure titanium(99.5%; Taixing Metal Co., LTD, Xi’an, China), Homeothermia shaker THZ-C (Experiment Instrument Company, Taicang,China), ophthalmology microsurgery instrument (Suzhou Mingren Medical Apparatus and Instruments Co., LTD,Suzhou, China), Leica optical microscope (Leica Microsystems Inc., Germany), HITACHIS-4800 scanning electron microscope(SEM), Su-Mian-Xin II (Institute of Military Veterinary Medicine, The Academy of Military Medical Sciences,Changchun, Jilin, China); transmission electron microscopy(TEM; HITACHI-7000).
Design and Preparation of Improved HA/SB-Ti ImplantThe titanium implant was dissected into only 0.13 mm thick and was the sector-shaped (Figure 1A).The diameter of the titanium implant was 4.25 mm; the curvature radius of the front surface was 8.0 mm.The titanium implant was abraded in sand blast (250 grits, 180°, 5s, 5 cm, 5 Pa) to rough the surface.The sandblasted titanium implants (SB-Ti), which were coated in HAp by the acid-base two step methods, can be processed into the improved titanium implants (HAp/SB-Ti).The lens column of the artifcial cornea was designed according to the principle of geometric optics, and OSLO, an optical design software developed by Lambda Research Corporation, USA was used for design calculation.The material was hand-ground by polymethyl methacrylate (PMMA).
The diameter of the artifcial corneal column was 3.2 mm, the thickness was 3.8 mm, the curvature radius of the front surface was 8.874 mm, the back surface was planar, the focal length was 23.99 mm, the refraction was 55.40d, and the modified titanium implant with silicone rubber cap (diameter was 3.2 mm)was rotated into the column (Figure 1B).
In VivoExperimentsAll procedures used in this study were compliant with the locally approved protocols of the Administration Office Committee of Laboratory Animal and Ear Infirmary and performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.Twelve New Zealand white rabbits (mean 2.0-2.5 kg) were purchased from animal center of PLA General Hospital (Beijing, China).
Anesthesia was induced by intramuscular injections of ketamine 35 mg/kg and xylazine 5 mg/kg.Surgeries were performed on the right eye of each rabbit.Once the rabbits were anesthetized, the lamellar cornea pocket was made to approximately two-thirds the thickness of the cornea with a knife to accommodate the rabbit cornea, whose diameters were 7.0-7.5 mm and whose thicknesses were 0.1 mm.The HAp/SB-Ti implant was inserted with forceps into the corneal lamellar pocket.The fap was closed by two stitch of interrupted suturing with a 10-0 nylon suture.There were two groups, HAp/SB-Ti and SB-Ti, and six rabbits in each group.We used to exam the rabbit cornea with slit-lamp examination to assess infammatory reaction and neovascularization.
Histology, Transmission Electron Microscopy and Scanning Electron MicroscopyAfter 1mo, the rabbits were euthanatized, and the corneal specimens were processed for histology [hematoxylin-eosin (HE) staining] and analyzed by light microscopy.
After 1mo, the rabbits were euthanatized, and the corneal specimens were processed for TEM, take about 1 mm×1 mm in size, and immediately put into 2.5% glutaraldehyde for internal fixation.Different concentrations of ethanol and acetone were dehydrated step by step, and Epon812 epoxy resin was infltrated and embedded, a semi-thin section with a thickness of 2 μm was prepared, the flms were stained with 5% uranium acetate and citric acid, and were stained with lead, the flm was observed under TEM.

Figure 1 The structure of keratoprosthesis A: Top view diagram and cross-section diagram of titanium bracket; B: Artificial keratoplasty (including lens column and implant).
After 1, 2wk, 1 and 3mo, the rabbits were euthanatized, and the KPro implant pulled away manually from the cornea.The devices then were immersed in half-strength Karnovsky’s fixative (2% paraformaldehyde; 2.5% glutaraldehyde) in 0.1 mol/L phosphate buffer pH 7.4 overnight, dried in a critical point dryer, and coated with gold/palladium for SEM imaging.
Clinical ObservationThe secretions were increased within 3d after the operation, without red eyes, photophobia and tears.Observation of slit lamp: there was no infammatory response in anterior chamber with slight corneal edema after operation.The corneal edema disappeared three days after operation, and the implant was stable (Figure 2A).No corneal ulcer, cataract,retinal detachment and other complications.However, there was corneal neovascularization in the cornea implanted SB-Ti implant (Figure 2B).
HistologyHistologic sections 1mo after implantation, there were fibroblasts, inflammatory cells and a number of new blood vessels at the interface between the scaffold and cornea(Figure 3).
Transmission Electron MicroscopyMost of the stromal cells around the normal animal model were intact and the organelle structure was normal.The diameter of the collagen fbers around the implant is different, the implant and corneal junction were surrounded by irregular round cells with abundant cytoplasm, which were corneal fibroblasts.The collagen fbers around the implant of HA/SB-Ti implant were arranged perpendicular to or at a certain angle (Figure 4A),while the arrangement of collagen fbers around the implant of SB-Ti implant was almost parallel to the implant (Figure 4B).
Scanning Electron MicroscopySEM showed that the surface of the titanium implant was rough, uneven and some sharp edges after sandblasting (Figure 5A).After sanding and acidbase treatment, the titanium implant surface formed a complete micropore structure (Figure 5B).The HA coating consists of a large number of sheet crystals, compared with the implant removed 1mo after implantation, the surface adhesion of the implant removed 3mo after implantation was increased.There were lots of the corneal tissues on the HAp/SB-Ti implant,while the amount of adhesion of the corneal tissue on the surface of the HAp/SB-Ti was more than that of the SB-Ti(Figure 6).Compared with the implant removed 1wk after implantation, the surface adhesion of the implant removed 2wk after implantation was signifcantly increased, while the amount of adhesion of the corneal tissue on the surface of the HAp/SB-Ti was more than that of the SB-Ti (Figure 7).

Figure 2 Clinical Observation A: HAp/SB-Ti implants in the rabbits eyes.There was no infammation reaction; B: SB-Ti implant in the rabbits as control.The corneal neovascularization occurred.
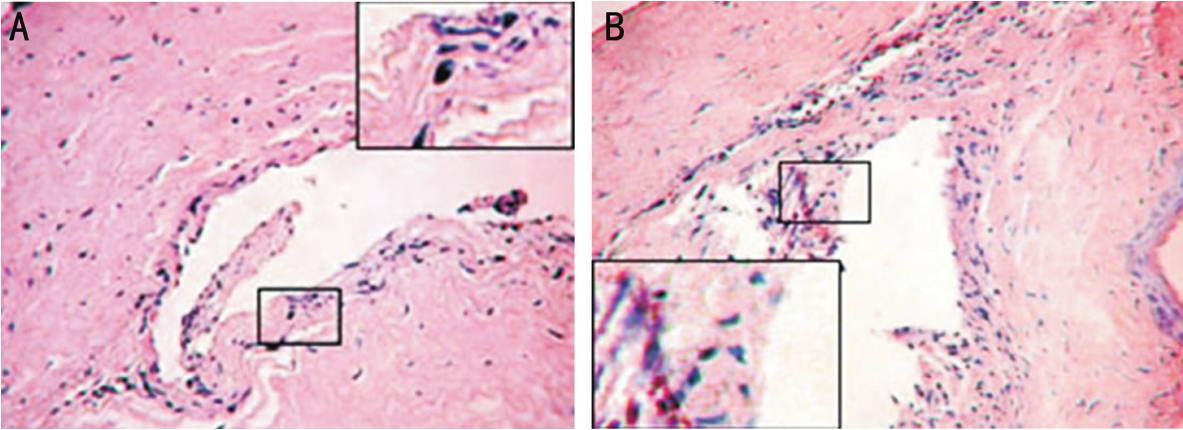
Figure 3 Histologic analysis A: The cornea of rabbit implanted with HAp/SB-Ti can be seen under light after 1mo; B: The cornea of rabbit implanted with SB-Ti can be seen under light after 1mo.
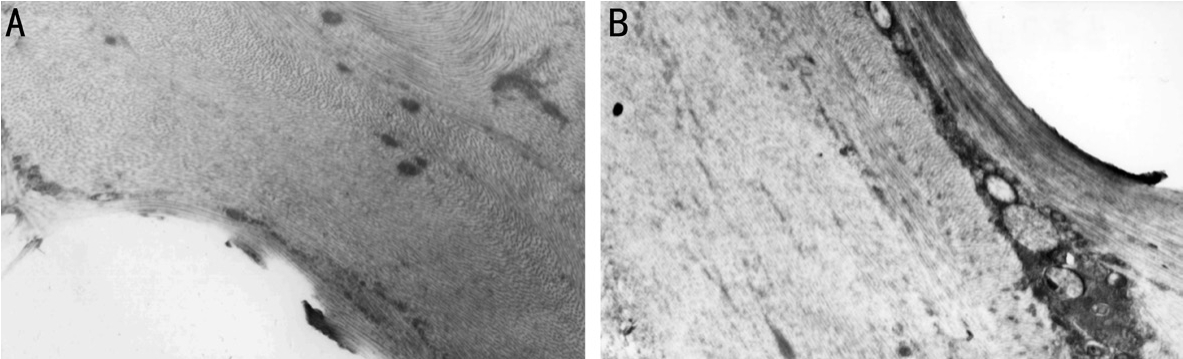
Figure 4 The corneal tissues were observed under TEM A: After three months, collagenous fbers in the corneal tissue with HAp/SB-Ti were arranged vertically or at a certain angle; B: After three months, collagenous fbers in the corneal tissue with SB-Ti were almost parallel to the scaffold.

Figure 5 The titanium implant was observed under SEM A: The rugosity surface had keen borderline before implanted with SB treatment(×20 000); B: A great quantity scale-shaped lamellar crystal on the surface before implanted with HAp/SB treatment (×20 000).
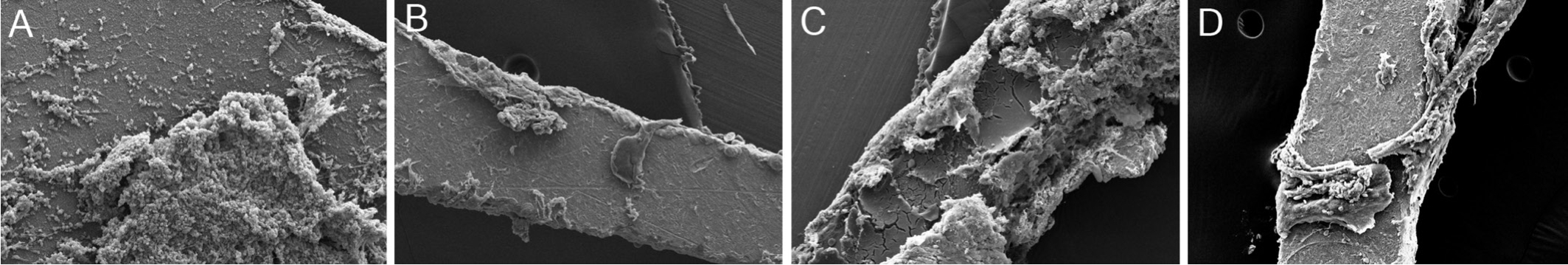
Figure 6 The corneal tissues on the implant were observed under SEM at postoperative 1 and 3mo A: After implanted with HAp/SBTi at postoperative 1mo and the lateral surface adhered with lots of corneal tissues (×150); B: After implanted with SB-Ti at postoperative 1mo and the lateral surface adhered with corneal tissues (×150); C: The implant adhered with lots of corneal extracellular matrix after implanted with HAp/SB-Ti at postoperative 3mo (×150); D: The implant adhered with corneal tissues after implanted with SB-Ti at postoperative 3mo (×150).

Figure 7 The corneal tissues on the implant were observed under SEM at postoperative 1 and 2wk A: After implanted with SB-Ti at postoperative 2wk, there were small corneal tissues on the implant surface (×150); B: After implanted with HAp/SB-Ti at postoperative 2wk,there were some corneal tissues on the surface (×150); C: After implanted with SB-Ti at postoperative 1wk, the implant surface adhered with corneal tissues (×150); D: After implanted with HAp/SB-Ti at postoperative 1wk, there were some corneal tissues on the implant surface (×150).
Penetrating keratoplasty has a poor prognosis in certain corneal eye diseases.The safety and effcacy of KPro surgery as a primary penetrating corneal surgery were evaluated for patients with corneal blindness and poor prognosis for penetrating keratoplasty[10].The path KPro has not always been an easy one.Initially discarded for its devastating complications, the introduction of new materials and the discovery of antibiotics in the last century gave new life to the field[11].Although microbial endophthalmitis after KPro has been drastically reduced in the last decade by use of daily lowdose prophylactic antibiotics[12-13], infectious complications still occur, especially in the developing world[14-15].It has long been suspected that the main contributing factor facilitating the increase in the risk of endophthalmitis could be inadequate integration between the KPro and the surrounding corneal tissue[16].For all these reasons, any method that can enhance the adhesion of corneal tissue around the KPro on a long-term basis might reduce the incidence of infection.Alteration of the surface of the device to allow better biointegration could be of clinical benefit.Biointegration or a tight attachment between implanted medical devices and body tissues directly influences clinical outcomes, including tissue breakdown and infection, ultimately having an impact on safety and efficacy[1-2].It has been demonstrated that materials with a highly hydrophilic surface and high surface roughness induce better tissue attachmentin vivo[6].Titanium as the material of KPro was implanted into some severe corneal patients.However, there some implications like KPro movement or retroprosthetic membrane, retroprosthetic membrane formation is the most common complication after Boston type 1 KPro implantation, Three of 11 eyes with titanium KPro that had a visually significant retroprosthetic membrane required surgical membranectomy[17].For all these reasons, any method that can enhance the adhesion of corneal tissue around the KPro on a long-term basis might reduce the incidence of infection.Alteration of the surface of the device to allow better biointegration could be of clinical beneft.Here we have modifed the titanium surface with HAp.We found that HAp promoted superior keratocyte adhesion and proliferation.In our study, titanium samples modified with HAp improved the bioactivity.Titanium is more resistant to inflammatory degradation and has a higher corrosion resistance as compared with HAp.This would reduce resorption rates for KPro surgery[18].Hydroxyapatite is a main component of bone and teeth and has been widely used for surface modification of bone implants, because it can bind electrostatically with charged biological molecules.However, the HAp layers were easily collapse and shedding.So we blasted with sand first to rough the surface and then coated with HAp by acid-base two step methods.The external shape is designed as a tri-loop scaffold, which is theoretically more conducive to its fxation in the cornea.
The composition of HAp is similar with main organic principle of human skeletal; simultaneously the scale-shaped structure of HAp could increase the surface area of implants and the conjunction area of implant-cornea.Grosset al[19]suggested that porosity surface could obtain vertical connection of bone trabecula by the study of the binding between HAp layer and implants.The other way to improve the implants’ bioactivity is to mimesis a nature physio-condition which was suitable for cell adhesion, proliferation and differentiation[20-21].In our study, the SEM that observed at the postoperative 1,2wk, 1, 3mo suggested that most area of HAp/SB-Ti surface was covered with corneal extracellular matrix and cells.The implant-cornea interface was compact connection.SB-Ti sleeves greatly enhanced adherence of the KPro to the rabbit cornea[16].
In present study, both HAp/SB-Ti and SB-Ti could induce slight inflammation which was the common reaction of implant for the host.HAp coating significantly increased the force and work required to pull PMMA cylinders out of porcine corneasex vivo.HAp coating of implants reduced the infammatory response around the PMMA implantsin vivo[22].In our study, HE staining showed that the fbroblasts composed by the implant of the HAp/SB-Ti were signifcantly increased.The metabolic function of the cells was enhanced.The physicochemical property and surface texture of biomaterial could influence the process of inflammation directly, and the extension and duration could also impact the stability of biomaterial[23].In our study, the interface inflammation was slight both in the HAp/SB-Ti and the SB-Ti.
Under the TEM, fibroblasts in the surrounding and pores of implant of the HAp/SB-Ti are more abundant and proliferative than those in the control group.
In conclusion, titanium implant coated with HAp greatly improved cell viability, implant adhesion to tissue, and biocompatibility compared with unmodifed titanium implant.Our study showed better tissue attachment when titanium implant coated with HAp, compared to a rough surface titanium implant.
Foundations:Supported by National Stem Cell and Translational Medicine Key Project (No.2017YFA0103204);the National Natural Science Foundation Project(No.81670830); the Capital Clinical Key Project (No.Z161100000516012); the Military Logistics Technology Project (No.CWS13C057); the PLA General Hospital Transformation Medicine Project (No.2016TM-025).
Conflicts of Interest:Li L, None; Jiang H, None; Wang LQ,None; Huang YF, None.
REFERENCES
1 Hacking SA, Pauyo T, Lim L, Legoux JG, Bureau MN.Tissue response to the components of a hydroxyapatite-coated composite femoral implant.J Biomed Mater Res A2010;94:953-960.
2 Theis JC, Gambhir S, White J.Factors affecting implant retention in infected joint replacements.ANZ J Surg2007;77:877-879.
3 Baril E, Lefebvre LP, Hacking SA.Direct visualization and quantifcation of bone growth into porous titanium implants using micro computed tomography.J Mater Sci Mater Med2011;22:1321-1332.
4 Tan XW, Beuerman RW, Shi ZL, Neoh KG, Tan D, Khor KA, Mehta JS.In vivoevaluation of titanium oxide and hydroxyapatite as an artifcial cornea skirt.J Mater Sci Mater Med2012;23:1063-1072.
5 Tan XW, Perera AP, Tan A, Tan D, Khor KA, Beuerman RW, Mehta JS.Comparison of candidate materials for a synthetic osteo-odonto keratoprosthesis device.Invest Ophthalmol Vis Sci2011;52:21-29.
6 Hacking SA, Boyraz P, Powers BM, Sen-Gupta E, Kucharski W,Brown CA, Cook EP.Surface roughness enhances the osseointegration of titanium headposts in non-human primates.J Neurosci Methods2012;211:237-244.
7 De Groot K, Geesink R, Klein CP, Serekian P.Plasma sprayed coatings of hydroxylapatite.J Biomed Mater Res1987;21(12):1375-1381.
8 Colman A, Byers MJ, Primrose SB, Lyons A.Rapid purification of plasmid DNAs by hydroxyapatite chromatography.Eur J Biochem1978;91(1):303-310.
9 Schröder E, Jönsson T, Poole L.Hydroxyapatite chromatography:altering the phosphate-dependent elution profle of protein as a function of pH.Anal Biochem2003;313(1):176-178.
10 Fadous R, Levallois-Gignac S, Vaillancourt L, Robert MC, Harissi-Dagher M.The Boston Keratoprosthesis type 1 as primary penetrating corneal procedure.Br J Ophthalmol2015;99(12):1664-1668.
11 Salvador-Culla B, Kolovou P.Keratoprosthesis: a review of recent advances in the feld.J Funct Biomater2016;7(2):13.
12 Behlau I, Martin KV, Martin JN, Naumova EN, Cadorette JJ, Sforza JT, Pineda R, Dohlman CH.Infectious endophthalmitis in Boston keratoprosthesis: incidence and prevention.Acta Ophthalmol2014;92:e546-e555.
13 Durand ML, Dohlman CH.Successful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesis.Cornea2009;28:896-901.
14 Aldave AJ, Sangwan VS, Basu S,et al.International results with the Boston type I keratoprosthesis.Ophthalmology2012;119:1530-1538.
15 Jain V, Mhatre K, Shome D, Pineda R.Fungal keratitis with the type 1 Boston keratoprosthesis: early Indian experience.Cornea2012;31:841-843.
16 Salvador-Culla B, Jeong KJ, Kolovou PE, Chiang HH, Chodosh J, Dohlman CH, Kohane DS.Titanium coating of the Boston keratoprosthesis.Transl Vis Sci Technol2016;5(2):17.
17 Talati RK, Hallak JA, Karas FI, de la Cruz J, Cortina MS.Retroprosthetic membrane formation in boston keratoprosthesis: a casecontrol-matched comparison of titanium versus PMMA backplate.Cornea2018;37(2):145-150.
18 Chen JQ, Zhai JJ, Gu JJ, Shao YF, Liu YM, Yuan J, Zhou SY.Preliminary study of Boston keratoprosthesis in treatment of severe late stage ocular chemical burns.Zhonghua Yan Ke Za Zhi2012;48(6):537-541.
19 Gross UM, Müller-Mai CM, Voigt C.The interface of calciumphosphate and glass-ceramic in bone, a structural analysis.Biomaterials1990;11:83-85.
20 Lange R, Lüthen F, Beck U, Rychly J, Baumann A, Nebe B.Cellextracellular matrix interaction and physico-chemical characteristics of titanium surfaces depend on the roughness of the material.Biomol Eng2002;19(2):255-261.
21 Balto H, Al-Nazhan S.Attachment of human periodontal ligament fibroblasts to 3 different root-end filling materials: Scanning electron microscope observation.Oral Surg Oral Med Oral Pathol Oral Radiol Endod2003;95(2):222-227.
22 Wang L, Jeong KJ, Chiang HH, Zurakowski D, Behlau I, Chodosh J,Dohlman CH, Langer R, Kohane DS.Hydroxyapatite for keratoprosthesis biointegration.Invest Ophthalmol Vis Sci2011;52(10):7392-7399.
23 Robert MC, Arafat SN, Ciolino JB.Collagen cross-linking of the Boston keratoprosthesis donor carrier to prevent corneal melting in highrisk patients.Eye Contact Lens2014;40(6):376-381.
Citation:Li L, Jiang H, Wang LQ, Huang YF.Experimental study on the biocompatibility of keratoprosthesis with improved titanium implant.Int J Ophthalmol2018;11(11):1741-1745
DOl:10.18240/ijo.2018.11.02
Accepted:2018-09-27
Received:2017-12-10
Correspondence to:Yi-Fei Huang.Department of Ophthalmology, Chinese People’s Liberation Army General Hospital,No.28 Fuxing Road, Haidian District, Beijing 100853, China.yhqqkid@vip.sina.com