Clinical features of posterior microphthalmic and nanophthalmic eyes
Jing-Jing Liu, Yi-Ye Chen, Xiang Zhang, Pei-Quan Zhao
Department of Ophthalmology, Xin Hua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China
Abstract
● AlM:To clinically differentiate nanophthalmos (NO)and posterior microphthalmos (PM) and to explore the mechanisms related to papillomacular folds (PMF).
● METHODS:Medical records of 34 unrelated patients with microphthalmos (54 eyes) from April 2009 to October 2017 were retrospectively reviewed.
● RESULTS:Fourteen eyes of 7 unrelated patients with NO and PM were included in the study.The presenting age of the NO cohort was signifcantly higher compared with the PM cohort (NO: 27±16y; PM: 3.7±0.6y).PMF was more likely to occur in cases with PM than in NO (25% in NO,100% in PM).The anatomic features of PMF from optical coherence tomography (OCT) included: ganglion cell layer,inner plexiform layer, inner nuclear layer, outer plexiform layer and outer nuclear layer.ln eyes without an apparent PMF (these were all NO eyes), rudimentary fovea without a foveal pit was noted.Four eyes that were NO developed angle closure glaucoma.Three NO eyes developed exudative retinal detachment and were successfully treated with lamellar sclerectomy.
● CONCLUSlON:Posterior segment changes are pervasive both in PM and NO.Complications like angle closure glaucoma and exudative retinal detachment are likely to occur in eyes with NO but not with PM.Detailed OCT analysis found that PMF was partially a neural retinal issue, suggesting that redundancy of retinal issues involved only inner retinal layers.
● KEYWORDS:nanophthalmos; non-rhegmatogenous retinal detachment; optical coherence tomography; papillomacular folds; posterior microphthalmos; rudimentary fovea
INTRODUCTION
M icrophthalmos is a developmental arrest of ocular growth, defned as eyes with a total axial length (TAL)at least two standard deviations shorter than the mean axial length of a normal control age group[1].Study showed genetic factors are related to the development of microphthalmos[2].
The clinical spectrum of microphthalmos includes a heterogenous group of conditions[3]including nanophthalmos (NO) and posterior microphthalmos (PM), both rare conditions with decreased TAL and high hyperopia without additional malformations[4].While PM primarily affects the posterior segment, NO is microphthalmos with a small-sized anterior segment[5].A retinal papillomacular fold (PMF) in the posterior segment is associated with PM and NO[6-9].PMF formation was speculated to be due to the redundancy of retinal tissue as a result of the disparity between the normal growth of the retina and the halted growth of the sclera[10-11].A thickened sclera consisting of abnormal deposits of glycosaminoglycans and elevated levels of fbronectin[12-14]is also associated with PM and NO.Recessive mutations in the membrane-type frizzled-related protein and the serine protease PRSS56 have been found to cause both PM and NO[15-18].The extent of overlapping of phenotype and genotype in PM and NO make it diffcult to differentiated them in clinical practice[15-18].
In the current study, we documented various features and clinical management of NO and PM to better understand and differentiate these rare conditions and their prognosis.The mechanisms of PMF formation was studied using optical coherence tomography (OCT).
SUBJECTS AND METHODS
The medical records of 34 patients with microphthalmos (54 eyes) from April 2009 to October 2017 were retrospectively reviewed.The inclusion criteria of this study included an axial length of <20 mm, high hyperopia >+7.00 D sphere and no other ocular or systemic abnormalities such as congenital cataract, anterior synechiae, coloboma of iris, retina, choroid and optic disc.Patients who were too young to cooperate with OCT examination were excluded.All the included subjects underwent a full clinical evaluation and a complete ophthalmologic examination including: best-corrected visual acuity (BCVA), intraocular pressure measurement, cycloplegic refraction, axial length determination, slit-lamp biomicroscopy,A-mode and B-mode ultrasonographic examination, dilatedfundus photography and OCT (RTVue-100, Optovue Inc, Fremont, CA, USA).The OCT scan modes included radial lines (12×9 mm2), horizontal lines (12×9 mm2).The corneal diameter was also measured in cooperative patients using Lenstar examination (Lenstar LS900; Haag-Streit International, New Orleans, Louisiana, USA).Foveal retinal thickness (FRT) was defined as the distance between retinal pigment epithelium/Bruch membrane (RPE/BM) complex hyper-reflective band and the apex of PMF on the vertical OCT scan.In eyes without a PMF, FRT was defined as the distance of the vertical line though the apex of the bunched out nuclear layer from retinal surface to RPE/BM complex hyperrefective band on the vertical OCT scan.Lamellar sclerectomy was performed in eyes presented with retinal detachment by a single experienced surgeon (Zhao PQ).
Table 1 Demographics and clinical parameters of patients

NO: Nanophthalmos; PM: Posterior microphthalmos; D: Diopters; BCVA: Best-corrected visual acuity; NLP: No light perception; HM: Hand motion; PMF: Papillomacular fold; OCT: Optical coherence tomography; RPE: Retinal pigment epithelium; FRT: Foveal retinal thickness.
Patient code/diagnosis Gender Age(y)Axial length(mm)Spherical quivalent BCVA Fundus fndings and complications 1/NO Female 33 OD 15.61 OS 15.63 OD +13.00 D OS +13.00 D OD 20/40 OS HM Bilateral angle closure glaucoma; PMF in both eyes; exudative retinal detachment in left eye; FRT: OD 562 mm; OS 329 mm.2/NO Female 5 OD 14.00 OS 14.23 OD +18.00 D OS +19.50 D OD 20/80 OS 20/80 Bilateral crowded optic disc and vascular tortuosity; absence of foveal pit in both eyes; FRT: OD 447 mm; OS 462 mm 3/NO Male 41 OD 16.33 OS 16.10 OD +14.00 D OS +14.00 D OD 20/125 OS 20/125 Bilateral crowded optic disc, tortuous retinal vessels, thickened optic nerve layers and delicate chorioretinal folds; exudative retinal detachment in both eyes; absence of foveal pit in both eyes; FRT: OD 296 mm; OS 253 mm 4/NO Male 30 OD 15.59 OS 15.66 OD +14.00 D OS +14.00 D OD NLP OS 20/63 Bilateral pale and cupped optic disc, angle closure glaucoma; absence of foveal pit in both eyes; FRT: OD 268 mm; OS 279 mm 5/PM Male 4 OD 16.20 OS 15.40 OD +16.00 D OS +15.25 D OD 20/200 OS 20/200 Bilateral tortuous retinal vessels and crowded cupless optic discs; PMF in both eyes with evident intraretinal cavities in left eye; FRT: OD 596 mm;OS 635 mm 6/PM Female 4 OD 15.00 OS 15.00 OD +18.25 D OS +18.50 D OD 20/125 OS 20/160 Bilateral PMF; FRT: OD 506 mm; OS 538 mm 7/PM Female 3 OD 16.00 OS 16.40 OD +8.25 D OS +8.00 D OD 20/63 OS 20/63 Bilateral tortuous retinal vessels, crowded optic disks and PMF; FRT: OD 532 mm; OS 508 mm
Patients were diagnosed with PM if they had a normalappearing anterior segment with a horizontal corneal diameter≥11 mm or NO if the horizontal corneal diameter was <11 mm.BCVA, recorded as decimal visual acuity, was converted to Snellen acuity and to logarithm of minimal angle of resolution(logMAR) value for statistics.SPSS software, version 22.0(SPSS, Inc, Chicago, IL, USA) was used for all statistical analyses.All continuous variables were represented as the mean and standard deviation.The Student’st-test was used to test the difference of independent samples.The binary variables were compared using the Chi-square test or Fisher’s exact test.APvalue of <0.05 was considered statistically signifcant.
The study was approved by the Ethics Committee of Xin Hua Hospital Affliated to Shanghai Jiao Tong University School of Medicine and adhered to the tenets of the Declaration of Helsinki.Informed consent was obtained from the patients or their legal guardians.
RESULTS
Seven patients (14 eyes) were included in this study.There were 3 male and 4 female patients and all were sporadic cases without a consanguineous relationship.Demographics and clinical parameters of patients are summarized in Table 1.
The presenting age of the NO cohort was signifcantly higher and with a larger range compared with the PM cohort (NO:27±16y, range: 5-41y; PM: 3.7±0.6y, range: 3-4y).All of the affected eyes showed compromised visual acuity (no light perception to 20/40), high hyperopia (+8.00 to +19.50 D),decreased TAL (14.00 to 16.40 mm) and increased FRT (253 to 635 μm).Fundus photos revealed PMF in 3 eyes that was confrmed by OCT.OCT also showed 5 eyes had retinal folds not apparent from fundus examinations.The anatomic contents of PMF consisted of a thickened ganglion cell layer (GCL),inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL) and a highly bunched up outer nuclear layer (ONL) on OCT imaging (Figures 1-4).The external limiting membrane (ELM), ellipsoid zone layer (EZL) and RPE/Bruch’s complex (RBC) were found to be normal.In eyes without PMF, rudimentary fovea with increased thickness were noted.Four eyes that were NO developed angle closure glaucoma.Three eyes that were NO developed exudative retinal detachment and were successfully treated with lamellar sclerectomy.Clinical parameters of eyes with and without PMF are listed in Table 2.FRT was significantly lower in patients with a fat macula compared with patients with PMF.Patient demographics and clinical parameters of patients with NO and microphthalmos are listed in Table 3.FRT and the number of eyes with an absence of foveal depression were signifcantly higher in patients with NO compared with patients with PM (Table 3).The number of eyes with macular folds were signifcantly lower in patients with NO compared with patients with PM (Table 3).

Figure 1 Multimodality imaging of patient 1 with NO A-C: Vertical spectral-domain optical coherence tomography (SD-OCT) scans of the left eye; A: SD-OCT demonstrated macular involved retinal detachment and intraretinal edema in the left eye.Note the increased visibility of the vitreous cavity and (B) retinal reattachment after lamellar sclerotomy.Intraretinal edema remained.Note the retinal fold consisted only of partial neural retina with apical surface corrugations; C: SD-OCT showed the resolution of the retinal fold in the left eye at the latest follow-up; D:Fundus photographs showed a horizontal PMF (arrow) in the right eye; E: SD-OCT revealed a retinal fold with apical surface corrugations in the right eye.Note the bunched up ONL; F, G: Ultra-wide feld scanning laser ophthalmoscopy (UWF SLO) of patient 1’s eyes at the latest followup showed attached retina and clearly visible ciliary processes on the nasal side.

Figure 2 UWF SLO of patient 3 A: A cupless optic disk, macular wrinkles, vascular tortuositas, peripheral retinal hemorrhage (yellow arrow), retinal detachment on the nasal and inferior side (empty arrow head), discrete round white pigment lesions (solid arrow head) in the right eye, note the bilateral increased refex of the optic nerve fibers; B: Cupless optic disk, tortuous vessels, peri-palillary hemorrhage (yellow arrow), white pigment lesions (solid arrow head)and chorioretinal folds (empty arrow) in left eye.Note the bilateral increased refex of the optic nerve fbers.
Table 2 Clinical parameters of eyes with and without PMF

aP<0.05.PMF: Papillomacular folds; TAL: Total axial length; FRT:Foveal retinal thickness.
Parameters PMF Flat maculaPRefraction (D) 14±4 16±2 0.357 TAL (mm) 15.7±0.5 15±1 0.419 FRT (μm) 526±91 334±94 0.002aNo.of eyes 8 6
DISCUSSION
In our study, all eyes with NO and PM had compromised visual acuity, high hyperopia (+8.00 to +19.50 D), decreased TAL (14.00 to 16.40 mm) and increased FRT (253 to 635 μm)compared with normative ocular parameters[19].The presenting age of the NO cohort was signifcantly higher and with a larger range compared with the PM cohort, a fnding similar to that of another study[20].However, TAL and high hyperopia were not statistically different in patients with NO and PM.
In the current study, PMF with a higher FRT was more likely to occur in cases with PM (25% in NO, 100% in PM).It has been hypothesized that PMF formation is due to the redundancy of retinal tissue compared with small-sized eyeballs[6].However,we found that TAL in 2 groups was not signifcantly different which implies that there may be another explanation other than the disparity of retinal tissue and sclera.OCT was superior in finding small PMF that were difficult to find in fundus photographs.The PMF involved GCL, IPL, INL, OPL and ONL.This is different from the normal structure of the fovea which is only comprised of ONL.An abnormal or rudimentary fovea was part of the reason for poor vision[21].It has been speculated that PMF formation is due to a thickened sclera that impedes the development of the choroid and the RPE but does not influence the growth of the neurosensory retina, thereby causing it to fold[22].We believed this contributed to PMF formation because scleral thickening was observed in all of our patients and PMF was located only in the neural retina without involvement of the RPE or choroid.However, as part of the neural retina, ELM and ellipsoid are not involved in the fold.In addition, signifcantly increasing thickness of inner retinal layers in the foveal region were also found.So the redundancy of retinal tissue and poor differentiation of the macula may contribute to PMF formation.The left eye of patient 1 after 2 surgical procedures for retinal detachment showed resolution of PMF and disturbance of ELM, IS/OS and ONL.This may be related to the degeneration of retinal layers after the long duration of retinal detachment.Intraretinal cystlike cavities located in the INL were seen in 25% of the eyes with PMF.In eyes without an apparent PMF (these were all NO eyes),abnormal macula without a foveal pit was observed.GCL, IPL,INL and OPL were also seen in the foveal area, so the fovea was thicker compared with normal eyes.
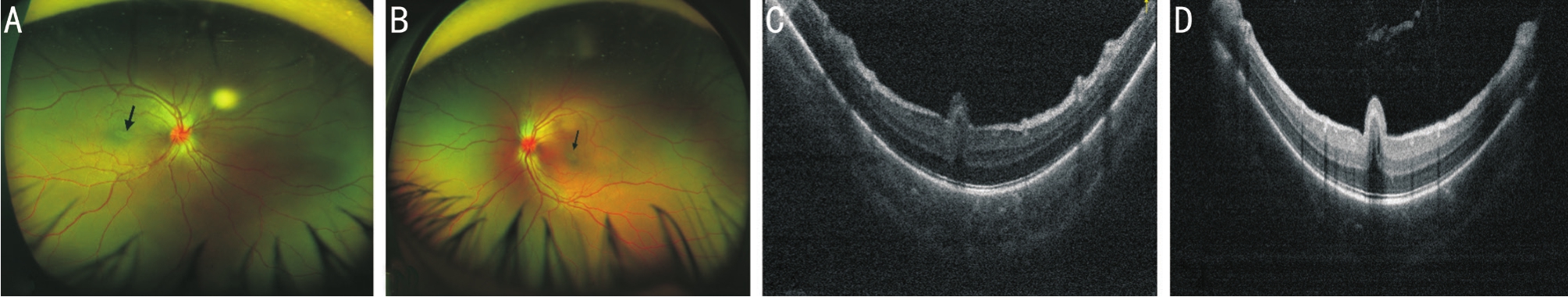
Figure 3 SD-OCT and UWF SLO of patient 5’s eyes A, B: UWF SLO showed bilateral crowed and congestive optic disc, tortuous vessels,and elevated papillomacular folding (arrow) in both eyes; C, D: SD-OCT of the macular region revealed bilateral retinal folds with smooth apical surface.Inner retinal layer cyst cavities were noted in the left eye (D) and a vitreous cavity was found with high refection which may be attributed to the condensed content.

Figure 4 SD-OCT and UWF SLO of patient 7’s eyes A, B: UWF SLO showed bilateral crowded optic disk and tortuous vessels.No apparent PAF were found; C, D: SD-OCT images show bilateral retinal folding with apical surface corrugations in both eyes.
Table 3 Patient demographics and clinical parameters of patients with NO and microphthalmos

aP<0.05.NO: Nanophthalmos; BCVA: Best-corrected visual acuity; FRT: Foveal retinal thickness.
Parameters NO (range) Microphthalmos (range)PNo.of patients (2 eyes each) 4 3 Age (y) 27±16 (5 to 41) 3.7±0.6 (3 to 4) 0.05aGender (% male) 100 50 0.629 BCVA (logMAR) 0.86±0.65 (2.3 to 0.4) 0.78±0.23 (0.5 to1.0) 0.798 TAL (mm) 15.4±0.8 (14.00 to 16.33) 15.7±0.6 (15.00 to 16.40) 0.514 Refraction (D) 15±2 (13.00 to 19.50) 14±5 (8.00 to 18.50) 0.686 FRT (μm) 362±114 (253 to 562) 552±52 (506 to 635) 0.002aGlaucoma (No.of eyes) 4 0 0.085 Virtuous vessels (No.of eyes) 4 4 0.627 Macular folds (No.of eyes) 2 6 0.01aAbsence of foveal depression (No.of eyes) 6 0 0.01aCrowded optic disk (No.of eyes) 4 4 0.627 Retinal detachment (No.of eyes) 3 0 0.209 End-stage glaucoma (No.of eyes) 2 0 0.473
We also found that complications such as angle closure glaucoma and exudative retinal detachment were more likely to occur in NO cases.Exudative retinal detachment was found in 3 nanophthlamic eyes and increased resistance to both protein movement and venous outfow through the abnormal sclera was suggested as the main cause, so we performed lamellar sclerectomy in these eyes.In their latest follow up,the retina was attached.All of these were cases were adults supporting the previous findings of the reduced permeability of the sclera with advancing age[23].Besides, angle closure glaucoma and choroidal folds were also found in our study.All of the individuals presented with these features were adult NO cases.It has been suggested that NO eyes have thicker lenses and a high lens/eye volume ratio, which may cause a higher uveal effusion risk[20].In addition, abnormal thickened sclera which can cause angle closure glaucoma and choroidal folding were found in every patient.Though no complications were found in the PM cohort, it is important to notice that in this study, patients in PM cohort were significantly younger compared with the NO group.Complications may not develop until late in their life, so the importance of a regular follow up should be noted.
We found crowded optic discs and virtuous retinal vessels were the most frequently found fundus features in both NO and PM cohorts.The formation of a crowded optic disc may be due to the dense arrangement of the optic nerve fibers into a small scleral canal in small eyes[24].Patient 5 with a hyperemic and crowded optic discs, was misdiagnosed as papillitis.Patient 3,with bilateral late-phase angle closure glaucoma due to uveal effusion syndrome, had cupped optic disks.This suggests that ocular structure changes due to complications.Therefore, it is important to perform a detailed ophthalmologic evaluation and provide a close follow-up.
The limitations of our study include the small sample and short duration of follow-up.In addition, some biometric data were not available because of the poor cooperation of the pediatric patients.
In conclusion, eyes with NO and PM have poor vision due to the high refractive amblyopia and structural macular changes.In our study, patients with PM were younger compared with patients with NO, and PM was unrecognized frequently due to these eyes presented with normal anterior segment dimensions[4-5].Therefore, careful examination at presentation and appropriate ancillary tests are required for the diagnosis of PM.In addition to the previous hypothesis that PMF was due to the redundancy of retinal tissue as a result of a disparity between the normal growth of the retina and the halted growth of the sclera, we proposed that the PMF and fattened macula in PM and NO may also develop as a result of a poorly differentiated macula because the presence of inner retinal layers in this area.Complications such as angle closure glaucoma and exudative retinal detachment occur mainly in NO compared with PM.Therefore, close follow-up should be scheduled for early detection of for exudative retinal detachment and angle closure glaucoma.Scleral surgery may be useful in attaching the retina in these eyes.
ACKNOWLEDGEMENTS
We sincerely thank all the patients and their families for their participation.
Authors’ contributions:Design and conduct of the study (Liu JJ, Zhao PQ); collection, analysis and interpretation of data(Liu JJ, Chen YY, Zhang X); preparation of manuscript (Liu JJ);critical review and fnal approval of the manuscript (Zhao PQ).
Conflicts of Interest:Liu JJ, None; Chen YY, None; Zhang X, None; Zhao PQ, None.
REFERENCES
1 Elder MJ.Aetiology of severe visual impairment and blindness in microphthalmos.Br J Ophthalmol1994;78(5):332-334.
2 Patel N, Khan AO, Alsahli S,et al.Genetic investigation of 93 families with microphthalmia or posterior microphthalmos.Clin Genet2018;93(6):1210-1222.
3 Zheng T, Chen Z, Xu J, Tang Y, Fan Q, Lu Y.Outcomes and prognostic factors of cataract surgery in adult extreme microphthalmos with axial length <18 mm or corneal diameter <8 mm.Am J Ophthalmol2017;184:84-96.
4 Khan AO.Posterior microphthalmos versus nanophthalmos.Ophthalmic Genet2008;29(4):189.
5 Khan AO.Recognizing posterior microphthalmos.Ophthalmology2006;113(4):718.
6 Serrano JC, Hodgkins PR, Taylor DS, Gole GA, Kriss A.The nanophthalmic macula.Br J Ophthalmol1998;82(3):276-279.
7 Nowilaty SR, Mousa A, Ghazi NG.The posterior pole and papillomacular fold in posterior microphthalmos: novel spectral-domain optical coherence tomography findings.Ophthalmology2013;120(8):1656-1664.
8 Park SH, Ahn YJ, Shin SY, Lee YC.Clinical features of posterior microphthalmos associated with papillomacular fold and high hyperopia.Clin Exp Optom2016;99(6):590-593.
9 Tekin K, Teke MY, Citirik M.Clinical appraisal and retinal imaging in posterior microphthalos.Semin Ophthalmol2018;33(3):412-418.
10 Bijlsma WR, van Schooneveld MJ, Van der Lelij A.Optical coherence tomography fndings for nanophthalmic eyes.Retina2008;28(7):1002-1007.
11 Boynton JR, Purnell EW.Bilateral microphthalmos without microcornea associated with unusual papillomacular retinal folds and high hyperopia.Am J Ophthalmol1975;79(5):820-826.
12 Trelstad RL, Silbermann NN, Brockhurst RJ.Nanophthalmic sclera.Ultrastructural, histochemical, and biochemical observations.Arch Ophthalmol1982;100(12):1935-1938.
13 Yue BY, Duvall J, Goldberg MF, Puck A, Tso MO, Sugar J.Nanophthalmic sclera.Morphologic and tissue culture studies.Ophthalmology1986;93(4):534-541.
14 Uyama M, Takahashi K, Kozaki J, Tagami N, Takada Y, Ohkuma H,Matsunaga H, Kimoto T, Nishimura T.Uveal effusion syndrome: clinical features, surgical treatment, histologic examination of the sclera, and pathophysiology.Ophthalmology2000;107(3):441-449.
15 Aldahmesh MA, Nowilaty SR, Alzahrani F, Al-Ebdi L, Mohamed JY, Rajab M, Khan AO, Alkuraya FS.Posterior microphthalmos as a genetically heterogeneous condition that can be allelic to nanophthalmos.Arch Ophthalmol2011;129(6):805-807.
16 Orr A, Dube MP, Zenteno JC,et al.Mutations in a novel serine protease PRSS56 in families with nanophthalmos.Mol Vis2011;17:1850-1861.
17 Nowilaty SR, Khan AO, Aldahmesh MA, Tabbara KF, Al-Amri A,Alkuraya FS.Biometric and molecular characterization of clinically diagnosed posterior microphthalmos.Am J Ophthalmol2013;155(2):361-372.e367.
18 Wasmann RA, Wassink-Ruiter JS, Sundin OH, Morales E, Verheij JB,Pott JW.Novel membrane frizzled-related protein gene mutation as cause of posterior microphthalmia resulting in high hyperopia with macular folds.Acta Ophthalmol2014;92(3):276-281.
19 Ostrin LA, Yuzuriha J, Wildsoet CF.Refractive error and ocular parameters: comparison of two SD-OCT systems.Optom Vis Sci2015;92(4):437-446.
20 Relhan N, Jalali S, Pehre N, Rao HL, Manusani U, Bodduluri L.High-hyperopia database, part I: clinical characterisation including morphometric (biometric) differentiation of posterior microphthalmos from nanophthalmos.Eye (Lond)2016;30(1):120-126.
21 Walsh MK, Goldberg MF.Abnormal foveal avascular zone in nanophthalmos.Am J Ophthalmol2007;143(6):1067-1068.
22 Meire F, Leys M, Boghaert S, de Laey JJ.Posterior microphthalmos.Bull Soc Belge Ophtalmol1989;231:101-106.
23 Gass JD.Uveal effusion syndrome: a new hypothesis concerning pathogenesis and technique of surgical treatment.Retina1983;3(3):159-163.
24 Khairallah M, Messaoud R, Zaouali S, Ben Yahia S, Ladjimi A, Jenzri S.Posterior segment changes associated with posterior microphthalmos.Ophthalmology2002;109(3):569-574.
Citation:Liu JJ, Chen YY, Zhang X, Zhao PQ.Clinical features of posterior microphthalmic and nanophthalmic eyes.Int J Ophthalmol2018;11(11):1829-1834
DOl:10.18240/ijo.2018.11.15
Accepted:2018-05-15
Received:2018-03-13
Correspondence to:Pei-Quan Zhao.Department of Ophthalmology, Xin Hua Hospital, 1665 Kongjiang Road,Shanghai 200092, China.zhaopeiquan@xinhuamed.com.cn






