Table 1 Patient baseline demographics and disease characteristics(safety population)n(%)

AMD: Age-related macular degeneration; BMI: Body mass index;ICGA: Indocyanine green angiography; PCV: Polypoidal choroidal vasculopathy; SD: Standard deviation.
San-Ni Chen1,2, Cheng-Kuo Cheng3, Ling Yeung4, Jiann-Torng Chen5, Wei-Chun Chan6, Jorn-Hon Liu7,Shwu-Jiuan Sheu8, Wen-Chuan Wu9, Chi-Chun Lai10
1Changhua Christian Hospital, Changhua, Taiwan 50094,China
2College of Medicine, Chung Shan Medical University,Taichung City, Taiwan 40246, China
3Shin Kong Wu Ho-Su Memorial Hospital, Shilin District,Taipei City, Taiwan 11101, China
4Chang Gung Memorial Hospital-Keelung, Anle District,Keelung City, Taiwan 204, China
5Tri-Service General Hospital, Chenggong Road, Taipei City,Taiwan 11490, China
6Mackay Memorial Hospital, Zhongshan District, Taipei City,Taiwan 813, China
7Cheng Hsin General Hospital, Beitou District, Taipei City,Taiwan 112, China
8Kaohsiung Veterans General Hospital, Zuoying District,Kaohsiung City, Taiwan 81362, China
9Kaohsiung Medical University Chung-Ho Memorial Hospital,Sanmin District, Kaohsiung City, Taiwan 807, China
10Chang Gung Memorial Hospital-Linkou, Guishan District,Taoyuan City, Taiwan 333, China
Abstract
● AlM:To assess the effectiveness and safety of ranibizumab 0.5 mg in Taiwanese patients with polypoidal choroidal vasculopathy (PCV) by performing a retrospective exploratory subgroup analysis of the REAL study.
● METHODS:REAL was a 12-month, observational,prospective, non-interventional phase lV post-marketing surveillance study conducted at 9 centers in Taiwan.The study collected data as part of the routine patient visits from the medical records of patients with neovascular agerelated macular degeneration treated with ranibizumab 0.5 mg according to local standard medical practice and local label and/or reimbursement guidelines.The presence of PCV at baseline was determined using indocyanine green angiography.
● RESULTS:At baseline, PCV was diagnosed in 64 of the 303 enrolled patients (21.1%).Of these, 41 patients(64.1%) had received prior treatment; 15 (23.4%)patients had received ranibizumab.The intent-to-treat population included 58 patients; 47 (80%) who received ranibizumab and 11 (20%) who received ranibizumab plus photodynamic therapy (PDT; 9 patients received once, 2 patients received twice).Bevacizumab was used as a concomitant medication in a similar percentage of patients who received ranibizumab (43%,n=20) or ranibizumab plus PDT (45%,n=5).ln patients who received ranibizumab, visual acuity (VA) at baseline was 50.1±12.9 Early Treatment Diabetic Retinopathy Study letters, and the gain at month 12 was 1.1±17.8 letters.ln patients who received ranibizumab plus PDT, VA at baseline was 51.4±15.9 letters, and there was a marked gain in VA at month 12 (14.0±9.2 letters,P=0.0009).ln the intent-totreat population, the reduction in central retinal subfield thickness from baseline at month 12 was 69.6±122.6 µm(baseline: 310.8±109.8 µm,P=0.0004).The safety results were consistent with the well-characterized safety profle of ranibizumab.
● CONCLUSlON:ln real-world settings, ranibizumab 0.5 mg treatment for 12mo results in maintenance of VA and reduction in central retinal subfeld thickness in Taiwanese patients with PCV.lmprovements in VA are observed in patients who received ranibizumab plus PDT.There are no new safety fndings.
● KEYWORDS:observational study; polypoidal choroidal vasculopathy; ranibizumab; Taiwan; visual acuity
Polypoidal choroidal vasculopathy (PCV), a subtype of neovascular age-related macular degeneration (nAMD),is an exudative maculopathy characterized by polypoidal subretinal vascular lesions associated with hemorrhagic detachments of the retinal pigment epithelium[1-3].The frequency of PCV diagnosis in patients with nAMD has increased with the advent of indocyanine green angiography(ICGA), which is considered the gold standard for PCV diagnosis[4-5].
The prevalence of PCV is reportedly higher among Asians compared with Caucasians, and ranges from 23% to 55%among Asians initially diagnosed with nAMD[6-8].In Taiwan,PCV has been reported in nearly 50% of patients with an initial diagnosis of nAMD, and the consequences of PCV appear to be worse among Taiwanese patients than in other ethnic groups[9-11].However, only limited studies have assessed the treatment patterns and outcomes for PCV in the Taiwanese population[9,11].Anti vascular endothelial growth factors (anti-VEGFs) are the standard of care for nAMD, while the optimal treatment for PCV is still under consideration, as verteporfn photodynamic therapy (vPDT), anti-VEGFs, or a combination of the two therapies are all under investigation[4-5].
REAL, a 12-month, prospective, observational multicenter study conducted in Taiwan, was the first study to assess the real-world outcomes of ranibizumab 0.5 mg treatment in Taiwanese patients with nAMD.The primary results from the study in nAMD patients are reported elsewhere[12].Here, we present the results from a retrospective exploratory analysis in the subgroup of Taiwanese patients with PCV.
REAL was an open-label, prospective, observational, noninterventional phase IV post-marketing surveillance study conducted from July 22, 2010, to April 02, 2013, at 9 centers in Taiwan.The study collected data as part of the routine patient visits from the medical records of patients treated with ranibizumab 0.5 mg according to local standard medical practice and local label and/or reimbursement guidelines in Taiwan.The observational period for each patient was 12mo after initiation of treatment with ranibizumab 0.5 mg.After enrolment, data were collected from the patients’ medical records on day 1 and at months 3, 6 [best-corrected visual acuity (BCVA) assessment only], and 12.Patients could voluntarily withdraw from the study for any reason at any time.The investigators could also discontinue study treatment for a given patient or withdraw the patient from study if they considered continuation to be detrimental to the patient’s well-being for any reason.The study adhered to the tenets of the Declaration of Helsinki, the International Conference on Harmonization and Good Clinical Practice guidelines.The protocol and amendments were approved by the independent ethics committee or institutional review board for each participating center.Patients provided written informed consent.The study included male or female Taiwanese patients with newly diagnosed or previously treated primary or recurrent subfoveal choroidal neovascularization (CNV) secondary to nAMD and a BCVA score between 73 and 20 letters (inclusive;approximately 20/40 to 20/400 Snellen equivalent) in the study eye.
Efficacy endpoints included the following parameters: mean change in BCVA and central retinal subfeld thickness (CSFT)from baseline to months 3, 6 (only BCVA), and 12; proportion of patients with an increase (gain of ≥5 letters), no change(change of ≤4 letters), or decrease (loss of ≥5 letters) in BCVA at months 3, 6, and 12; and presence of subretinal fuid (SRF),CNV, hemorrhage, hemorrhagic retinal pigment epithelial detachment (HRPED), pigment epithelial detachment (PED),lipids, SRF (apparent), and scar at baseline, month 3 and month 12.The BCVA measurements were performed in a sitting position using Early Treatment Diabetic Retinopathy Study (ETDRS) testing charts for visual acuity (VA) at a testing distance of 4 meters.CSFT and SRF were assessed usingoptical coherence tomography (OCT).The occurrence of CNV, its type and location at baseline, and regression of CNV lesions were assessed using fluorescein angiography(FA).Other parameters were assessed using color fundus photography.Safety endpoints included monitoring and recording of all adverse events (AEs) and serious AEs, and monitoring of intraocular pressure (IOP).The efficacy and safety results were summarized descriptively.
Patients who received at least one dose of ranibizumab during the observational period and had baseline and at least one postbaseline assessment of the effectiveness variable (BCVA) were included in the intent-to-treat (ITT) population.Patients who received at least one dose of ranibizumab and had at least one post-baseline safety assessment for the observational period were included in the safety population.The presence of PCV at baseline was diagnosed using ICGA.
Overall, 303 Taiwanese patients with nAMD were enrolled,of whom 228 (75.2%) completed the study, and 75 (24.8%)withdrew from the study.The mean age of the patients was 72.4y, and a higher proportion (65.6%) was male.At baseline,PCV was diagnosed in 64 (21.1%) patients of whom 48(75.0%) completed the study.The reasons for discontinuation were withdrawal of consent (n=15) and lost to follow-up(n=1).The mean±standard deviation (SD) age of the patients was 68.7±9.7y and a higher proportion (64.1%) were male(Table 1).ICGA was not performed in 83 (27.4%) patients.
Prior treatment was defned as medication for AMD in the 3mo prior to frst injection.Patients could have received more than one type of prior treatment for AMD during this period.
Table 1 Patient baseline demographics and disease characteristics(safety population)n(%)

AMD: Age-related macular degeneration; BMI: Body mass index;ICGA: Indocyanine green angiography; PCV: Polypoidal choroidal vasculopathy; SD: Standard deviation.
EfficacyThe ITT population included 58 patients with PCV.In these patients, the BCVA at baseline was 50.4±13.4 letters.The change in BCVA from baseline at month 3 was +2.7±12.4 letters (P=0.114), at month 6 was +2.4±17.1 letters (P=0.336),and at month 12 was +3.8±17.1 letters (P=0.131).
Of the 58 patients, 47 (80%) received only ranibizumab 0.5 mg and 11 (20%) received ranibizumab 0.5 mg plus PDT during the study.In the 47 patients who received only ranibizumab 0.5 mg, the BCVA at baseline was 50.1±12.9 letters and the change in BCVA from baseline at month 12 was +1.1±17.8 letters (P=0.714; Figure 1).Similar mean changes in BCVA from baseline were observed at months 3 and 6 (Figure 1).In the patients who received ranibizumab 0.5 mg plus PDT, the mean baseline BCVA was 51.4±15.9 letters and the change in BCVA from baseline at month 12 was +14.0±9.2 letters(P=0.0009; Figure 1).Improvements in mean BCVA were also observed at months 3 and 6 (Figure 1).
At month 12, the proportion of patients with a gain in BCVA of ≥5 letters [n=27 (57.5%)] was higher than those with a loss of ≥5 letters [n=12 (25.5%)]; 8 (17.0%) patients had no change in BCVA (Figure 2).
The CSFT at baseline was 310.8±109.8 µm, and the change from baseline at month 3 was -77.3±95.5 µm (P<0.0001) and at month 12 was -69.6±122.6 µm (P=0.0004).The proportion of patients with SRF in the study eye decreased from baseline(77.6%) to month 12 (19.0%,P<0.0001; Table 2).Similarly, at month 12, there was a decrease from baseline in the presence of hemorrhage (12.1%vs69.0%,P<0.0001), HRPED (3.5%vs25.9%,P=0.003), PED (22.4%vs55.2%,P=0.02), SRF apparent (13.8%vs72.4%,P<0.0001), and lipids (29.3%vs56.9%,P=0.013) but an increase in the presence of scar(39.7%vs19.0%,P=0.0005; Table 2).
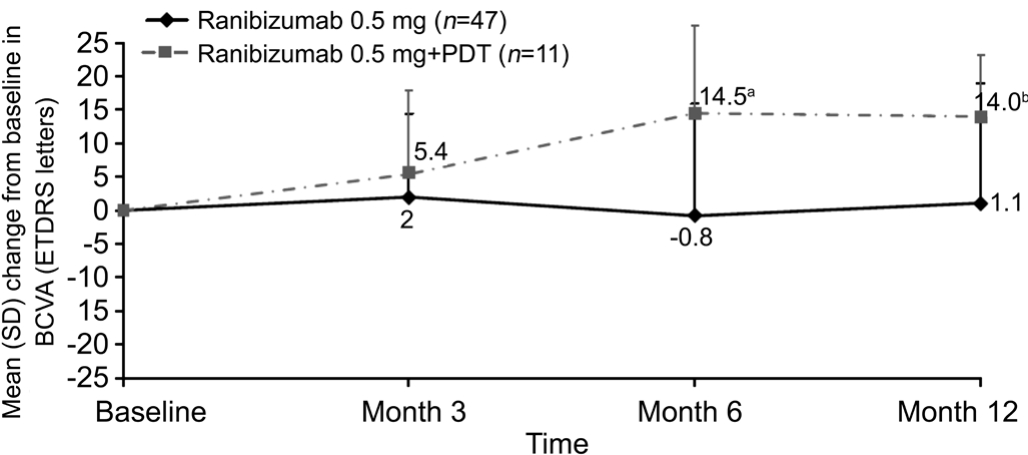
Figure 1 Mean change from baseline in BCVA in patients with PCV by treatment received (ITT population)
aP=0.007vsbaseline;bP=0.0009vsbaseline.

Figure 2 Proportion of patients with categorical changes in BCVA at months 3, 6 and 12 (ITT population).
At baseline, CNV was present in 51 (87.9%) patients; most had occult CNV, and most CNVs were located in the subfoveal region (Table 3).At month 12, complete regression of lesions was observed in 4 (6.9%) patients (Table 3).
Treatment ExposureThe number of ranibizumab 0.5 mg injections was 3.2±1.5; the exposure was similar in those who received additional PDT and those who did not (3.5±1.8 and 3.1±1.4 injections, respectively).The number of bevacizumab injections received as concomitant medication during the study was similar between the ranibizumab (1.1±1.8 injections, range 0-7) and ranibizumab plus PDT groups (0.5±0.5 injections,range 0-1).A similar percentage of patients in the ranibizumab and ranibizumab plus PDT groups received bevacizumab as concomitant medication during the study [43% (n=20/47) and 45% (n=5/11), respectively].Of the 5 patients who received bevacizumab in the ranibizumab plus PDT group, all received only 1 injection, and mostly on the same day that they received PDT.Of the 20 patients in the ranibizumab group who received bevacizumab, the number of injections ranged from 1 to 7 (mean±SD: 2.7±1.9).The difference in the number of bevacizumab injections shows that patients treated with ranibizumab plus PDT need fewer additional treatments than those treated with ranibizumab alone.Of the 11 patients who received PDT, two received PDT twice and the remaining nine received PDT once during the study.
SafetyAt least one AE was reported in 22 (34.4%) patients,and serious AEs were reported in 6 (9.4%) patients (Table 4).None of the serious AEs were considered by the investigator tobe related to ranibizumab.The most frequently reported ocular and non-ocular AEs (reported in ≥2% of patients) are shown in Table 4.All other ocular and non-ocular AEs were reported in one (1.6%) patient each.There were no reports of retinal hemorrhage.
Table 2 Categorical changes from baseline at months 3 and 12 in optical coherence tomography and color fundus photography parameters in the study eye (ITT population)n(%)

BCVA: Best-corrected visual acuity; HRPED: Hemorrhagic retinal pigment epithelial detachment; ITT: Intent-to-treat; NA/ND: Not assessed/not determined; PCV: Polypoidal choroidal vasculopathy;PED: Pigment epithelial detachment; SRF: Subretinal fluid.The ITT population included patients who received at least one dose of ranibizumab 0.5 mg during the observational period and had a baseline and at least one post-baseline BCVA assessment.
Chest pain and myositis were the treatment-related AEs reported, and both occurred in the same patient [n=1 (1.6%)].There were no deaths or discontinuations due to AEs.The IOP at baseline was 13.5±2.4 mm Hg.There was no significantmean change in IOP from baseline at months 3 (0.0±2.7 mm Hg,P=0.97) and 12 (0.6±2.5 mm Hg,P=0.20).
Table 3 Categorical changes from baseline at months 3 and 12 in fluorescein angiography parameters in the study eye (ITT population)n(%)
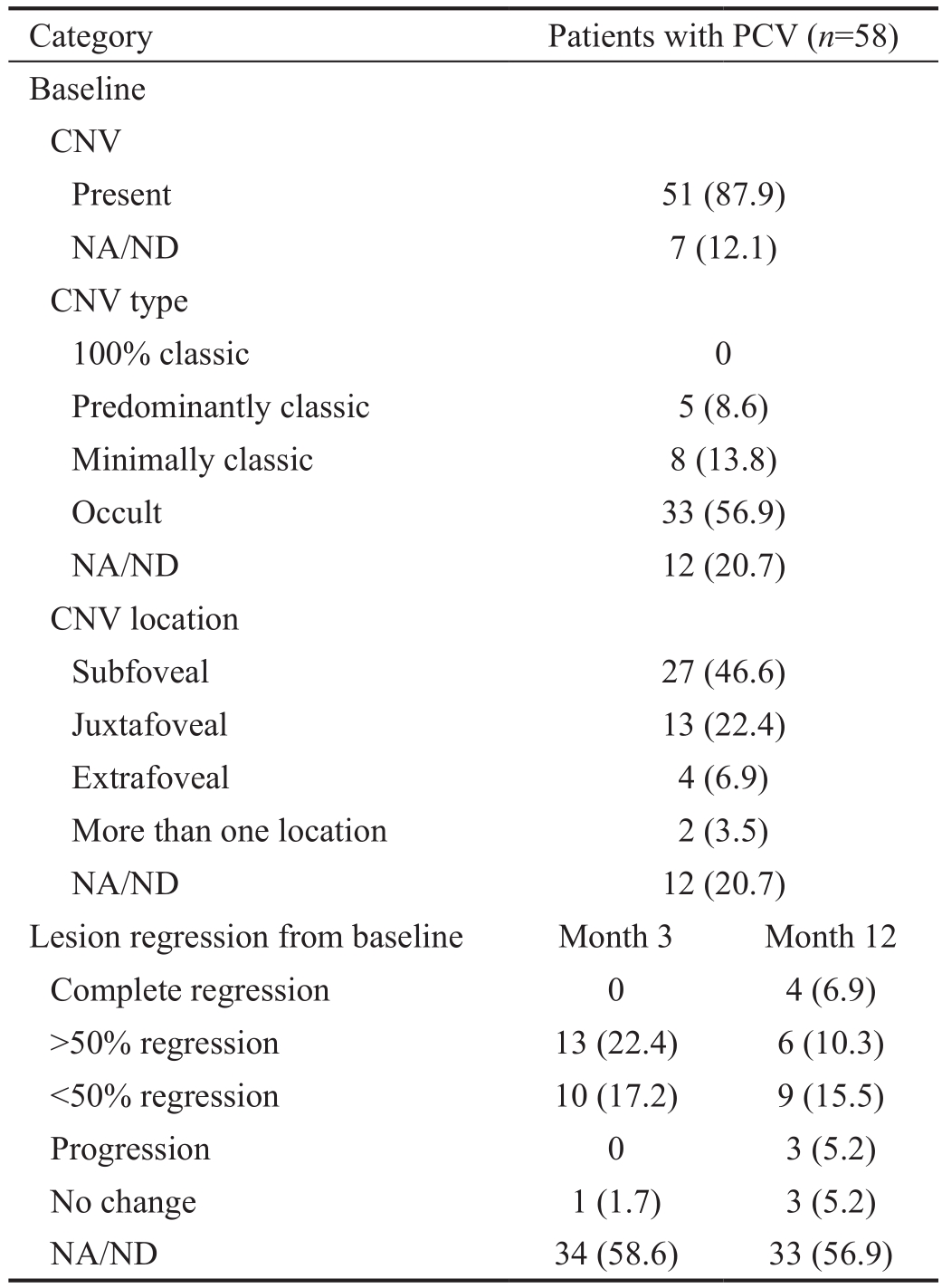
BCVA: Best-corrected visual acuity; CNV: Choroidal neovascularization; ITT: Intent-to-treat; NA/ND: Not assessed/not determined; PCV: Polypoidal choroidal vasculopathy.
REAL was the first study assessing the effectiveness of ranibizumab 0.5 mg in a real-life setting in Taiwanese patients with nAMD, and the study demonstrated that ranibizumab 0.5 mg maintained VA and delayed disease progression for up to 12mo.Consistent with these fndings, the exploratory analysis of the REAL study showed that ranibizumab 0.5 mg resulted in numerical increase in VA, reductions in the mean CSFT and a delay in disease progression at month 12 in patients with PCV.In the REAL study, of the 219 patients in whom ICGA was performed, PCV was diagnosed in 64 (29%) patients.Higher proportions have been reported in other real-life setting studies, but those studies had small sample sizes[9-10].Also,the diagnosis of PCV still remains a challenge, in spite of the use of ICGA for diagnosis, because of the possibility of PCV being misdiagnosed for conditions such as stage 1 retinal angiomatous proliferation or micro-aneurysms with similar presentation on ICGA[13], or under diagnosed if the polyps are masked by PED or hemorrhage at baseline.
Table 4 Incidence of adverse events during the study (safety population)n(%)

AE: Adverse event; PCV: Polypoidal choroidal vasculopathy.The safety population included patients who received at least one dose of ranibizumab 0.5 mg during the observational period and had at least one post-baseline safety assessment.The serious AEs of hypertensive heart disease and prostate cancer occurred in one and the same patient.Medical Dictionary for Regulatory Activities (MedDRA)version 15.0.
In our analysis, the mean age of patients diagnosed with PCV was lower (68.7y) than those of the overall population with nAMD (72.4y), and most patients (64.1%) were male.This fnding is consistent with previous studies in Asians, including Taiwanese patients, which show that PCV is generally diagnosed at an earlier age ranging between 60 to 70y than nAMD (≥70y), with predominance (71%) in males[5,10-11,14].
Most patients (64.1%) in this study had received prior treatment for AMD in the 3mo before the first ranibizumab 0.5 mg injection.In these mostly treatment-exposed patients,ranibizumab 0.5 mg treatment for 12mo maintained VA and decreased CSFT.These results are of relevance, as treatmentexposed patients generally do not show as much treatment gains as treatment-naive patients[15-16].Consistent with this observation, an interim analysis of LUMINOUS, a 5-year prospective observational global study showed that VA and CSFT gains at 1-year were better in treatment-naïve than treatment-exposed PCV patients[17].Similarly, visual and anatomic improvements with ranibizumab 0.5 mg treatment were observed in treatment-naive Japanese patients with PCV in the LAPTOP study[18-20].
In the REAL study, there were marked improvements in VA at month 12 in the subgroup of patients with PCV who received ranibizumab 0.5 mg plus PDT, suggesting additional benefits with combined ranibizumab plus PDT treatment.These findings are consistent with previous reports[21-23].Similar findings were observed at month 12 in the phase IV 24-month EVEREST II study (8.3vs5.1 ETDRS letters,P=0.013[24]; NCT01846273[25]).Complete regression of polyps was also higher with combination therapy versus ranibizumab monotherapy in EVEREST[22]and EVEREST II[24]studies;similar findings have been reported in other studies in Asian patients[21,23].These findings were also confirmed by a Metaanalysis that showed polyp regression and maintained or improved VA with the combination therapy compared with the respective monotherapies in patients with PCV[26].
The mean number of ranibizumab injections in this study was similar between those who received ranibizumab monotherapy(3.1) or combination therapy with PDT (3.5), which was lower than those reported in other observational studies in Asian patients (range, 4.0-4.5) where VA improvements were observed over 12mo[23,27-28].However, as the number of injections administered in the REAL study was driven by reimbursement decisions, the study did not answer if ranibizumab 0.5 mg plus vPDT would have been associated with a reduction in ranibizumab injection, compared with ranibizumab monotherapy, as observed at month 6 in EVEREST[22]and at month 12 in the EVEREST II study[24],considering the time frame this study observed.Further studies are required on the optimal treatment options as well as the optimal treatment intervals for PCV[5,29].
Results from randomized clinical studies, in which patients are regularly monitored and access to anti-VEGF agents is not a limiting factor, show that VA gains are higher than those in real-world studies.In the phase IV DRAGON study that assessed ranibizumab monotherapy in Chinese patients with nAMD for up to 2y, in the subset of patients with PCV, notable VA gains of 12.7/9.4 (baseline, 54.1/54.6) ETDRS letters at the end of 1y and 12.3/9.7 ETDRS letters at the end of 2y were observed in monthly/PRN group, respectively; the mean number of injections was 11.2/8.4 and 4.9/6.0 at 1 and 2y respectively[30].Similarly, in the phase IIIb/IV PLANET study,afibercept monotherapy for 1-year resulted in BCVA gains of 10.7 ETDRS letters with a mean 8.1 injections[31].
The strength of this exploratory analysis of the REAL study is that it was the first to provide real-life data in Taiwanese patients with PCV, thereby addressing to some extent the gap of limited information in such populations.It also provides further evidence regarding the need for accurate diagnosis and appropriate treatment of PCV.The analysis was limited by the short duration and observational nature of the study,lack of a control group, exploratory nature of the analysis,and the limited sample size.In addition, because the study was observational, ICGA was investigator-graded and there was no protocol-specified criterion; the machines used for assessments were not standardized and were those available at the participating sites as part of routine clinical practice;and the exact timing and fuence of PDT was not collected per protocol.
To conclude, in this frst real-life study of ranibizumab 0.5 mg in Taiwanese patients, ranibizumab 0.5 mg treatment for 12mo maintained VA and decreased CSFT in patients with PCV,consistent with the fndings observed in the overall population with nAMD.Improvement in VA was observed in patients with PCV who received PDT in addition to ranibizumab 0.5 mg.There were no new safety fndings.These real-world fndings confirm the benefits of ranibizumab across all patients with nAMD, including those with PCV, and add to the evidence on treatment of PCV in Taiwanese patients.
The authors thank Lakshmi Venkatram an (Scientifc Services Practice–Product Lifecycle Services, Novartis Healthcare Pvt.Ltd., Hyderabad, India) for medical writing and editorial assistance.
Previous Presentation:Data from the analysis was previously presented at the 11thAsia-Pacifc Vitreo-retina Society (APVRS)Congress, 8-10 December 2017 Kuala Lampur, Malaysia.
Foundation:Supported by Novartis (Taiwan) Co., Ltd.
Conflicts of Interest:Chen SN receives grants from Novartis and Bayer; Cheng CK, None; Yeung L, None; Chen JT,None; Chan WC, None; Liu JH, None; Sheu SJ is principal investigator for clinical studies sponsored by Novartis, Bayer,Alcon and Allergan; Wu WC, None; Lai CC receives grants from Novartis.
REFERENCES
1 Yannuzzi LA, Sorenson J, Spaide RF, Lipson B.Idiopathic polypoidal choroidal vasculopathy (IPCV).Retina1990;10(1):1-8.
2 Honda S, Matsumiya W, Negi A.Polypoidal choroidal vasculopathy:clinical features and genetic predisposition.Ophthalmologica2014;231(2):59-74.
3 Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E,Schlingemann R, Eldem B, Monés J, Richard G, Bandello F, European Society of Retina Specialists.Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA).Br J Ophthalmol2014;98(9):1144-1167.
4 Koh AH, Chen LJ, Chen SJ,et al.Polypoidal choroidal vasculopathy:evidence-based guidelines for clinical diagnosis and treatment.Retina2013;33(4):686-716.
5 Wong CW, Wong TY, Cheung CM.Polypoidal choroidal vasculopathy in Asians.J Clin Med2015;4(5):782-821.
6 Imamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA.Polypoidal choroidal vasculopathy: a review.Surv Ophthalmol2010;55(6):501-515.
7 Maruko I, Iida T, Saito M, Nagayama D, Saito K.Clinical characteristics of exudative age-related macular degeneration in Japanese patients.Am J Ophthalmol2007;144(1):15-22.
8 Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T, Nishikawa M, Mitsuma Y, Yamazaki Y, Matsumura M, Uyama M.Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics.Arch Ophthalmol2003;121(10):1392-1396.
9 Chang YC, Wu WC.Polypoidal choroidal vasculopathy in Taiwanese patients.Ophthalmic Surg Lasers Imaging2009;40(6):576-581.
10 Chen PJ, Chen SN.Clinical characteristics of exudative age-related macular degeneration in Taiwan.Taiwan J Ophthalmol2012;2(4):127-130.
11 Yeung L, Chen SN.Polypoidal choroidal vasculopathy in Taiwan.Chang Gung Med J2004;27(5):366-372.
12 Chen S, Lai CC, Cheng CK, Yeung L, Liu JH, Chen JT, Sheu SJ, Chan WC, Wu WC.Real-world outcomes of ranibizumab treatment in Taiwanese patients with neovascular age-related macular degeneration: 12-month results from the REAL study.2017 Euretina, Sep 08, Free Paper Session 9 AMD III.
13 Tan CS, Ngo WK, Lim LW, Tan NW, Lim TH; EVEREST Study Group.EVEREST study report 3: diagnostic challenges of polypoidal choroidal vasculopathy.Lessons learnt from screening failures in the EVEREST study.Graefes Arch Clin Exp Ophthalmol2016;254(10):1923-1930.
14 Kwok AK, Lai TY, Chan CW, Neoh EL, Lam DS.Polypoidal choroidal vasculopathy in Chinese patients.Br J Ophthalmol2002;86(8):892-897.
15 Singh RP, Fu EX, Smith SD, Williams DR, Kaiser PK.Predictive factors of visual and anatomical outcome after intravitreal bevacizumab treatment of neovascular age-related macular degeneration: an optical coherence tomography study.Br J Ophthalmol2009;93(10):1353-1358.
16 Levy J, Shneck M, Rosen S, Klemperer I, Rand D, Weinstein O,Pitchkhadze A, Belfair N, Lifshitz T.Intravitreal bevacizumab (avastin)for subfoveal neovascular age-related macular degeneration.Int Ophthalmol2009;29(5):349-357.
17 Lai T, Lacey S, Margaron P.Baseline characteristics and 1-year outcomes of patients with polypoidal choroidal vasculopathy: results from the third interim analysis of the LUMINOUS study.Available at http://2016.apvrs.org/wp-content/uploads/2016/11/APVRS-2016_finalprogram.pdf.page 90 Accessed on October 10, 2018.
18 Oishi A, Kojima H, Mandai M, Honda S, Matsuoka T, Oh H, Kita M, Nagai T, Fujihara M, Bessho N, Uenishi M, Kurimoto Y, Negi A.Comparison of the effect of ranibizumab and verteporfn for polypoidal choroidal vasculopathy: 12-month LAPTOP study results.Am J Ophthalmol2013;156(4):644-651.
19 Oishi A, Miyamoto N, Mandai M, Honda S, Matsuoka T, Oh H, Kita M,Nagai T, Bessho N, Uenishi M, Kurimoto Y, Negi A.LAPTOP study: a 24-month trial of verteporfn versus ranibizumab for polypoidal choroidal vasculopathy.Ophthalmology2014;121(5):1151-1152.
20 Hikichi T.Individualized ranibizumab therapy strategies in year 3 after as-needed treatment for polypoidal choroidal vasculopathy.BMC Ophthalmol2015;15(1):37.
21 Gomi F, Oshima Y, Mori R, Kano M, Saito M, Yamashita A, Iwata E,Maruko R, Fujisan Study G.Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: The Fujisan Study.Retina2015;35(8):1569-1576.
22 Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S,Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH.EVEREST study:effcacy and safety of verteporfn photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy.Retina2012;32(8):1453-1464.
23 Lai TY, Lee GK, Luk FO, Lam DS.Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy.Retina2011;31(8):1581-1588.
24 Koh A, Lai TYY, Takahashi K, Wong TY, Chen LJ, Ruamviboonsuk P, Tan CS, Feller C, Margaron P, Lim TH, Lee WK; EVEREST II Study Group.Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial.JAMA Ophthalmol2017;135(11):1206-1213.
25 ClinicalTrials.gov.Visual outcome in patients with symptomatic macular PCV treated with either ranibizumab as monotherapy or combined with verteporfin photodynamic therapy.(EVEREST II,NCT01846273).https://clinicaltrials.gov/ct2/show/NCT01846273.2015 Accessed on 18 June 2015.
26 Tang K, Si JK, Guo DD, Cui Y, Du YX, Pan XM, Bi HS.Ranibizumab alone or in combination with photodynamic therapy vs photodynamic therapy for polypoidal choroidal vasculopathy: a systematic review and Meta-analysis.Int J Ophthalmol2015;8(5):1056-1066.
27 Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, Ohtsuka H, Ariga H.One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients.Am J Ophthalmol2012;154(1):117-124 e1.
28 Song MH, Ryu HW, Roh YJ.One-year results of intravitreal ranibizumab with or without photodynamic therapy for polypoidal choroidal vasculopathy.Ophthalmologica2011;226(3):119-126.
29 Wang W, He M, Zhang X.Combined intravitreal anti-VEGF and photodynamic therapy versus photodynamic monotherapy for polypoidal choroidal vasculopathy: a systematic review and meta-analysis of comparative studies.PLoS One2014;9(10):e110667.
30 Li X, Chang L, Weisberger A, Xu X, Zhu A.Ranibizumab 0.5 mg in Chinese patients with polypoidal choroidal vasculopathy: results of the 2-year DRAGON study.Available at http://2016.apvrs.org/wp-content/uploads/2016/11/APVRS-2016_fnal-program.pdf.page 97 Accessed on October 10, 2018.
31 Lee WK, Iida T, Ogura Y, Chen SJ, Wong TY, Mitchell P, Cheung GCM, Zhang Z, Leal S, Ishibashi T, Planet Investigators.Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial.JAMA Ophthalmol2018;136:786-793.
Citation:Chen SN, Cheng CK, Yeung L, Chen JT, Chan WC, Liu JH, Sheu SJ, Wu WC, Lai CC.One-year real-world outcomes of ranibizumab 0.5 mg treatment in Taiwanese patients with polypoidal choroidal vasculopathy: a subgroup analysis of the REAL study.Int J Ophthalmol2018;11(11):1802-1808
DOl:10.18240/ijo.2018.11.11
Accepted:2018-08-01
Received:2017-12-15
Correspondence to:Chi-Chun Lai.Chang Gung Memorial Hospital-Linkou, Guishan District, Taoyuan City, Taiwan 333,China.chichun.lai@gmail.com