Table 1 Muscarinic acetycholine 1 receptor and β-actin oligonucleotide primers

Yuan Tao1, Xiao-Li Li2, Li-Yuan Sun3, Yu-Hua Wei3, Xiao-Ting Yu3, Hong Wang3
1Department of Ophthalmology, the Second People’s Hospital of Jinan City, Jinan 250001, Shandong Province, China
2Department of Laboratory Medicine, Qilu Hospital of Shandong University, Jinan 250001, Shandong Province, China
3Department of Ophthalmology, Qilu Hospital of Shandong University, Jinan 250001, Shandong Province, China
Abstract
● AlM:To investigate the effects of green flickering light on refractive development and expression of muscarinic acetylcholine receptor (mAChR) M1 in the eyes of guinea pigs.
● METHODS:Thirty guinea pigs (15-20 days old) were randomly divided into three groups (n=10/group).Animals in group l were raised in a completely closed carton with green fickering light illumination.Those in group ll were kept in the open top closed carton under normal natural light.Guinea pigs were raised in a sight-widen cage under normal natural light in group lll.The refractive status and axial length were measured before and after 8 weeks'illumination.Moreover, total RNA extracted from retinal,choroidal, and scleral tissues were determined by real-time reverse transcription polymerase chain reaction (RT-PCR).The expressions of the receptor M1 were also explored in the retina, choroid, and sclera using immunohistochemistry.
● RESULTS:There was a remarkable reduction in refractive error and increase in axial length after 8-weeks' green flickering light stimulation (P<0.001).The expression of M1 receptor mRNA in sclera and retina in myopia group were remarkably lower than that in group ll and lll (P<0.01).Signifcant reduced expression of M1 receptor stimulated by green fickering light in retina and sclera tissues were also observed (P<0.05).However, there was no M1 receptor expression in choroid in 3 groups.
● CONCLUSlON:Myopia can be induced by 8 weeks' green fickering light exposure in the animal model.M1 receptor may be involved causally or protectively in myopia development.
● KEYWORDS:guinea pigs; green flashing light; myopia model; muscarinic acetylcholine 1 receptor
M yopia, one of the most common human visual disorders,is characterized by excessive axial elongation of the eye and negative refractive error[1-2].The number of myopia presents a dramatically increasing tendency over the past decades, with a reported rate of 80% in urban areas in Asia alone[3-5].The underlying pathogenesis of myopia is poorly understood, but increasing evidences have demonstrated that genetic and environmental factors are critical for myopia development[6].Exposure to flickering light has long been considered as one of important environmental risks for myopia progression to which people are extensively used electronics during their lives and work.It has been reported that chronic exposure to low-frequency fickering light induces myopia in guinea pigs with the associated histological and concurrent electrophysiological changes[7].Aslo, previous studies have revealed that flickering light is closely associated with refractive error development[8].By contrast, Crewtheret al’s[9]research reported that luminance modulation had no effect on refraction or ocular parameters in no-lens conditions.Thus,controversy still exists at present regarding flickering light induced myopia.
However, considerable evidences from studies in animal models demonstrated that many retinal neurotransmitters have been involved in ocular development, also implicated in myopia[10-11].Five distinct muscarinic acetylcholine receptors(mAChRs) subtype (M1-M5) have been identified and mediated most of the actions of the neurotransmitter ACh in the central and peripheral nervous systems[12].Clinical application of muscarinic receptor antagonists, such as atropine (a non-selective mAChR antagonist), pirenzepine (an M1-selective antagonist) and himbacine (an M4-selective antagonist)etc.can play a critical role in the inhibition of myopia development[13].The M1 receptor is widely distributed in the eyes of guinea pigs, which is might has the most important role in the “stop” signal of myopic progression[14].M1 is the most effective in preventing myopic eye change.Moreover, retina,choroid, and/or sclera are implicated potential sites of action for muscarinic-active drugs.Although some studies revealed that the high concentrations of the M1 or M4 receptor may suggest involvement of a non-cholinergic receptor mechanism such as the nicotinic system[15-16], their underlying mechanism remains unclear.The objective of current study was to investigate the effectiveness of green fickering light on myopia and expression of M1 receptor in the eyes of guinea pigs.
Animals and GroupsThirty male and female guinea pigs(black, brown and white), aged from 15 to 20d, 70-90 g were obtained from the Experimental Animal Center of Shandong University.The refraction ranged from +2.50 to +3.50 D was examined by retinoscopy.All experimental protocols conformed to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research.This work was approved by the Animal Care and Ethics Committee in Shandong University.Animals were randomly divided into 3 groups(n=10 for each group).Animals in group I were irradiated with 5 Hz green fickering light (515-530 nm, peak value 525 nm, bright 2s, dark 2s) for 8wk, placed in a completely sealed carton.The light source is installed above the carton, and the experimental animals were exposed to an illumination intensity of 800 lx with a 50% duty cycle (0.1s and then in the dark for 0.1s, with this analogize).Animals in group II were kept in the carton under normal natural light by leaving the cap of the carton open.Animals in group III were raised in an animal room with windows and good lighting, avoid interference from artifcial light sources.All the cages were well ventilated to maintain constant temperature inside, food and water free access.
Measurement of Ocular Refraction and Axial LengthThe refraction was examined prior to the experiment and 8wk after the experiment.Before examination, the eyes were applied with compound tropicamide eye drops (per 1 mL containing tropicamide 5 mg, phenylephrine hydrochloride 5 mg, Santen Pharmaceutical Co., Ltd., Japan) 2 times to paralyze the ciliary muscle.After half an hour, horizontal and vertical diameter of the retinoscopy was respectively performed to examine the refraction in the dark with a streak retinoscope.The gradient was 0.25 D, and astigmatism was represented by an equivalent spherical mirror.The axial length was measured prior to the experiment and 8wk after the experiment.Oxybuprocaine hydrochloride eye drops 0.4% (Santen Pharmaceutical Co.,Ltd., Japan) were administered in conjunctival sac 2 times, and the axial length was measured with A-scan ultransonography.A continuous measurement was made 10 times and presented as mean values±standard deviation (SD).
Table 1 Muscarinic acetycholine 1 receptor and β-actin oligonucleotide primers

Collection of TissueAnimals were sacrificed by excessive anesthesia (3% sodium pentobarbital).The eyeballs were enucleated and hemisected after carefully removal of residual orbital tissue (conjunctiva, fascia, extraocular muscles, fat and optic nerve).Then anterior ocular tissues and vitreous body were removed with no teeth tweezers, and posterior retina,choroid and sclera were collected.All dissected tissue samples were placed in eppendorf tubes, immediately frozen in liquid nitrogen and stored at -80℃.
RNA Extraction and Reverse TranscriptionTo prevent RNA degradation by RNA enzymes during the extraction process, glassware, pipette tips and other experimental consumables were pretreated with diethypyrocarbonate water(DEPC).Total RNA was routinely extracted from the tissue samples by Trizol (Shanghai shenggong bio-engineering technology Co., Ltd., Shanghai, China) according to the manufacturer’s instructions.The A260/A280 value was determined between 1.8 and 2.0 by the ZF type ultraviolet spectrophotometer analyzer (Shanghai Hong Wah biochemical instrument factory, Shanghai, China).Agarose 1% gel electrophoresis results showed that 28S and 18S RNA bands were clear observed, indicating that the total RNA extracted was intact and no obvious degradation.cDNA was then obtained by reverse transcription and cDNA was used as a template to amplify the target gene for PCR.Amplifcation and detection of mRNA were performed in a TaqMan real-time RT-PCR (TaqMan rRT-PCR).
Real-time Polymerase Chain ReactionOligonucleotide primers for M1 receptors of guinea pigs were summarized in Table 1.The internal reference gene was murine β-actin.Reaction system was totally 20 μL.Amplifcation of the M1 receptor product was performed according to RT-PCR kit(Dalian TakaRa Biological Engineering Co., Ltd., Dalian,China) instructions.After an initial denaturation at 94℃ for 5min, the samples underwent 35 cycles of denaturation (94℃,40s), annealing (60℃, 40s), and extension (72℃, 1min).This was followed by fnal extension at 72℃ for 7min.Of 15 g/L PCR product was subjected to gel electrophoresis to determine the amount of M1 receptor product.The PCR product was scanned and quantified by using a Gel Imager System (Bio-Rad Laboratories, Hercules, CA).

Figure 1 The refraction and axial length changes of all guinea pigs in three groups before and after 8 weeks’ exposure A: Signifcant refractive changes in the eyes of three groups appeared after the 8thweek’s exposure; B: Signifcant axial length changes in the eyes of three groups appeared after the 8thweek’s exposure.aP<0.001 compared between before and after 8 weeks’ exposure;bP<0.05 compared with any two of three groups.
Table 2 The refraction and changes before and after exposure of guinea pigs in the three groups

ImmunohistochemistryStrept avidin-biotin complex (SABC)assay was used to detect the expression of M1 receptor.Briefy,5 μm tissue sections were obtained from several representative areas of each specimen and were mounted on to glass slides.Slides were incubated at room temperature for 10min.Xylene was used to dewaxing for 15min and anhydrous alcohol was applied for 7min twice.Tissue sections were incubated with 90% alcohol for 10min twice, 80% and 70% alcohol for 5min respectively to block endogenous peroxidase activity.After washing, sections were incubated with a repairing solution for 5min.Immunostaining was then carried out by incubation with 50 μL M1 antibody (Santa Cruz, California, USA, diluted by 1.5% goat serum, and the fnal concentration was 1:100) at 4℃for one night.After washing three times with phosphate buffer saline (PBS) for 5min, the reaction was subsequently amplifed with primary antibody amplifier, followed by horseradish peroxidase (HRP; Santa Cruz, California, USA, diluted by 1.5% goat serum, and the fnal concentration was 1:200).Then SABC was added and incubated at 37℃ for 30min.Color was developed using Horseradish Peroxidase Color Development Kit (DAB; Santa Cruz, California, USA) substrate chromogenic system for 3-5min (Santa Cruz, California, USA).Finally,sections were analyzed with a microscope (BH-2 OLYMPUS,Tokyo, Japan).Sections were analyzed with a microscope(BH-2 OLYMPUS, Tokyo, Japan).For evaluation of M1 receptors expression, the percentage of positive cells (0: <5%positive cells; 1: 5%-10% positive cells; 2: 11%-50% positive cells; 3: 51%-75% positive cells; 4: >75% positive cells) were counted in the 400-fold feld of view using the Leica Qwin V3 image analysis system.
Statistical AnalysisStatistical analysis was performed by SPSS software (Version 12.0, Chicago, IL, USA).Continuous data was presented as mean±SD.One-way ANOVA followed by Tukey’s post hoc test was applied for analysis of differences between groups.
Measurement of Ocular Refraction and Axial LengthThe refraction and axial length of all guinea pigs in three groups were presented in Figure 1.There were no signifcant differences among the three groups with respect to the refraction and axial length before exposure.Refraction was found to be signifcantly reduced among the three groups over 8 weeks’ experiments, and the refractive changes in the group I was 6.74±0.68 (P<0.001), group II was 3.80±0.51 (P<0.001),group III was 1.20±0.22 (P<0.001; Table 2).Moreover, the refractive changes of any two of these three groups all showed significant difference (P<0.05; Figure 1A).Axial length was significantly longer after 8 weeks’ exposure in both group I (P<0.001) and group II (P<0.001; Table 3).Meanwhile,the axial length changes of any two of these three groups all showed signifcant difference (P<0.05; Figure 1B).
RNA Sample IdentificationThe RNA concentration was 2.5-4.5 g/L and the A260/A280 value was between 1.8 and 2.0.The results of 1% agarose gel electrophoresis showed that the total RNA of receptor extracted through this method has no obvious degradation and has a good purity.
Table 3 The axial length and changes before and after exposure of guinea pigs in the three groups


Figure 2 The images and expression of M1 receptor mRNA present in retina, sclera and choroid of the three groups A: The expression of M1 receptor mRNA was observed in retina and sclera of the three groups; B: The mRNA expression of M1 receptor in the three groups in retina tissues; C: The mRNA expression of M1 receptor in the three groups in sclera.cP<0.01 compared with group I.1: Group I; 2: Group II; 3: Group III; 4: DNA marker.
Qualitative Expression and Quantitative Analysis of M1 Receptor mRNA in Retina, Sclera and Choroid of Guinea PigAs shown in Figure 2A, the expression of acetylcholine M1 receptor mRNA was observed in retina and sclera of group I, II and III.However, there was no M1 receptor mRNA expression in choroid.According to the image analysis (Figure 2B, 2C), the mRNA expression of M1 receptor in group I was significant different from that in group II and group III(56.38±3.34vs70.16±2.45 and 73.34±2.83) in retina tissues(P<0.01).In sclera tissues, the content of M1 receptor mRNA in group I was 58.40±2.73, 70.59±1.89 in group II and 72.60±2.81 in group III, and mRNA content in group I had obvious difference from those in groups II and III (P<0.01).
Expression of M1 Receptor in Retina, Sclera and Choroid of Guinea Pig by ImmunohistochemistryM1 receptor was expressed in all layers of retina and sclera tissues in three groups and the tissue with positive expression of M1 receptor was brownish.No positive expression was found in choroid tissue (Figure 3A).As shown in Figure 3B, animals were stimulated by green flickering light showed significant reduced expression of M1 receptor in retina and sclera tissues compared with those exposed to normal natural light (P<0.05).The result was consistent with the results of mRNA expression.
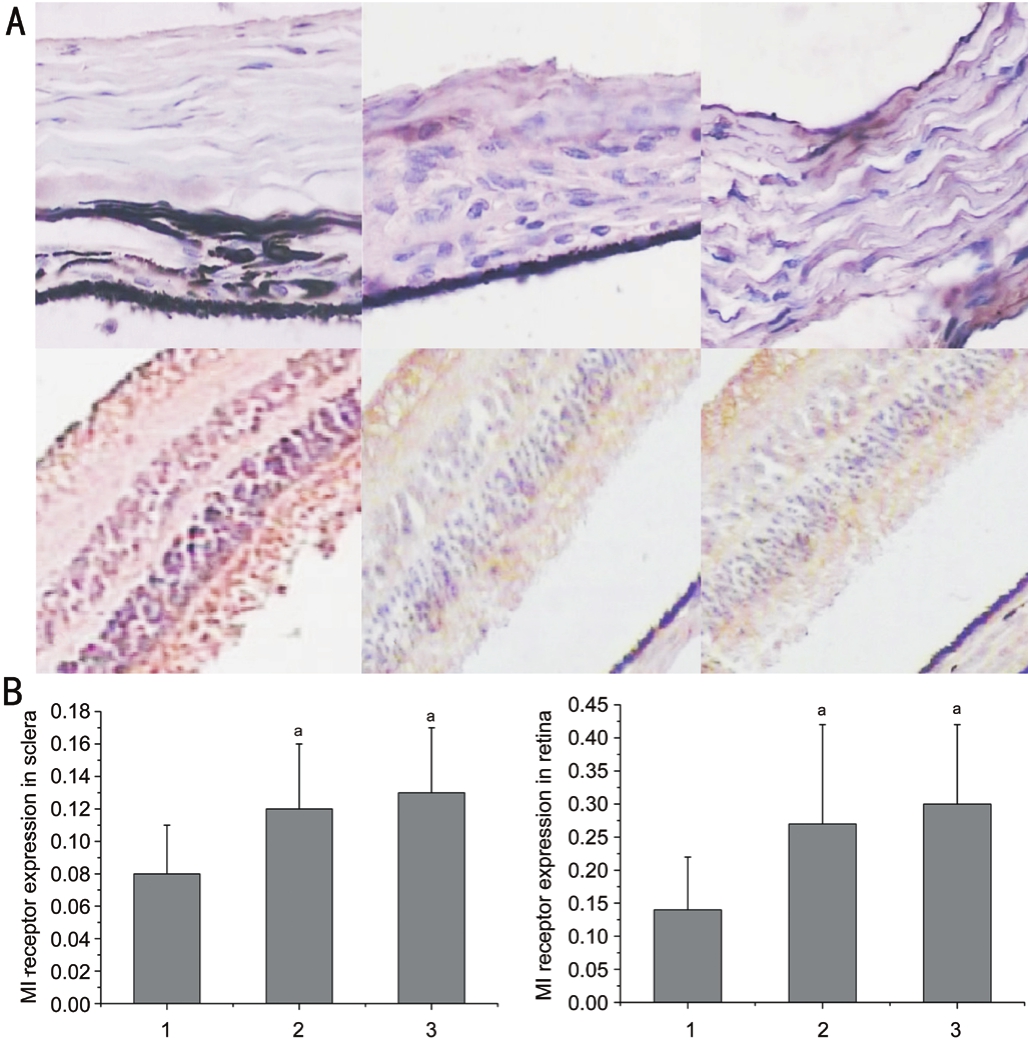
Figure 3 The immunohistochemistry images and expression of retina and sclera obtained by staining M1 receptors A: The images of retina and sclera obtained by staining M1 receptors; B: The expression of M1 receptor in retina and sclera in the three groups.
aP<0.05 compared with group I.
This study was undertaken to investigate the effects of green fickering light on refractive error development and expression of mAChRs subtype M1 during the development of myopia in the eyes of guinea pigs.Induction of refractive errors from green flickering light has been reported in different animal species[17-19].Wanget al’s study[20]indicated that in green light of 530 nm was not only to induce greater axial myopia, but also to increase secretion of melatonin.This study confrmed that axial myopia can be induced by long-wavelength green light.Findings of the present study are consistent with previous studies who reported a myopia shift after variable weeks’ of green flickering light stimulation[7-8].By contrast,Schwahn and Schaeffel’s[21]investigation demonstrated that the eyes of chicks kept under flickering light were more hyperopic.The reasons for this discrepancy were might due to light parameters, such as intensity, frequency and exposure timeetc, which consistent with the study results of Wanget al[20]and Cohenet al[22].Furthermore, axial length increased dramatically during the myopia development.It can be seen that the effects of flickering light on ocular refraction are mainly due to changes in axial elongation.Several researches have also revealed that there are mechanisms for eye growth in the anterior and posterior segment independently[23-24].Ocular circadian rhythm plays an important role in postnatal ocular growth, axial elongation, and emmetropization.The formation of myopia is the process of the eye’s active hyperplasia adapt to the new environment[13].The reason of scintillation light induces myopia is that when the retina does not get a clear phase, it will send out information to adjust the development of the eyeball, causing abnormal growth of the eyeball and fnally forming myopia.In our research the myopia was successfully induced by 8-week green flickering light stimulation in guinea pigs.Compared with guinea pigs in natural light,ocular refractive error and axial length were changed in green scintillation light environment guinea pigs.This indicates that green scintillation light has an important infuence on the development of the refractive error of guinea pig eyes.
ACh is a crucial neurotransmitter involved in diverse physiological functions of nervous system.Meanwhile,diverse functions of ACh are mediated by a variety of specific receptors[25].Although five distinct mAChRs have been identified by immunohistochemical technique, the role of M1 receptor in myopic development is still not fully understood.Many previous studies have been demonstrated that the mRNA expression of all mAChR were present in the guinea pig retinal pigment epithelium[13,26].Several other researches have also demonstrated the expression of M1, M2,M3, and M4 were found in retina in different animals[27-28].Similar receptor expression was also found in human sclera[26].In mammals, inhibition of form-deprivation myopia is caused by muscarinic antagonists involves both M4 and M1 muscarinic receptor signaling pathways[29].Pirenzepine as an M1 elective muscarinic antagonist, is effective in slowing the progression of myopia in both humans and experimental animals, including chick[30].In our research M1 receptor mRNA expression was found in the retina and sclera, and mRNA expression for M1 receptor in these sites signifcantly reduced during the induction of myopia.Moreover, the results of immunohistochemical staining revealed that the mRNA of M1 receptor was found to be distributed throughout the sclera and retina.Our study confrmed that mAChR signaling may participate in the induction of myopia in guinea pigs and that the retina and sclera may be potential sites for preventing myopia by using mAChR antagonists.However, our research was in consistent with the study conducted by Liuet al[13],where M1 gene and protein expression were increased in the guinea pig during myopia development.An important consideration in comparing the two studies is that Liuet al[13]induced myopia over a 21d period rather than the 8wk period used in the current study.Thus, the observed alterations in M1 and M4 receptor expressions may reflect later changes arising from the enlarged eye rather than reflecting a causal relationship with eye growth.A previous report showed that pirenzepine treatment inhibited myopia development through M1 and M4 regulation in retina, sclera and choroid[31].However, it still unclear that whether the pirenzepine-induced increase in the M1 and M4 receptors directly reduced the myopia or was merely a result of it.
This study is limited by its relatively small sample size, and lack of test for other receptors.Thus, we suggest a larger-scale study be conducted in future to evaluate the other receptors in different animal.In addition, the differences in the above conclusions might be due to different myopic model induced by different mechanisms.Therefore further studies of the relevant mechanisms are still needed.
In conclusion, our study provided a comprehensive profile of the expression of mAChRs in the ocular tissues of guinea pigs.Expression of the M1 subtype significantly decreased in the posterior retina and sclera of myopia induced by green flickering light.It is a better and in-depth understanding to further study the specific mechanism of M1 receptor in fickering light induced myopia.
Foundation:Supported by Qilu Hospital of Shandong University (No.201805049).
Conflicts of Interest:Tao Y, None; Li XL, None; Sun LY,None; Wei YH, None; Yu XT, None; Wang H, None.
REFERENCES
1 Li S, Wu J, Ding H, Liao A, He H, Stell WK, Zhong X.Flicker downregulates the content of crystallin proteins in form-deprived C57BL/6 mouse retina.Exp Eye Res2012;101:1-8.
2 Curtin BJ, Karlin DB.Axial length measurements and fundus changes of the myopic eye.Am J Ophthalmol1971;71(1):42-53.
3 Xiang F, He M, Zeng Y, Mai J, Rose KA, Morgan IG.Increases in the prevalence of reduced visual acuity and myopia in Chinese children in Guangzhou over the past 20 years.Eye (Lond)2013;27(12):1353-1358.
4 Wu HM, Seet B, Yap EP, Saw SM, Lim TH, Chia KS.Does education explain ethnic differences in myopia prevalence? A population-based study of young adult males in Singapore.Optom Vis Sci2001;78(4):234-239.
5 Lam CS, Lam CH, Cheng SC, Chan LY.Prevalence of myopia among Hong Kong Chinese schoolchildren: changes over two decades.Ophthalmic Physiol Opt2012;32(1):17-24.
6 Zhou X, Huang Q, An J, Lu R, Qin X, Jiang L, Li Y, Wang J, Chen J,Qu J.Genetic deletion of the adenosine A2A receptor confers postnatal development of relative myopia in mice.Invest Ophthalmol Vis Sci2010;51(9):4362-4370.
7 Di Y, Lu N, Li B, Liu R, Chu RY, Zhou XT, Zhou XD.Effects of chronic exposure to 0.5 Hz and 5 Hz flickering illumination on the eye growth of guinea pigs.Curr Eye Res2013;38(11):1182-1190.
8 Yu Y, Chen H, Tuo J, Zhu Y.Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice.Ophthalmic Res2011;46(2):80-87.
9 Crewther SG, Barutchu A, Murphy MJ, Crewther DP.Low frequency temporal modulation of light promotes a myopic shift in refractive compensation to all spectacle lenses.Exp Eye Res2006;83(2):322-328.
10 Li B, Luo X, Li T, Zheng C, Ji S, Ma Y, Zhang S, Zhou X.Effects of constant fickering light on refractive status, 5-HT and 5-HT2A receptor in guinea pigs.PLoS One2016;11(12):e0167902.
11 Schaeffel F, Burkhardt E, Howland HC, Williams RW.Measurement of refractive state and deprivation myopia in two strains of mice.Optom Vis Sci2004;81(2):99-110.
12 Caulfeld MP, Birdsall NJ.International Union of Pharmacology.XVII.Classification of muscarinic acetylcholine receptors.Pharmacol Rev1998;50(2):279-290.
13 Liu Q, Wu J, Wang X, Zeng J.Changes in muscarinic acetylcholine receptor expression in form deprivation myopia in guinea pigs.Mol Vis2007;13:1234-1244.
14 Wang F, Zhou J, Lu Y, Chu R.Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig.Cur Eye Res2011;36(2):103-111.
15 Stone RA, Sugimoto R, Gill AS, Liu J, Capehart C, Lindstrom JM.Effects of nicotinic antagonists on ocular growth and experimental myopia.Invest Ophthalmol Vis Sci2001;42(3):557-565.
16 Leech EM, Cottriall CL, McBrien NA.Pirenzepine prevents form deprivation myopia in a dose dependent manner.Ophthalmic Physiol Opt1995;15(5):351-356.
17 Guggenheim JA, Erichsen JT, Hocking PM, Wright NF, Black R.Similar genetic susceptibility to form-deprivation myopia in three strains of chicken.Vision Res2002;42(25):2747-2756.
18 Tejedor J, de la Villa P.Refractive changes induced by form deprivation in the mouse eye.Invest Ophthalmol Vis Sci2003;44(1):32-36.
19 Huang J, Hung LF, Smith EL 3rd.Effects of foveal ablation on the pattern of peripheral refractive errors in normal and form-deprived infant rhesus monkeys (macaca mulatta).Invest Ophthalmol Vis Sci2011;52(9): 6428-6434.
20 Wang F, Zhou J, Lu Y, Chu R.Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig.Curr Eye Res2011;36(2):103-111.
21 Schwahn HN, Schaeffel F.Flicker parameters are different for suppression of myopia and hyperopia.Vision Res1997;37(19):2661-2673.
22 Cohen Y, Belkin M, Yehezkel O, Avni I, Polat U.Light intensity modulates corneal power and refraction in the chick eye exposed to continuous light.Vision Res2008;48(21):2329-2335.
23 Backhouse S, Phillips JR.Effect of induced myopia on scleral myofbroblasts and in vivo ocular biomechanical compliance in the guinea pig.Invest Ophthalmol Vis Sci2010;51(12):6162-6171.
24 Lu F, Zhou X, Jiang L, Fu Y, Lai X, Xie R, Qu J.Axial myopia induced by hyperopic defocus in guinea pigs: a detailed assessment on susceptibility and recovery.Exp Eye Res2009;89(1):101-108.
25 Zenko D, Hislop JN.Regulation and trafficking of muscarinic acetylcholine receptors.Neuropharmacology2017;11(17):30526-30529.
26 Qu J, Zhou X, Xie R, Zhang L, Hu D, Li H, Lu F.The presence of M1 to M5 receptors in human sclera: evidence of the sclera as a potential site of action for muscarinic receptor antagonists.Curr Eye Res2006;31(7-8):587-597.
27 Wasselius J, Johansson K, Bruun A, Zucker C, Ehinger B.Correlations between cholinergic neurons and muscarinic M2 receptors in the rat retina.Neuroreport1998;9(8):1799-1802.
28 McKinnon LA, Nathanson NM.Tissue-specific regulation of muscarinic acetylcholine receptor expression during embryonic development.J Biol Chem1995;270(35):20636-20642.
29 Arumugam B, McBrien NA.Muscarinic antagonist control of myopia:evidence for M4 and M1 receptor-based pathways in the inhibition of experimentally-induced axial myopia in the tree shrew.Invest Ophthalmol Vis Sci2012; 53(9):5827-5837.
30 Yin GC, Gentle A, McBrien NA.Muscarinic antagonist control of myopia: a molecular search for the M1 receptor in chick.Mol Vis2004;10:787-793.
31 Ilien B, Glasser N, Clamme JP, Didier P, Piemont E, Chinnappan R,Daval SB, Galzi JL, Mely Y.Pirenzepine promotes the dimerization of muscarinic M1 receptors through a three-step binding process.J Biol Chem2009;284(29):19533-19543.
Citation:Tao Y, Li XL, Sun LY, Wei YH, Yu XT, Wang H.Effect of green fickering light on myopia development and expression of M1 muscarinic acetylcholine receptor in guinea pigs.Int J Ophthalmol2018;11(11):1755-1760
DOl:10.18240/ijo.2018.11.04
Accepted:2018-08-22
Received:2018-04-09
Correspondence to:Hong Wang.Department of Ophthalmology,Qilu Hospital of Shandong University, No.107, Wenhuaxi Road, Lixia District, Jinan 250001, Shandong Province, China.hongwang123456@yeah.net
Co-first authors:: Yuan Tao and Xiao-Li Li