
Figure 1 Images of the fibroblasts and fibrous tissue in the study groups A: Control group; B: Sham group; C: Steroid group; D:Bevacizumab group; E: Pazopanib group.
Glaucoma is a chronic and progressive optic neuropathy caused by various pathological processes, which is typically seen with visual field defects, causes degeneration of retina ganglion cells and is usually accompanied by elevated intraocular pressure (IOP)[1]. It is the 2ndmost preventable cause of blindness worldwide[2]. Glaucoma treatment: with changing according the type of glaucoma, treatment is frequently medical. Laser treatment and surgical treatment can be applied on patient who do not tolerate or in situations that treatment is insufficent. Currently, the most frequently used surgical treatment is trabeculectomy[3]. Following trabeculectomy surgery, rapid wound healing causes failure of the surgical procedure[4-5]. Scar tissue (subconjuctival fibrosis)which will form as a result of the progression of the wound healing process is the most important cause of failure in trabeculectomy[6].
The use of anti- fibrotic agents is generally accepted in the treatment following glaucoma surgery[7-8]. Topical corticosteroids are the drug groups most often used as anti- fibrotics. In the vast majority of cases, this drug groups alone is sufficient to obtain surgical success. However, in cases where the chance of success is low, such as patients of a younger age, African ethnicity, with a history of glaucoma surgery or with uveitis,steroids remain insufficient. Mitomycin C (MMC) and 5- fluorouracil (5-FU) are widely used to prevent fibrosis in the wound site in glaucoma filtration surgery (GFS) in cases where corticosteroids are inadequate[9]. However, both these drugs which can be classically accepted and other cytotoxic agents with similar effects can lead to hypotonia, increased infection risk, the development of staphyloma and other serious sideeffects, especially in the long-term and this has therefore led to research into new agents[10-15].
Bevacizumab (Avastin, F.Hoffmann-La Roche Ltd., Basel,Switzerland) is a long-term effect recombinant monoclonal mouse antibody which is synthesised against the vascular endothelial growth factor (VEGF) molecule and binds to all isoforms of VEGF-A. FDA approval was obtained in 2004 for its use in the treatment of metastatic colorectal cancers[16].It is used without licence in age-related macular degeneration(AMD), choroid neovascularisations which develop associated with high myopia, premature retinopathy, diabetes mellitus(DM) and retina and iris neovascularisations which develop associated with retinal vascular obstructions[17-20]. In a study by Ozgonulet al[21]of 48 rabbits, subconjuctival and intravitreal injections to the bleb region of 0.1 mL (1.25 mg) bevacizumab were compared with 5-FU and histopathological evaluation was made of in flammation, vascularisation and fibrosis formation.It was concluded that the effect on the bleb of subconjunctival administered bevacizumab was more successful in respect of these 3 parameters compared to the other groups[21]. In another study of 20 patients, the postoperative topical use of a corticosteroid+bevacizumab combination was seen to have a positive effect on bleb formation in high-risk patients and a preventative effect on vascularisation[22].
Pazopanib (Glaxo Smith Kline, King of Prussia, PA) is a small molecule multi-tyrosine kinase inhibitor. It provides VEGF, platelet derived growth factor (PDGF) and fibroblast growth factor (FGF) receptor inhibition. It is used in the treatment of renal cell carcinoma (RCC), ovarian cancer,metastatic melanoma and soft tissue sarcoma[23-26]. Topical pazopanib application in humans has been shown to provide a regression in cornea neovascularisation. There have also been experimental animal studies showing that topical and intravitreal application of pazopanib regressed retinal and choroid neovascularisation[27-29]. However, to the best of our knowledge, there is no study in literature which has evaluated the effect of pazopanib on wound healing in GFS.
The aim of the current study was to histopathologically and immunohistochemically investigate the effect on postoperative in flammation, fibrosis and wound healing using bevacizumab and pazopanib in trabeculectomy surgery and to compare the results with the use of corticosteroids.
The study was conducted in the Ophthalmology Department and the Pathology Department of Firat University Medical Faculty. Approval for the study was granted by the Animal Experiments Ethics Committee of Firat University (dated 08.01.2014). A single eye was used from each of 35 male,albino New Zealand rabbits, each weighing approximately 2500-3000 g. Throughout the study the animals were kept in special cages with suitable nutrition in the Experimental Animal Centre of Firat University.
With the exception of group I (control group), limbus-based trabeculectomy was applied to a single eye of each rabbit.In group I, no postoperative treatment was applied. To the animals in group II (sham group), physiological saline was administered, to group III, prednisolone acetate, to group IV, 5 mg/mL bevacizumab and to group V, 5 mg/mL pazopanib as 4×1 topically for 28d to the eye on which trabeculectomy had been performed.
Anaesthesia TechniqueIn the application of anaesthesia and analgesia, a combination of intramuscular 50 mg/kg ketamine hydrochloride (Ketalar, Eczacıbaşı, Turkey) and 6 mg/kg xylazine hydrochloride (Rompun, Bayer, Turkey) was used. Before the procedure, eyedrops of 0.5% proparacaine hydrochloride(Alcaine, Alcon, Turkey) were administered to the eyes of the animals.
Surgical TechniqueFollowing cleaning of the surgical site,by passing an 8/0 vicryl suture from the level of 12 o’clock with corneal traction, inferior and nasal rotation of the globe was provided. By cutting the conjunctiva and Tenon capsule from the limbus with blunt-tipped Westcott scissors at the level of 10 o’clock, fornix -based conjunctiva was opened.The conjunctiva and Tenon capsule were dissected with blunt dissection. A 3×3 mm2square scleral flap was marked using a 30° curved knife. Using the crescent knife, a half thickness scleral flap was raised until the trabeculum was seen. With a 15° curved knife, the trabeculum was excised so that trabeculectomy borders were formed of approximately 2×1 mm2.Peripheral iridectomy was applied to the trabeculectomy site formed. The scleral flap was sutured to the sclera with 2×10/0 nylon sutures from the corners. The conjunctiva and the Tenon capsule were closed with 8/0 vicryl suture. After closure, by applying irrigation fluid to the anterior chamber , the bleb was in flated and it was checked that there was no leakage.
Histopathological Preparation and Evaluation of the FindingsFollowing the enucleation of the operated eyes by applying intramuscular thiopental sodium at the end of the 28thpostoperative day, the animals were returned to the Experimental Animal Centre. The bleb regions were excised including the conjunctiva, Tenon and sclera from the enucleated eyes and were sent to the pathology laboratory for histopathological examination. The tissue samples were fixed in 10% formalin then routine procedures were applied. Slices of 5 µm thickness were obtained from the paraf fin blocks and stained with hematoxylin-eosin. The slices obtained were also stained with Masson-Trichrome and examined under a light microscope (Olymus BX-50) at ×400 magni fication. On each slice, the number of fibroblasts and mononuclear cells in a 25 µm2area was determined with the aid of a specially marked micrometer placed on the microscope.

Figure 1 Images of the fibroblasts and fibrous tissue in the study groups A: Control group; B: Sham group; C: Steroid group; D:Bevacizumab group; E: Pazopanib group.
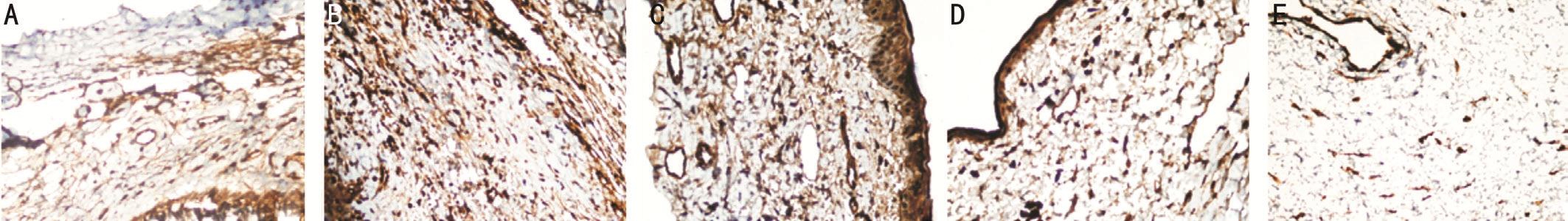
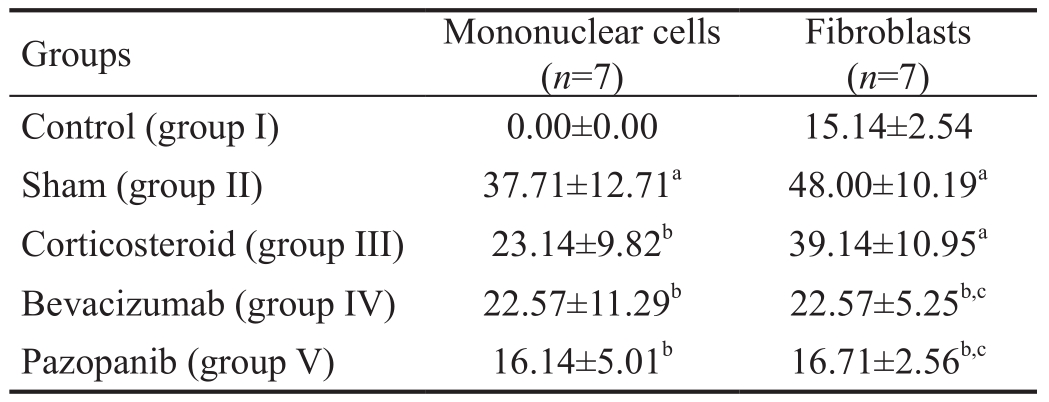
Figure 2 Immunohistochemical images of the FGF-β staining intensity in the bleb regions of the study groups A: Control group; B: Sham group; C: Steroid group; D: Bevacizumab group; E: Pazopanib group.
Immunohistochemical Staining of VEGF, FGF-β, and PDGF-βFor immunohistochemical staining, slices were prepared of 5 µm thickness passing from the centre of the bleb. The slices were stained using an automatic immunohistochemical staining device (Ventana Benchmark XT) and VEGF, FGF-β,PDGF-β kit (Biorbyt, Cambridge, UK). The preparates were covered with a special covering substance and examined randomly under a light microscope (Olympus BX-50).With a camera attachment on the same microscope, digital photographs were taken of the tissues at ×40 magnification.The intensity of nuclear positive stained cells was evaluated as weak (+), moderate (++) and strong (+++)[30-31].
Statistical AnalysisStatistical analyses of the data obtained were made using Windows Vista operating system and SPSS version 15 software. The data obtained were stated as mean±standard deviation (SD). The One-Way ANOVA test was applied for multiple comparisons. In paired comparisons between groups, the post hoc Tukey test was applied. A value ofP<0.05 was accepted as statistically signi ficant.
In the evaluation of fibroblast count, the values of groups II and III were statistically signi ficantly higher than those of the control group (bothP<0.05; Figure 1A-1C). The fibroblast count values of groups IV and V were statistically signi ficantly lower than those of groups II and III (AllP<0.05). No statistically significant difference was determined between groups IV and V (P>0.05; Figure 1D, 1E).
In respect of the evaluation of mononuclear cell count, the group II cell count was higher than that of group I (P<0.05;Figure 1A, 1B). In the comparison between the groups applied with treatment drugs, no signi ficant difference was determined in respect of mononuclear cell count (P>0.05; Figure 1C-1E).The mean and standard deviation values of the fibroblast andmononuclear cell counts found in the operation area of the study groups are shown in Table 1.
Table 1 Fibroblast and mononuclear cell counts in the operation area of the groups mean±SD
aP<0.05vscontrol group;bP<0.05vssham group;cP<0.05vscorticosteroid group.
?
The FGF-β, VEGF, PDGF-β, immunohistochemical staining was determined to be statistically signi ficantly lower in group I than in group II (P<0.05; Figures 2A-2B, 3A-3B, 4A-4B).The decrease in group III was not statistically significant in comparison with group II (P>0.05). The FGF-β and VEGF staining intensity in groups IV and V was significantly lower than that of groups II and III (allP<0.05; Figures 2B-2E, 3B-3E). No statistically significant difference was determined between groups IV and V (P>0.05). The PDGF-β immunohistochemical staining intensity in group V was lower than that of groups II, III and IV (allP<0.05; Figure 4B-4E).The PDGF-β, FGF-β, VEGF immunohistochemical staining intensity values are shown in Table 2.
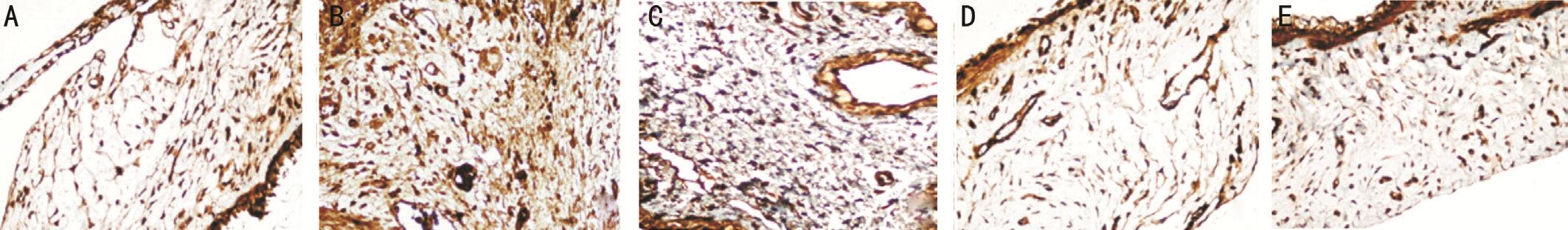
Figure 3 Immunohistochemical images of the VEGF staining intensity in the bleb regions of the study groups A: Control group; B: Sham group; C: Steroid group; D: Bevacizumab group; E: Pazopanib group.
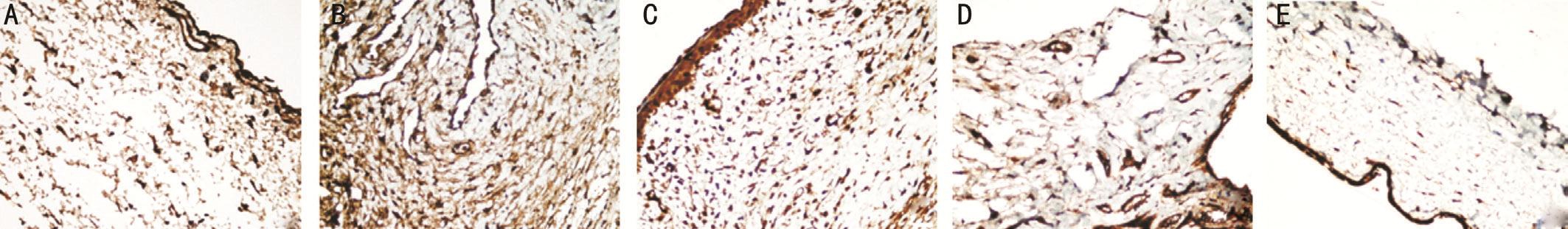
Figure 4 Immunohistochemical images of the PDGF-β staining intensity in the bleb regions of the study groups A: Control group; B:Sham group; C: Steroid group; D: Bevacizumab group; E: Pazopanib group.
Table 2 PDGF-β, FGF-β and VEGF immunohistochemical staining intensity mean±SD
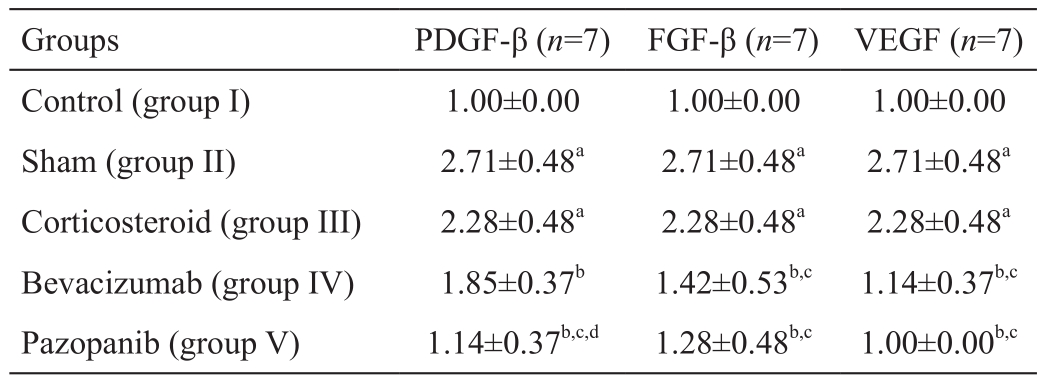
aP<0.05vscontrol group;bP<0.05vssham group;cP<0.05vscorticosteroid group;dP<0.05vsbevacizumab group.
?
Topical corticosteroids are used to inhibit wound healing following GFS[32]. It is known that topical corticosteroids increase the success of GFS by reducing bleb scarring[33].The drugs primarily used for this purpose are corticosteroids,dexamethasone, prednisolone acetate and triamnisolone acetone. In the current study, topical corticosteroid (prednisolone acetate) was used, as it is conventionally used to delay wound healing after trabeculectomy. Although the fibroblast count on the histology slices was reduced compared to the sham group, this had no statististically significant effect. This small reduction in fibroblast count suggested that the effect of corticosteroids on wound site healing could be limited.Corticosteroids reduce inflammatory cell migration to the wound site. In the current study the mononuclear cell count in the corticosteroid group was significantly reduced compared with the sham group. This finding suggested that the effects of corticosteroids on wound site healing could be on the anti-inflammatory activity. Therefore, despite occasional corticosteroid use in the clinic, rapid wound healing could be associated with it only being able to be one-way. There was no statistically significant difference between the corticosteroid group and the sham group in respect of PDGF-β, FGF-β and VEGF immunohistochemical staining intensity. This finding suggests that corticosteroids have no effect on growth factors in wound healing.
Corticosteroids, which are the most frequently used drug group in the clinic are suf ficient alone to provide successful surgery in the vast majority of cases. However, they remain inadequate for some risk groups[34-35]. Therefore, alternative agents are being sought to increase the success of GFS. MMC and 5-FU have started to be widely used in cases where corticosteroids are insuf ficient[9]. However, there are known to be serious sideeffects of these agents[10-15]. In the current study, the effect of corticosteroids on the parameters affecting wound healing was seen to be limited. From this starting point, groups were formed in the current study to compare the histopathological effects of bevacizumab and pazopanib with corticosteroids which are conventionally used.
VEGF is an important molecule in the response to wound healing. VEGF is not only a strong indicator for angiogenesis but is also important as a key regulator of fibroblast and in flammatory cell migration and proliferation[36]. In flammatory cells which will migrate to the wound site with increased vascular permeability in the early stage as a result of stimulation formed with surgical trauma, will reach the wound site in fewer numbers with reduced vascular permeability of anti-VEGF. This role of anti-VEGF in the blocking of wound healing and angiogenesis can lead to a reduction in the development of scar tissue and response to wound healing.
In a study by Ozgonulet al[21], subconjunctival 0.1 mL(1.25 mg) bevacizumab was applied in an experimental trabeculectomy model and vascularisation, inflammation and fibrosis in the bleb region were seen to be significantly decreased compared to the other groups. In another study, a postoperative topical corticosteroid+bevacizumab combination was seen to signi ficantly increase the formation of functional bleb and significantly decrease bleb vascularisation in high risk patients[22]. In a clinical study by Jonaset al[37]of 2 cases of neovascular glaucoma secondary to corticosteroid glaucoma and venous obstruction, success was reported to have been obtained with intravitreal 1.5 mg bevacizumab combined with surgery and this success was considered to be associated with angiogenesis inhibition. Previous studies have reported the rapid and marked reduction of abnormal new vessels and IOP after intraocular bevacizumab in neovascular glaucoma patients[18,38-40]. However, in a case by Kahooket al[41]of unsuccessful trabeculectomy because of encapsulated filtration bleb, bleb revision was made with a needle, the bleb became more diffuse following 1 mg bevacizumab and the neovascular structures in the conjunctiva over the bleb were observed to have regressed. Akkan and Cilsim[42]concluded that subconjunctival bevacizumab administration in primary open-angle glaucoma patients who underwent primary trabeculectomy was more effective and safe than MMC.
In the current study, the fibroblast count in the bevacizumab group was determined to be statistically significantly lower than that of the sham and corticosteroid groups. The reduction in mononuclear cell count was significant compared to the sham group but not to the corticosteroid group. The reduction in PDGF-β immunohistochemical staining intensity was significant compared to the sham group but not to the corticosteroid group, whereas the reduction in FGF-β and VEGF immunohistochemical staining intensity was signi ficant compared to both the sham and corticosteroid groups. These results suggest that the inhibitor effect of bevacizumab on wound healing has antifibrotic and anti-angiogenic effects in addition to the mononuclear cell inhibition effect of corticosteroids.
Bevacizumab, which is an anti-VEGF and is used in many ocular pathologies, inhibits wound healing at several stages. However, while positive effects can be formed in the pathological angiogenesis process with the inhibition of VEGF activity with anti-VEGF drugs, thereby reversing the VEGF efficacy, it may lead to negative side-effects in the physiological angiogenesis process[43]. Even if the eye is isolated from systemic circulation with the blood-brain barrier, in neovascular eye diseases, this barrier can be destroyed and there could be systemic transfer. Several cases of acute elevation of systemic blood pressure, cerebrovascular accidents, myocardial infarction, and transient ischemic attack were also reported[44].
The application of bevacizumab is absolutely not recommended in pregnancy and childhood, acute intraocular infection,acute myocardial infarct, angina pectoris, hypertensive crisis,kidney failure, active glomerulonephritis and known allergies and relatively not recommended in cases of uncontrolled hypertension, neurodegenerative diseases and respiratory failure[45-46]. Therefore, although this study obtained successful histological results with bevacizumab, these side-effects must always be kept in mind.
Pazopanib is a small molecule, multi-tyrosine kinase inhibitor that inhibits VEGFR, PDGFR, FGFR and c-kit and is used in patients with advanced stage RCC or soft tissue sarcoma[23-26].Previous studies have shown that oral and topical application of pazopanib in choroid and retina neovascularisations regressed the neovascularisation and improved visual acuity[27,47-48]. It has also been reported that patients with neovascularisation receiving topical 0.5% pazopanib have shown a decrease in neovascularisation area and vascular diameter. In that study,which also evaluated reliability and efficacy, no systemic or ocular side-effects were encountered[49].
In a study by Singhet al[50]topical 10 mg/mL pazopanib was applied 4 times per day for 12wk to a patient group with subfoveal choroid neovascularisation secondary to AMD and to a healthy group and reliability and ef ficacy were evaluated.Although no serious side-effects were seen, ocular irritation was reported as the most common side-effect and it was concluded that 10 mg/mL topical pazopanib was well tolerated.In a study by Csakyet al[51]topical 5 mg/mL pazopanib and 10 mg/mL pazopanib applied 4 times per day a patient group in neovascular AMD. As a result; pazopanib was well tolerated.Daily pazopanib eye drops in neovascular AMD subjects did not result in therapeutic benefit beyond that obtained with ranibizumab alone.
To the best of our knowledge, there is no study in literature that has evaluated the effects of pazopanib on wound healing in GFS. Therefore, in this study it was decided to make a topical application of pazopanib, which is effective on wound healing at several stages. Pazopanib was applied at a dose of 5 mg/mL, 4 times a day for 28d. A signi ficant reduction was seen in fibroblast count in the pazopanib group compared to the sham and corticosteroid groups. In respect of mononuclear cell count, a signi ficant reduction was observed compared to the sham group and the reduction was seen to be similar to that in the corticosteroid and bevacizumab groups. The decrease in FGF-β and VEGF immunohistochemical staining intensity was signi ficant in comparison with the sham and corticosteroid groups. The inhibitor effect of pazopanib on mononuclear cell and fibroblast count, VEGF and FGF levels was similar to that of bevacizumab. However, the decrease in PDGF-β immunohistochemical staining intensity was significant compared to the other treatment groups. With PDGF-β, which is an effective mediator on wound healing, migration of fibroblasts to the wound site is stimulated. Therefore, PDGF-β inhibition prevents fibroblast migration to the wound site and as a result, scar formation is reduced. The reduction in fibroblast count supports this view. The fact that pazopanib creates significant PDGF-β inhibition in addition to the anti fibrotic and anti-angiogenic effects of bevacizumab renders it superior to the other drugs.
The results of this study indicate that bevacizumab and pazopanib could be good alternatives for wound healing and because of the PDGF inhibition effect of pazopanib, this could be one step ahead of bevacizumab.
Conflicts of Interest: Kobat SG,None;Celiker FU,None;Daglı AF,None;Kasar K,None.
1 Terminology and guidelines for glaucoma.European Glaucoma Society.4th ed. Italy: PubliComm; 2014.
2 Quigley HA. The number of people with glaucoma worldwide in 2010 and 2020.Br J Ophthalmol2006;90(3):262-267.
3 Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma.Ophthalmology1994;101(10):1651-1657.
4 Reder JE. Update of adjunctive antimetabolities in glaucoma surgery.Contemporary Issues in Glaucoma1991;4:861-888.
5 Turaçlı ME. The use of fibroblast inhibitors in combined glaucoma surgery.Turkiye Klinikleri J Ophthalmol1997;6(4):280-290.
6 Fan Gaskin JC, Nguyen DQ, Ang GS, Oconnor J, Crowston JG. Wound healing modulation in glaucoma filtration surgery: conventional practices and new perspectives: the role of antifibrotic agents (part I).J Curr Glaucoma Pract2014;8:37-45.
7 Atreides SP, Skuta GL, Reynolds AC. Wound healing modulation in glaucoma filtering surgery.Int Ophthalmol Clin2004;44(2):61-106.
8 Joshi AB, Parrish RK II, Feuer WF. 2002 survey of the american glaucoma society.J Glaucoma2005;14(2):172-174.
9 Lama PJ, Fechtner RD. Anti fibrotics and wound healing in glaucoma surgery.Surv Ophthalmol2003;48(3):314-346.
10 Seibold LK, Sherwood MB, Kahook MY. Wound modulation after filtration surgery.Surv Ophthalmol2012;57(6):530-550.
11 Crowston JG, Akbar AN, Constable PH, Occleston NL, Daniels JT, Khaw PT. Antimetabolite-induced apoptosis in Tenon’s capsule fibroblasts.Invest Ophthalmol Vis Sci1998;39(2):449-454.
12 Palanca-Capistrano AM, Hall J, Cantor LB, Morgan L, Hoop J,Wudunn D. Long-term outcomes of intraoperative 5-fluorouracil versus intraoperative mitomycin C in primary trabeculectomy surgery.Ophthalmology2009;116(2):185-190.
13 Seah SK, Prata JA Jr, Minckler DS, Baerveldt G, Lee PP, Heuer DK.Hypotony following trabeculectomy.J Glaucoma1995;4(2):73-79.
14 Parrish R, Minckler D. “Late endophthalmitis”: filtering surgery time bomb?Ophthalmology1996;103(8):1167-1168.
15 Van Bergen T, Vandewalle E, Van de Veire S, Dewerchin M, Stassen JM, Moons L, Stalmans I. The role of different VEGF isoforms in scar formation after glaucoma filtration surgery.Exp Eye Res2011;93(5):689-699.
16 Mulcahy MF, Benson AB III. Bevacizumab in the treatment of colorectal cancer.Expert Opin Biol Ther2005;5(7):997-1005.
17 Wykoff CC, Clark WL, Nielsen JS, Brill JV, Greene LS, Heggen CL.Optimizing anti-VEGF treatment outcomes for patients with neovascular age-related macular degeneration.J Manag Care Spec Pharm2018;24(2-a Suppl):S3-S15.
18 Ha JY, Lee TH, Sung MS, Park SW. Efficacy and safety of intracameral bevacizumab for treatment of neovascular glaucoma.Korean J Ophthalmol2017;31(6):538-547.
19 Karkhaneh R, Khodabande A, Riazi-Eafahani M, Roohipoor R,Ghassemi F, Imani M, Dastjani Farahani A, Ebrahimi Adib N, Torabi H. Efficacy of intravitreal bevacizumab for zone-II retinopathy of prematurity.Acta Ophthalmologica2016;94(6):e417-e420.
20 Chhablani J, Paulose RM, Lasave AF, Wu L, Carpentier C, Maia M,Lujan S, Rojas S, Serrano M, Berrocal MH, Arevalo JF; Pan-American Collaborative Retina Study Group. Intravitreal bevacizumab monotherapy in myopic choroidal neovascularisation: 5-year outcomes for the PAN-American Collaborative Retina Study Group.Br J Ophthalmol2018;102(4):455-459.
21 Ozgonul C, Mumcuoglu T, Gunal A. The effect of bevacizumab on wound healing modulation in an experimental trabeculectomy model.Curr Eye Res2014;39(5):451-459.
22 Klos-Rola J, Tulidowicz-Bielak M, Zarnowski T. Effects of topical bevacizumab application on early bleb failure after trabeculectomy:observational case series.Clin Ophthalmol2013;7:1929-1935.
23 Park J, Jiao X, Ghate S, Wilson T, Ahmad QI, Vogelzang NJ. Predictors of long-term response with pazopanib in patients with advanced renal-cell carcinoma.Clin Genitouterin Cancer 2018;16(4):293-297.
24 Ferrero S, Leone Roberti Maggiore U, Aiello N, Barra F, Ditto A,Bogani G, Raspagliesi F, Lorusso D. Pharmacokinetic drug evaluation of pazopanib for the treatment of uterine leiomyosarcomas.Expert Opin Drug Metab Toxicol2017;13(8):881-889.
25 Richardson DL, Sill MW, Coleman RL, Sood AK, Pearl ML, Kehoe SM, Carney ME, Hanjani P, van le L, Zhou XC, Alvarez Secord A, Gray HJ, Landrum LM, Lankes HA, Hu W, Aghajanian C. Paclitaxel with and without pazopanib for persistent or recurrent ovarian cancer.JAMA Oncol2018;4(2):196-202.
26 Fruehauf JP, El-Masry M, Osann K, Parmakhtiar B, Yamamoto M,Jakowatz JG. Phase II study of pazopanib in combination with paclitaxel in patients with metastatic melanoma.Cancer Chemother Pharmacol2018;82(2):353-360.
27 Yafai Y, Yang XM, Niemeyer M, Nishiwaki A, Lange J, Wiedemann P,King AG, Yasukawa T, Eichler W. Anti-angiogenic effects of the receptor tyrosine kinase inhibitor, pazopanib, on choroidal neovascularization in rats.Eur J Pharmacol2011;666(1-3):12-18.
28 Iwase T, Oveson BC, Hashida N, Lima e Silva R, Shen JK, Krauss AH,Gale DC, Adamson P, Campochiaro PA. Topical pazopanib blocks VEGF-induced vascular leakage and neovascularization in the mouse retina but is ineffective in the rabbit.Invest Opthalmol Vis Sci2013;54(1):503-511.
29 Tran J, Craven C, Wabner K, Schmit J, Matter B, Kompella U,Grossniklaus HE, Olsen TW. A pharmacodynamic analysis of choroidal neovascularization in a porcine model using three targeted drugs.Invest Opthalmol Vis Sci2017;58(9):3732-3740.
30 Bishen KA, Radhakrishnan R, Satyamoorthy K. The role of basic fibroblast growth factor in oral submucous fibrosis pathogenesis.J Oral Pathol Med2008;37(7):402-411.
31 Pérez-Santonja JJ, Campos-Mollo E, Lledó-Riquelme M, Javaloy J,Alió JL. Inhibition of corneal neovascularization by topical bevacizumab(Anti-VEGF) and Sunitinib (Anti-VEGF and Anti-PDGF) in an animal model.Am J Ophthalmol2010;150(4):519-528.e1.
32 Araujo SV, Spaeth GL, Roth SM, Starita RJ. A ten-year follow-up on a prospective, randomized trial of postoperative corticosteroids after trabeculectomy.Ophthalmology1995;102(12):1753-1759.
33 Weinreb RN. Adjusting the dose of 5-fluorouracil after filtration surgery to minimize side effects.Ophthalmology1987;94(5):564-570.
34 Gressel MG, Heuer DK, Parrish RK 2nd. Trabeculectomy in young patients.Ophthalmology1984;91(10):1242-1246.
35 Broadway DC, Grierson I, Hitchings RA. Local effects of previous conjunctival incisional surgery and the subsequent outcome of filtration surgery.Am J Ophthalmol1998;125(6):805-818.
36 Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N,Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo.Circulation1995;92(9):365-371.
37 Jonas JB, Spandau UH, Schlichtenbrede F. Intravitreal bevacizumab for filtering surgery.Ophthalmic Res2007;39(2):121-122.
38 Sasamoto Y, Oshima Y, Miki A, Wakabayashi T, Song D, Matsushita K, Hamasaki T, Nishida K. Clinical outcomes and changes in aqueous vascular endothelial growth factor levels after intravitreal bevacizumab for iris neovascularization and neovascular glaucoma:a retrospective two-dose comparative study.J Ocul Pharmacol Ther2012;28(1):41-48.
39 Wolf A, von Jagow B, Ulbig M, Haritoglou C. Intracameral injection of bevacizumab for the treatment of neovascular glaucoma.Ophthalmologica2011;226(2):51-56.
40 Bhagat PR, Agrawal KU, Tandel D. Study of the effect of injection bevacizumab through various routes in neovascular glaucoma.J Curr Glaucoma Pract2016;10(2):39-48.
41 Kahook MY, Schuman JS, Noecker RJ. Needle bleb revision of encapsulated filtering bleb with bevacizumab. Ophthalmic Surg Lasers Imaging. 2006;37(2):148-150.
42 Akkan JU, Cilsim S. Role of subconjunctival bevacizumab as an adjuvant to primary trabeculectomy: a prospective randomized comparative 1-year follow-up study.J Glaucoma2015;24(1):1-8.
43 Ferrara N. Vascular endothelial growth factor: basic science and clinical progress.Endocr Rev2004;25(4):581-611.
44 Wu L, Martínez-Castellanos MA, Quiroz-Mercado H, Arevalo JF,Berrocal MH, Farah ME, Maia M, Roca JA, Rodriguez FJ, Pan American Collaborative Retina Group (PACORES). Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES).Graefes Arch Clin Exp Ophthalmol2007;246(1):81-87.
45 Michels S, Rosenfeld P, Puliafito C, Marcus E, Venkatraman A.Systemic bevacizumab (avastin) therapy for neovascular age-related macular DegenerationTwelve-week results of an uncontrolled open-label clinical study.Ophthalmology2005;112(6):1035-1047.e9.
46 Kilickap S, Abali H, Celik I. Bevacizumab, bleeding, thrombosis, and warfarin.J Clin Oncol2003;21(18):3542; author reply 3543.
47 Takahashi K. Suppression and regression of choroidal neovascularization by the multitargeted kinase inhibitor pazopanib.Arch Ophthalmol2009;127(4):494-499.
48 Danis R, McLaughlin MM, Tolentino M, Staurenghi G, Ye L, Xu CF,Kim RY, Johnson MW; Pazopanib Eye Drops Study Group. Pazopanib eye drops: a randomised trial in neovascular age-related macular degeneration.Br J Ophthalmol2014;98(2):172-178.
49 Amparo F, Sadrai Z, Jin YP, Alfonso-Bartolozzi B, Wang HB, Shikari H, Ciolino JB, Chodosh J, Jurkunas U, Schaumberg DA, Dana RZ. Safety and ef ficacy of the multitargeted receptor kinase inhibitor pazopanib in the treatment of corneal neovascularization.Invest Opthalmol Vis Sci2013;54(1):537-544.
50 Singh R, Wurzelmann JI, Ye L, Henderson L, Hossain M, Trivedi T,Kelly DS. Clinical evaluation of pazopanib eye drops in healthy subjects and in subjects with neovascular age-related macular degeneration.Retina2014;34(9):1787-1795.
51 Csaky KG, Dugel PU, Pierce AJ, Fries MA, Kelly DS, Danis RP,Wurzelmann JI, Xu CF, Hossain M, Trivedi T. Clinical evaluation of pazopanib eye drops versus ranibizumab intravitreal injections in subjects with neovascular age-related macular degeneration.Ophthalmology2015;122(3):579-588.