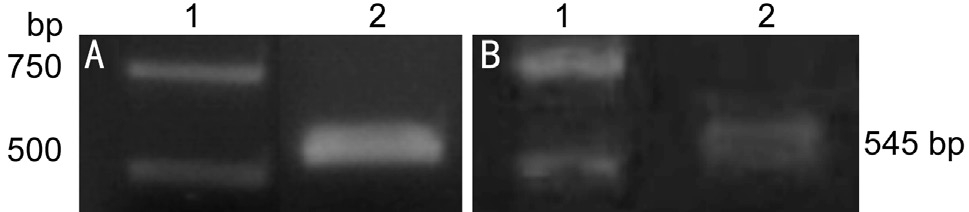
Figure 1 Gel electrophoresis following Ecor l and XhoI double enzymatic digestion A: Recombinant plasmid PMD19-T-αB-crystallin; B: Recombinant plasmid pET28a-αB-crystallin. Lane 1:Marker; Lane 2: Target gene fragment.
Alpha B-crystallin (αB-crystallin) is a subunit of alpha crystallin. In 2002, αB-crystallin was first isolated from the lens by Srivastava[1]. Later studies have shown that αB-crystallin is one of a small molecular heat shock protein (sHsps)family, which is sHspB5. αB-crystallin is 20 kD and has the commonness of sHsps family, including small molecular weight, participation in the process of cell development, and being able to resist stress responses and apoptosis induced by various factors[2]. The recent research demonstrated that αB-crystallin is related to age-related macular degeneration, optic neuropathy due to glaucoma, diabetic retinopathy, retinal tear,retinal ischemia-reperfusion injury, retinal dystrophy, and other pathological changes[3-9].
The abilities of αB-crystallin inhibiting protein aggregation,oxidation, in flammation, and apoptosis have been exploited for its therapeutic use in eye diseases. Exogenous administration of αB-crystallin inhibits microglial activation and rescues optic nerve oligodendrocytes in an experimental animal model of anterior ischemic optic neuropathy in mice[10]. In addition to the whole protein, a recent study showed that the administration of αB-crystallin derivatives have also shown promising results. The intraperitoneal injection of αB-crystallin derivatives inhibited drug-induced cataracts in rats, which was accompanied by their beneficial effects against protein aggregation and lens epithelial cell apoptosis[11].The therapeutic uses of αB-crystallin for other diseases outside the eye have also been reported. Following central nervous system injury in mice, the administration of human αB-crystallin led to an improvement in locomotor skills and an inhibition of secondary tissue damage during the acute stage[12].In many kinds of animal models, such as cerebral ischemia,autoimmune encephalomyelitis, multiple sclerosis, diabetes,autoimmune myocarditis, and cardiac ischemia, the exogenous administrations of αB-crystallin or its hexameric peptide showed signi ficant therapeutic bene fits[13-19].
However, complete αB-crystallin is unable to be separated from alpha crystallin, which limits further research and application. This study used an Escherichia coli (E. coli)prokaryotic expression system to recombine human αB-crystallin, as well as to study the protective effect of the recombinant αB-crystallin, and lay a foundation for its clinical application in treating optic nerve injury.
Experimental Materials
Plasmids, strains and cellsE. coli BL21 (DE3) (Novagen company, USA); PMD19-T vector (Takara company, Japan);E.coli DH5α strain (Novagen company, USA); pET28a(Novagen company, USA).
The main enzymes and reagentsReverse transcription Kit(Toyobo company, Japan); DNA gel extraction kit (Tiangen company, Germany); plasmid DNA extraction kit (Tiangen company, Germany); Luria Bertani medium (Difco, USA);KOD plus DNA polymerase (Toyobo company, Japan);restriction endonucleases Nco I, hind I and EcoRI and xhoi(Toyobo company, Japan); DNA ligase solution I (Takara company, Japan); polymerase chain reaction (PCR) reagents Taq polymerase (Japan Takara company, Japan).
Methods
Obtain human alpha B-crystal protein geneThe sequences of human αB-crystallin was retrieved from the GenBank:mdiaihhpwi rrpffpfhsp srlfdqffge hllesdlfpt stslspfylr ppsflrapsw fdtglsemrl ekdrfsvnld vkhfspeelk vkvlgdviev hgkheerqde hgfisrefhr kyripadvdp ltitsslssd gvltvngprk qvsgpertip itreekpavt aapkk. According to the codon usage bias of E. coli, the amino acid sequence of human αB-crystallin was transformed into DNA sequence.
Polymerase chain reaction amplification of target geneAccording to the gene sequence of human αB-crystallin and the multiple cloning sites of the vector, the speci fic primer of human αB-crystallin gene was designed by Primer 5 software.Upstream primer: 5’-GGAATTGATCGCCATCCACCAC-3’and downstream primer: 5’-CCGCTCGAGCTATTTCTT GGGGGCTGCGG-3’. The primers were synthesized by the biotechnology Service Co., Ltd of Shanghai, China,and the length of the amplified fragment was 545 bp. After PCR amplification, the amplified products were analyzed by electrophoresis in 1 g/L agarose gel, and the results were analyzed by gel auto imaging system.
Construction and identification of target gene vector plasmidRecovery and purification of target gene from PCR products after agarose gel electrophoresis (referring the specification of Tiangen company general agarose gel DNA Extraction Kit), purified DNA fragment of 50 μL(0.96 ng/μL), dNTP 5 uL (2 mmol/L) and Taq enzyme 1 μL(5 μ/μL), at 72℃, 20min, Poly A tail is added to the 3’ end of the DNA. The synthesized gene fragment was then inserted into the PMD19-T vector at restriction enzyme sites EcoRI and XhoI. The products were transformed into E. coli BL21(DE3) pLysS. A single colony of positive PMD19-T-αB-crystallin. E.coli BL21 (DE3) pLysS was inoculated into 5 mL LB medium containing ampicillin and incubated overnight at 37℃, in a 150-rpm shaker. The target gene PCR was identi fied by taking the bacterial fluid, and the recombinant vector was sent to the sequencing company for sequencing. At the same time, the recombinant plasmid of PMD19-T-αB-crystallin was extracted and identi fied by EcoR I and Xho I double enzyme digestion.
Inducible expression of recombinant proteinspET28a-αB-crystallin plasmid was transformed into E. coli BL21 (DE3)pLysS. A single colony of positive pET28a-αB-crystallin.E. coli BL21 (DE3) pLysS was inoculated into LB medium and incubated overnight at 37℃ in a 150-rpm shaker. The overnight culture was inoculated into fresh LB medium and incubated at 37℃, at 150 rpm until the culture reached an optical density at 600 nm of 0.6-0.8. This was followed by addition of isopropyl-β-D-thiogalactoside (IPTG) solution to induce protein expression (in a 150-rpm shaker, 30℃,overnight), and a number of final concentrations of IPTG(0, 0.2, 0.4, 0.6 and 0.8 mmol/L) of recombinant protein expression induction were evaluated. The cells were collected after recombinant protein expression was terminated. Then the harvested cells were resuspended in phosphate-buffered saline(PBS) and lysed. The lysate was centrifuged in a 150-rpm shaker,at 4℃, for 15min. The supernatant was analyzed using 10%sodium dodecyl sulfate polyacrylamide gel electrophoresis(SDS-PAGE).
Purification of recombinant Trx-αB-crystallin fusion proteinThe recombinant protein contains Thioredoxin and His Tags, which was purified by Ni2+/IDA metal chelating affinity column chromatography followed by Q-Sepharose ion-exchange column to obtain high purity fusion protein. The purity of the target protein was evaluated using SDS-PAGE.
Identification of recombinant human αB-crystallin and detection of molecular chaperone activityThe recombinant human αB-crystallin was identi fied by PAGE, Western-blot and protein peptide mass fingerprinting. Insulin reduction assay was used to detect the chaperone activity of the recombinant human αB-crystallinin vitro, which can inhibit the aggregation of insulin B chain caused by reducing agent and suppressing sediment formation[20].
The Amplification of the Target Gene FragmentThe synthesized target DNA fragment was ligated into the PMD19-T vector in order to construct the recombinant plasmid PMD19-T-αB-crystallin, which was then digested by restriction endonuclease Ecor l and XhoI. Gel electrophoresis revealed that the cleavage of Ecor l and XhoI generated the target fragment, which was consistent with the expected fragment size of 545 bp (Figure 1A). This indicates that the target gene fragment was successfully inserted into the PMD19-T vector.
Expression and Purification of the Recombinant Alpha B-crystallin
Construction of the expression vectorThe synthesized target DNA fragment was ligated into the pET-28a vector in order to construct the expression vector pET-28a-αB-crystallin,which was then digested by restriction endonuclease Ecor l and XhoI. Gel electrophoresis revealed that the cleavage of Ecor l and XhoI generated the target fragment, which was consistent with the expected fragment size of 545 bp (Figure 1B). The endonuclease cleavage fragment was veri fied by sequencing,and the results of this were consistent with the αB-crystallin sequence, indicating successful construction of the expression vector.
Expression of the recombinant αB-crystallinThe pET-28aαB-crystallin recombinant plasmid was transformed into E.coli BL21 (DE3) pLysS, protein expression was induced using 0.4 and 0.6 mmol/L IPTG under varying conditions. Upon terminating the expression, 10% SDS-PAGE was used to verify differing protein expression (Figure 2). The target protein was expressed in the pellet, which showed the recombinant αB-crystallin expression was successfully induced in E. coli BL21(DE3) pLysS.
Purification of the recombinant αB-crystallinNi2+/IDA metal chelating affinity column chromatography and Q-Sepharose ion-exchange column were conducted to purify the target protein (Figure 3). SDS-PAGE revealed that the size of the puri fied target protein matched its predicted size of 20 kDa,with above 95% purity (Figure 4).
Identi fication of Recombinant Proteins
Coomassie Brilliant Blue staining of the recombinant αB-crystallinThe recombinant human αB-crystallin during PAGE was stained with Coomassie Brilliant Blue. A recombinant human αB-crystallin band with the size of about 20 kD can be observed (Figure 5A). The size of the recombinant human αB-crystallin was similar to that found in GenBank, suggesting that the size of the recombinant protein was correct.
Western-blot analysis of the recombinant αB-crystallinAnti-αB-crystallin monoclonal antibody was used in Western blot analysis to assess the expressed recombinant αB-crystallin.The recombinant protein was demonstrated to speci fically bind the anti-αB-crystallin monoclonal antibody, which showed the recombinant αB-crystallin were successfully constructed(Figure 5B).

Figure 1 Gel electrophoresis following Ecor l and XhoI double enzymatic digestion A: Recombinant plasmid PMD19-T-αB-crystallin; B: Recombinant plasmid pET28a-αB-crystallin. Lane 1:Marker; Lane 2: Target gene fragment.
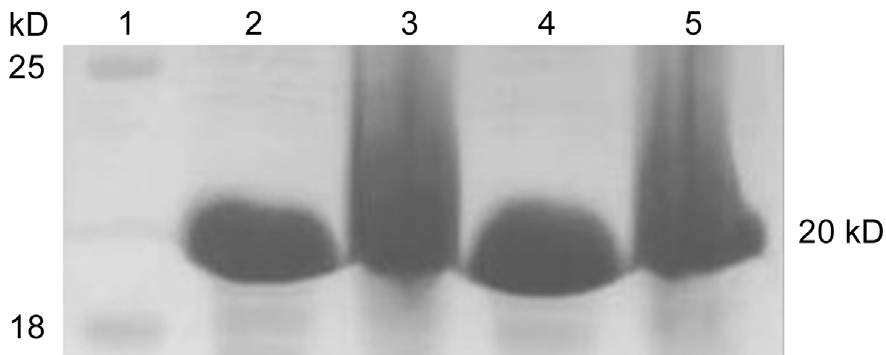
Figure 2 Recombinant protein expression SDS-PAGE Lane 1:Marker; Lane 2-5: 0.4 mmol/L and 0.6 mmol/L IPTG (supernatant and pellet).

Figure 3 Purification of the recombinant αB-crystallin by Q-Sepharose ion-exchange column.
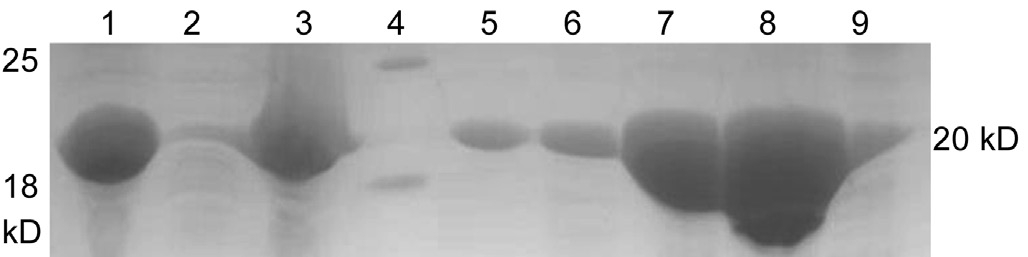
Figure 4 Recombinant protein puri fied by chromatography Lane 1-3: Simples, supernatant, pellet; Lane 4: Marker; Lane 5-9: p1, p2,p3, p4, p5 puri fied protein.
Peptide mass fingerprinting analysis of the recombinant αB-crystallinSearching in MATRIX SCIENCE database and using Mowse Score, when the score is greater than 83, the peptide fragments of the identified peptides match with the known protein peptide fragments in the database, there was significant difference (P<0.05; Figure 6). The peptide mass fingerprinting showed that the recombinant protein bands is αB-crystallin (Figure 7).
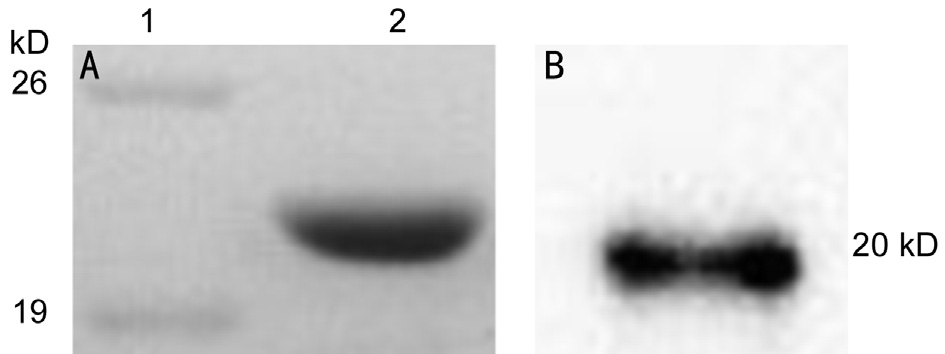
Figure 5 Coomassie Brilliant Blue staining (A) and Western blot analysis (B) of the recombinant protein Lane 1: Marker; Lane 2:Recombinant human αB-crystallin.

Figure 6 Peptide mass fingerprinting analysis of the recombinant protein.
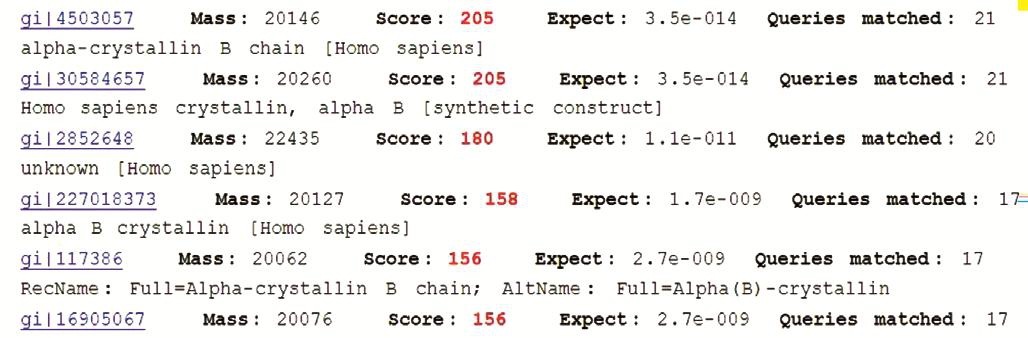
Figure 7 Identification of the recombinant protein by peptide mass fingerprint.
Identification of molecular chaperone activity of the recombinant αB-crystallinThe molecular chaperone activity of the recombinant human αB-crystallin was determined by insulin reduction assay, and compared with the negative control group (Figure 8). The results showed that the recombinant human αB-crystallin inhibited the aggregation and precipitation of insulin in the presence of reducing agent DTT. The experimental results show that the recombinant human αB-crystallin has molecular chaperone activity (Figure 9).
Human αB-crystallin is an sHsps; the molecular weight is small, about 20 kD and 170 to 180 amino acids[21]. αB-crystallin is a widely expressed sHsp first identi fied in the lens,and has been implicated in the pathogenesis of many diseases,including neurodegenerative disorders, myopathies, cancer,and cataracts[22-30]. The molecular chaperone activity of αB-crystallin is the core of its biological function, which plays an important part in the activity of life. In ophthalmology,the molecular chaperone activity of αB-crystallin protects the optic nerve and prevents the lens from ultraviolet radiation injury[31]. Because human αB-crystallin cannot be separated from the alpha crystallin, limiting its research and application.
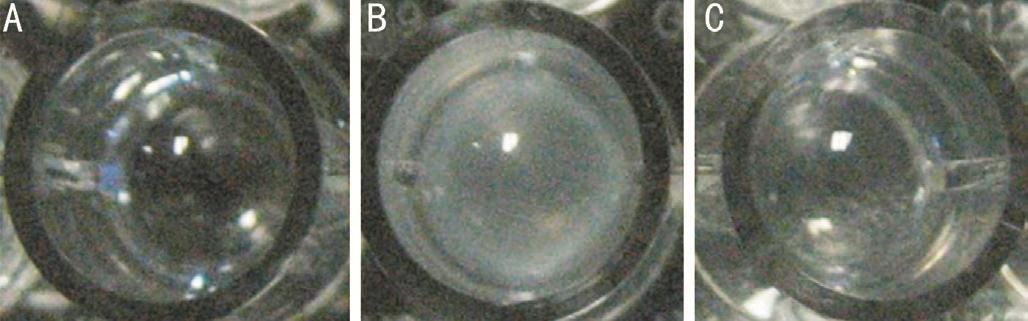
Figure 8 Insulin reduction assay A: Negative group (Deionized water); B: Positive group (Insulin+DTT); C: Recombinant human αB-crystallin group (Insulin+DTT+αB-crystallin).
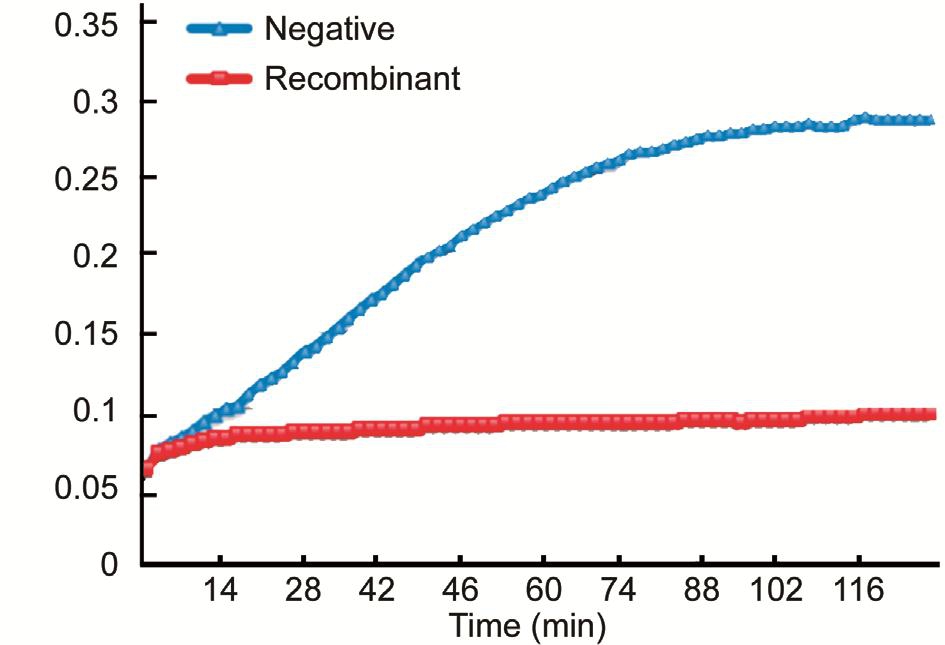
Figure 9 Recombinant human αB-crystallin molecular chaperone activity test.
Recombinant αB-crystallin with molecular chaperone activity is the basis for its further application. In this study, the gene engineering method was used to clone the human αB-crystallin gene fragment into the expression vector, the recombinant protein was then expressed and puri fied.
At present, the E. coli expression system is the most mature;it is also the first and most widely used of the recombinant protein expression systems. E. coli expression system offers several advantages such as high expression level, stability,and simple operation. Therefore, a recombinant human B-crystallin was prepared in this study using the E. coli prokaryotic expression system, and the biological activity of the recombinant protein was evaluated.
pET system is a widely used expression vector system for recombinant protein production. pET vector makes the cloning, expression, and puri fication of recombinant proteins easier[32-34]. A pET vector system using T7RNA polymerase and promoter matching system allows the cloning and expression to successfully leave. Many of the genes that are dif ficult to be expressed can ef ficiently and stably be cloned and expressed by the PET system.
On the basis of the above experiments, the double enzyme digestion method was used to insert the target protein gene into the pET28a plasmid vector. The vector pET-28a also has a T7 promoter, as well as exhibiting the advantages of low induced leakage, simplicity, convenience, rapid induction, and ef ficient expression of multiple genes. The biggest advantage for this study is that the pET28a plasmid vector can completely express the target protein. Further, the expressed recombinant protein has no label and can be puri fied without a restriction enzyme, which not only simpli fies the experimental procedure,but also protects the recombinant protein activity. In this study, identified pET28a-αB-crystallin recombinant plasmid was transformed into E. coli. After inducing expression, a significant recombinant protein molecular mass appeared at 20 kD band. This was revealed by protein electrophoresis and Western blot analysis, which is consistent with the target protein αB-crystal protein. The expression level of the recombinant protein is high, and the target protein was more than 30% of the total protein. The researches above indicated that the prokaryotic expression system of recombinant human αB-crystallin was successfully constructed. The recombinant protein was then purified by Q column chromatography and microfilter, and the purity of which reached over 95%. The recombinant protein was identi fied by peptide mass fingerprint mass spectrometry, which indicated that the target protein was human αB-crystallin. In conclusion, the prokaryotic expression system of pET28a is a stable and ef ficient method to prepare recombinant human αB-crystallin.
αB-crystallin (sHspB5) is one of the ten well-known members of sHsps belonging to mammalian heat shock proteins; it has the general characteristics of small molecule heat shock protein-molecular chaperone activity. In 1978 Laskey first proposed the concept of molecular chaperone,which is also known as molecular chaperone. Ellis and van der Vies[35]extended it to a class of proteins with molecular chaperone activity that is widely distributed in the body, its function is to mediate the accurate folding and assembly of proteins, and to protect the protein activity, but itself is not a functional component of the final assembly. Hendrick and Hartl[36]proposed that the molecular chaperone is a class of proteins that combine with other proteins in an unstable conformation and make them stable. Molecular chaperone by controlling the binding and separation help it’s binding protein to fold, assembly, transport and degradationin vivo[36-37].In 1992, Horwitz[38]confirmed that the alpha crystallin had molecular chaperone function, and which inhibited the thermal aggregation of b and g-crystalline protein, maintaining the transparency of the lens. Moreover, the molecular chaperone function of alpha crystallin has an inhibitory effect on the nonspecific agglutination reaction when the lens is exposed to UV irradiation, chemical modi fication, embellishment, and other kinds of denaturing agents, which is essential to maintain the transparency of the lens[39]. The alpha crystalline has two subunits, alpha A and alpha B, both of which have molecular chaperone activity. However, the molecular chaperone activity of αB-crystallin is 3 times of alpha A-crystallin in human normal temperature condition. In this study, we con firmed that the recombinant human αB-crystallin has molecular chaperone activity by the classical insulin chaperone activity test[40].
In summary, this study successfully constructed the prokaryotic expression vector carrying exogenous human αB-crystallin gene, obtained recombinant human αB-crystallin through expression and puri fication, and proved that the recombinant human αB-crystallin has distinct molecular chaperone activityin vitro. The conclusion of this study has established a solid foundation for further applications of the recombinant human αB-crystallin in the treatment of optic nerve injury.
Foundations:Supported by National Natural Science Foundation of China Grant (No.81270996); Science and Technology Project Foundation of Hainan Province (No.ZDYF201631); Health Science and Technology Innovation Project Foundation of Sanya (No.2016YW22).
Conflicts of Interest: Wang R,None;Chen ZH,None;Wang Y,None;Huang HB,None;Fan SJ,None;Chen LL,None.
1 Srivastava OP, Srivastava K. Existence of deamidated alphaB-crystallin fragments in normal and cataractous human lenses.Mol Vis2003;9:110-118.
2 Mao YW, Liu JP, Xiang H, Li DW.Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis.Cell Death Differ2004;11(5):512-526.
3 Sakaguchi H, Miyagi M, Darrow RM,Crabb JS, Hollyfield JG,Organisciak DT, Crabb JW.Intense light exposure changes the crystallin content in retina.Exp Eye Res2003;76(1):131-133.
4 Vázquez-Chona F, Song BK, Geisert EE Jr. Temporal changes in gene expression after injury in the rat retina.Invest Ophthalmol Vis Sci2004;45(8):2737-2746.
5 Kumar PA, Haseeb A, Suryanarayana P, Ehtesham NZ, Reddy GB.Elevated expression of alphaA- and alphaB-crystallins in streptozotocininduced diabetic rat.Arch Biochem Biophys2005;15;444(2):77-83.
6 Yoshimura N, Kikuchi T, Kuroiwa S, Gaun S.Differential temporal and spatial expression of immediate early genes in retinal neurons after ischemia-reperfusion injury.Invest Ophthalmol Vis Sci2003;44(5):2211-2220.
7 Iwaki T, Iwaki A, Tateishi J,Sakaki Y, Goldman JE.Alpha B-crystallin and 27-kd heat shock protein are regulated by stress conditions in the central nervous system and accumulate in Rosenthal fibers.Am J Pathol1993;143(2):487-495.
8 Renkawek K, de Jong WW, Merck KB, Frenken CW, van Workum FP,Bosman GJ. Alpha B-crystallin is present in reactive glia in Creutzfeldt-Jakob disease.Acta Neuropathol1992;83(3):324-327.
9 Renkawek K, Stege GJ, Bosman GJ. Dementia gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease.Neuroreport1999;10(11):2273-2276.
10 Pangratz-Fuehrer S, Kaur K, Ousman SS, Steinman L, Liao YJ.Functional rescue of experimental ischemic optic neuropathy with alphaB-crystallin.Eye (Lond)2011;25(6):809-817.
11 Nahomi RB, Wang B, Raghavan CT, Voss O, Doseff AI,Santhoshkumar P, Nagaraj RH. Chaperone peptides of α-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts.J Biol Chem2013;288(18):13022-13035.
12 Klopstein A, Santos-Nogueira E, Francos-Quijorna I, Redensek A,David S, Navarro X, López-Vales R. Bene ficial effects of αB-crystallin in spinal cord contusion injury.J Neurosci2012;32(42):14478-14488.
13 Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP,Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation.Proc Natl Acad Sci USA2011;108(32):13287-13292.
14 Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination.Nature2007;448(7152):474-479.
15 Rothbard JB, Kurnellas MP, Brownell S, Adams CM, Su L, Axtell RC, Chen R, Fathman CG, Robinson WH, Steinman L. Therapeutic effects of systemic administration of chaperone αB-crystallin associated with binding proin flammatory plasma proteins.J Biol Chem2012;287(13):9708-9721.
16 Kurnellas MP, Adams CM, Sobel RA, Steinman L, Rothbard JB. Amyloid fibrils composed of hexameric peptides attenuate neuroin flammation.Sci Transl Med2013;5(179):179ra42.
17 Velotta JB, Kimura N, Chang SH, Chung J, Itoh S, Rothbard J, Yang PC, Steinman L, Robbins RC, Fischbein MP. αB-crystallin improves murine cardiac function and attenuates apoptosis in human endothelial cells exposed to ischemia-reperfusion.Ann Thorac Surg2011;91(6):1907-1913.
18 Park H, Park H, Hwang HJ, Hwang HS, Kim H, Choi BR, Pak HN,Lee MH, Chung JH, Joung B. Alpha B-crystallin prevents ventricular arrhythmia by attenuating in flammation and oxidative stress in rat with autoimmune myocarditis.Int J Cardiol2015;182:399-402.
19 Reddy VS, Reddy GB. Role of crystallins in diabetic complications.Biochim Biophys Acta2016;1860(1 Pt B):269-277.
20 Saha S, Das KP. Relationship between chaperone activity and oligomeric size of recombinant human alphaA- and alphaB-crystallin: a tryptic digestion study.Proteins2004;57(3):610-617.
21 Sax CM, Piatigorsky J. Expression of the alpha-crystallin/small heatshock protein/molecular chaperone genes in the lens and other tissues.Adv Enzymol Relat Areas Mol Biol1994;69:155-201.
22 Boelens WC. Cell biological roles of αB-crystallin.Prog Biophys Mol Biol2014;115(1):3-10.
23 Hochberg GK, Benesch JL. Dynamical structure of αB-crystallin.Prog Biophys Mol Biol2014;115(1):11-20.
24 Thanos S, Böhm MR, Meyer zu Hörste M, Prokosch-Willing V, Hennig M, Bauer D, Heiligenhaus A. Role of crystallins in ocular neuroprotection and axonal regeneration.Prog Retin Eye Res2014;42;42145-42161.
25 van der Smagt JJ, Vink A, Kirkels JH, Nelen M, ter Heide H, Molenschot MM, Weger RA, Schellekens PA, Hoogendijk J, Dooijes D. Congenital posterior pole cataract and adult onset dilating cardiomyopathy: expanding the phenotype of αB-crystallinopathies.Clin Genet2014;85(4):381-385.
26 Bakthisaran R, Tangirala R, Rao ChM. Small heat shock proteins: role in cellular functions and pathology.Biochim Biophys Acta2015;1854(4):291-319.
27 Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis.J Mol Biol2015;427(7):1537-1548.
28 Haslbeck M, Peschek J, Buchner J, Weinkauf S. Structure and function of α-crystallins: traversing from in vitro to in vivo.Biochim Biophys Acta2016;1860(1 Pt B):149-166.
29 Nagaraj RH, Nahomi RB, Mueller NH, Raghavan CT, Ammar DA,Petrash JM. Therapeutic potential of α-crystallin.Biochim Biophys Acta2016;1860(1 Pt B):252-257.
30 Treweek TM, Meehan S, Ecroyd H, Carver JA. Small heat-shock proteins: important players in regulating cellular proteostasis.Cell Mol Life Sci2015;72(3):429-451.
31 Liu Z, Zhang S, Li D, Liu C. A structural view of αB-crystallin assembly and amyloid aggregation.Protein Pept Lett2017;24(4):315-321.
32 Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes.J Mol Biol1986;189(1):113-130.
33 Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes.Meth Enzymol1990;185:60-89.
34 Rosenberg AH, Studier FW. T7 RNA polymerase can direct expression of influenza virus cap-binding protein (PB2) in Escherichia coli.Gene1987;59(2-3):191-200.
35 Ellis RJ, van der Vies SM. Molecular chaperones.Annu Rev Biochem1991;60:321-347.
36 Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins.Annu Rev Biochem1993;62:349-384.
37 Hartl FU. Molecular chaperones in cellular protein folding.Nature1996;381(6583):571-579.
38 Horwitz J. Alpha-crystallin can function as a molecular chaperone.Proc Natl Acad Sci USA1992;89(21):10449-10453.
39 Hook DW, Harding JJ. Protection of enzymes by alpha-crystallin acting as a molecular chaperone.Int J Biol Macromol1998;22(3-4):295-306.
40 Ghahghaei A, Rekas A, Carver JA, Augusteyn RC. Structure/function studies of dogfish alpha-crystallin, comparison with bovine alphacrystallin.Mol Vis2009;20(15):2411-2420.