
Figure 1 IVCM image showing reduced subbasal nerves density and dendritic cells in patients with non-Sjögren dry eye disease.
Dry eye disease (DED) is defined as a complicated disorder of the tear film and ocular surface that results in symptoms of discomfort such as pain, heaviness, grittiness,burning, dryness, itchiness, foreign body sensation, visual disturbances, and tear film instability[1]. It is important to note that treating dry eye symptoms leads to a higher level of patient satisfaction. When DED is diagnosed, we evaluate not only subjective symptoms but also objective various clinical tests including the tear break-up time (TBUT), Schirmer’s wetting test, tear osmolarity, and vital dye staining of the ocular surface by fluorescein, Rose Bengal and Lissamine Green.In vivoconfocal microscopy (IVCM) is a relatively novel technology for evaluating cellular changes in DED.Using IVCM, numerous studies[2-6]have demonstrated ocular surface changes in various conditions including DED. DED is associated with comorbidities such as hypothyroidism,systemic lupus erythematosus, depression, and psychoses to state a few[7]. Patients with DED have been found to be more anxious and depressed than those without DED[8]. The association between dry eye symptoms and objective DED parameters or psychiatric disorders has been investigated in retrospective studies[6,9-11]. No comprehensive evaluation of this association, including confocal parameters and depression scale, has been conducted. The purpose of this study is twofold: to retrospectively investigate the association between dry eye symptoms and clinical or IVCM parameters in patients with DED, and to compare these parameters between eyes with DED and normal subjects.
Study PopulationThis retrospective, cross-sectional, controlled study included 25 eyes of 25 patients with DED not associated with Sjögren syndrome and 25 eyes of 25 age and gender matched healthy controls. The charts and IVCM images were reviewed for these individuals who had been seen in the University of Kitasato School of Medicine, Japan between 2015 and 2016. Patient data was anonymized before access and/or analysis. This retrospective review of the clinical charts was approved by the Institutional Review Board at Kitasato University and followed the tenets of the Declaration of Helsinki. Our Institutional Review Board waived the requirement for informed consent for this retrospective study.DED was de fined as the presence of dry eye symptoms (one or more symptoms of dry eye at least every day) associated with a Schirmer I test <5 mm and/or TBUT<10s[12]. Our patients with non-Sjögren DED were consecutively selected from the Cornea Unit of Kitasato University Hospital. The control group selection criteria were subjects with a Schirmer I test ≥5 mm and TBUT≥10s. Eyes with ocular surface discomfort or anterior segment abnormality were not included in the control group.They also were recruited from patients presenting to our ophthalmology clinic for a preoperative examination before corneal refractive or cataract surgery. Exclusion criteria for both groups were: under 18 years of age; subjects unable to complete the questionnaire or understand the procedures;subjects using topical medications that might affect the ocular surface and the cornea (except the use of nonpreserved tear substitutes in the DED group); previous ocular surgery;or contact lens wear. Patients with severe chronic diseases requiring medication such as diabetes mellitus, thyroid disease,and rheumatologic disease were also excluded. We chose one eye with more severe subjective symptoms from both eyes,when we evaluated clinical parameters in DED.
Clinical ExaminationAll patients underwent a complete examination of the ocular surface in the following order: tear osmolarity measurements, InflammaDry test, TBUT, corneal fluorescein staining, Schirmer I test, subjective symptoms questionnaires, and IVCM evaluations of the central cornea.The standard TBUT was measured. The interval between the last complete blink and the appearance of the first corneal black spot in the stained tear film was measured three times and the mean value of the measurements was calculated, after 1% fluorescein dye was instilled into the conjunctival sac. The fluorescein score was also evaluated with a 1% fluorescein solution by the 0-3 score in three different sections of the cornea as done by Shimmuraet al[13]. The staining of the superior cornea, mid-cornea, and inferior cornea was graded on a scale of 0 (no staining) to 3 (intense staining) using a slit-lamp microscope. Schirmer I test was performed without anesthetic and with the eye closed for 5min after the wetting strip was inserted into the lower conjunctival sac at the junction of the lateral and middle thirds, while avoiding contact with the cornea. The length of the wetting strips was recorded in millimeters 5min later. In flammaDry test also was performed to detect the presence of matrix metalloproteinase 9 level in the tear film. Osmolarity was measured using the TearLab system(TearLab Inc., San Diego, CA, USA). This device is intended to analyze the electrical impedance of a 50 nL tear sample collected from the inferior lateral tear meniscus. The subjective symptoms were assessed using a questionnaire of Dry Eye-Related Quality-of-Life Score (DEQS), which consists of 15 items related to dry eye symptoms and in fluence on daily life, and the overall degree of quality of life impairment is calculated as a summary score (0 to 100)[14]. We used the summary score of DEQS as subjective symptoms of DED. The tests were performed in a controlled temperature (22℃±3℃)and humidity (40%±4%). All measurements were performed by the same experienced examiner.
The history of psychiatric disorders, including depression or anxiety disorders, was determined and all regular systemic medications were recorded. Depressive symptoms were identified utilizing the Hungarian validated version of the Shortened Beck Depression Inventory (BDI)[15], which contains nine items that measure characteristic symptoms of depression(social withdrawal, indecisiveness, sleep disturbance, fatigability,somatic preoccupation, work inhibition, pessimism, lack of satisfaction and joy, and self-accusation).
In VivoConfocal MicroscopyIVCM of the cornea was performed using confocal microscopy [Rostock Cornea Module of the Heidelberg Retina Tomograph (HRT/RCM);Heidelberg Engineering GmbH, Heidelberg, Germany][3].The images comprised 384×384 pixels covering an area of 400 μm×400 μm with a transversal optical resolution of 2 μm, an axial optical resolution of 4 μm, and an acquisition time of 0.024s (Heidelberg Engineering GmbH). Images of subbasal nerves and dendritic cells of the central cornea were acquired using the same illumination intensity (manual mode)and by focusing the microscope beneath the basal epithelium.Approximately 20 images of the corneal subbasal nerve layer were captured in the central cornea for each eye and the five images (400 μm×400 μm) with most of the subbasal nerve fibers were included in quantitative analysis. We morphologically identi fied dendritic cells as bright individual dendritiform structures with cell bodies at posterior to the basal epithelial layer and anterior to Bowman’s layer (Figure 1). To evaluate the density of dendritic cell in IVCM images, ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD,USA) was used. As described previously, images of corneal subbasal nerves were analyzed retrospectively using NeuronJ(Biomedical Imaging Group) by a single researcher (Kobashi H), who was anonymized in patient identity and the results of ocular surface investigations[3]. The following parameters were determined for each image, as elaborated below: density of corneal nerves; the mean (Lmean), minimum (Lmin), and maximum length (Lmax) of corneal nerves; and dendritic cell density classi fied according to a semiquantitative scale[3,16].
Statistical AnalysisAll statistical analyses were performed using SPSS (SPSS Inc, Chicago, IL, USA). The normality of all data samples was first checked by the Kolmogorov-Smirnov test. Since the data did not fulfill the criteria for normal distribution, the Spearman’s rho coefficient was calculated to assess the relationships of dry eye symptoms with dry eye clinical parameters and comorbidities. Variables with aP-value of <0.05 in univariate analysis were entered into a multivariate analysis. Stepwise multiple regression analysis was also performed to investigate the strength of associations between several variables and the DEQS because this multivariate analysis is useful for eliminating confounding variables. The dependent variable was the DEQS. The explanatory variables included demographics, dry eye and IVCM parameters. The Mann-WhitneyUtest was used to compare each parameter between DED and control groups and the Fisher’s exact test, to compare patient sex between the two groups. A value ofP<0.05 was considered statistically significant. Power calculation was performed using PASS 2008 software (NCSS).
In univariate analysis, DEQS was associated with TBUT(ρ=-0.48,P=0.01), oral medications, such as hypotensive drug(ρ=0.57,P=0.003) and anti-depressant (ρ=0.56,P=0.004),and BDI (ρ=0.61,P=0.001; Table 1). With shorter TBUT,more frequent use of hypotensive and anti-depressant drugs,and higher BDI, dry eye symptoms signi ficantly deteriorated in eyes with DED. The results of multivariate analysis are shown in Table 1. The explanatory variables relevant to the DEQS were the anti-depressant medications (P=0.04, partial regression coefficient B=21.04) and BDI (P=0.02, B=0.76;adjustedR2=0.54). Multiple regression was expressed by the following equation: DEQS=(21.04×anti-depressant medications)+(0.76×BDI)+34.28. There was no significant correlation with other factors in multivariate analysis. The standardized partial regression coefficient was calculated to determine the magnitude of each variable’s influence. We found a signi ficant association between depression and dry eye symptoms. The demographics of the study population were shown in Table 2.

Figure 1 IVCM image showing reduced subbasal nerves density and dendritic cells in patients with non-Sjögren dry eye disease.
DED patients had significantly lower TBUT (P<0.001);higher fluorescein score (P<0.001); lower Schirmer test score (P<0.001); more symptoms: DEQS (P<0.001); higher percentage of subjects with In flammaDry positive (P=0.001);higher tear osmolarity (P<0.001); higher percentage of subjects taking anti-depressant drug (P=0.02); and higher BDI score(P<0.001) compared with the control group.
The IVCM parameters of the study population are shown in Table 3. Compared with the control group, the eye with DED group had significantly lower density of subbasal nerves(P<0.001); shorter Lmax of subbasal nerves (P=0.005); and higher density of dendritic cell (P<0.001).
To confirm the correlation between DEQS and dendritic cell density, 97.2% statistical power was offered based on the actual values; Spearman rho of 0.51, signi ficance level of <0.001, and sample size of 50.
In the present study, we found a significant association between depression and dry eye symptoms in the non-Sjögren dry eye disease patients. Several studies have evaluated independent factors affecting dry eye symptoms in DED[6,9-11].Our results were in accordance with previous epidemiological studies that showed a positive association between DED and depression[9,11]. Labbéet al[9]reported that depression score was significantly associated with dry eye symptoms using univariate and multivariate regression analysis. Although anti-depressant medications are known risk factors inducing DED[17-18], it has been shown that depression itself is involved in the pathophysiology of DED and not just its treatments[17].Previous reports showed a dysregulation of neuropeptides and an increased production of inflammatory cytokines in patients with depression[19-20], which are also mechanisms involved in DED[17]. The TBUT and the percentage of patients taking hypotensive medications were correlated with dryeye symptoms in eyes with DED in the univariate analysis.It is suggested that with shorter TBUT, more frequent use of hypotensive medication, dry eye symptoms significantly deteriorate. Our results were not in line with the previous studies[10,21]. In our patients with non-Sjögren dry eye disease,TBUT and hypotensive drug were found to have some impact on dry eye symptoms in the current study. In our study, dry eye symptoms had no signi ficant associations with each IVCM parameter, InflammaDry, and tear osmolarity. Correlations between IVCM and dry eye symptoms have been reported by some studies, but not others[5,10,22-23]. We assume that this discrepancy may be attributed to some confounding factors orsubtypes of DED. The association itself between each clinical variable might also be confounding factors when we analyze in fluential factors on the dry eye symptoms in DED. Further comprehensive studies evaluating the factors affecting dry eye symptoms in DED.
Table 1 Univariate and multivariate analysis evaluating contributors to dry eye symptoms in patients with non-Sjögren dry eye disease

DEQS: Dry eye related quality of life score; TBUT: Tear breakup time; BDI: Beck depression inventory.aAdjustedR2is a corrected goodness-of- fit measure for linear models. A value of 1 indicates a model that perfectly predicts values in the target field.
VariablesρPPartial regression coef ficient Standardized partial regression coef ficientPAge 0.03 0.90 - - -Gender -0.05 0.81 - - -TBUT -0.48 0.01 -4.82 -0.23 0.16 Fluorescein score 0.39 0.05 - - -Schirmer I test 0.02 0.91 - - -In flammaDry test 0.14 0.50 - - -Tear osmolarity -0.03 0.89 - - -Current smoking 0.20 0.34 - - -Hypotensive drug 0.57 0.003 9.93 0.20 0.27 Anti-anxiety 0.39 0.05 - - -Anti-depressant 0.56 0.004 21.04 0.36 0.04 Anti-histamine 0.10 0.64 - - -BDI 0.61 0.001 0.76 0.39 0.02 Subbasal nerves density -0.28 0.18 - - -Lmean -0.10 0.63 - - -Lmin -0.29 0.15 - - -Lmax 0.01 0.96 - - -Dendritic cell density 0.03 0.90 - - -Multiple regression - - 34.28 Constant AdjustedR²=0.54a
Table 2 Demographic characteristics of study subjects

DED: Dry eye disease; SD: Standard deviation; BDI: Beck depression inventory; TBUT: Tear breakup time; DEQS: Dry eye related quality of life score.
 Age (y) 61.8±14.9 (31-87) 67.0 61.3±13.6 (31-79) 67.0 0.97 Gender (female%) 88.0 - 88.0 - 0.99 TBUT (s) 3.8±1.1 (2.0-6.0) 4.0 10.6±2.3 (10.0-14.0) 11.0 <0.001 Fluorescein score 3.4±1.7 (0.0-6.0) 3.0 0.1±0.3 (0.0-1.0) 0.00 <0.001 Schirmer I test (mm) 12.2±2.5 (2.0-16.0) 12.0 21.7±3.9 (12.0-27.0) 23.0 <0.001 DEQS score 36.1±25.0 (8.3-90.0) 25.0 10.4±9.0 (0.0-38.3) 10.0 <0.001 In flammaDry positive (%) 72.0 - 20.0 - 0.001 Tear osmolarity (mOsm/L) 315.0±6.0 (299.3-322.7) 314.2 302.4±2.7 (294.7-307.8) 302.2 <0.001 Current smoking (%) 4.0 - 4.0 - 0.99 Medications Hypotensive drug (%) 20.0 - 16.0 - 0.99 Anti-anxiety (%) 8.0 - 12.0 - 0.99 Anti-depressant (%) 32.0 - 4.0 - 0.02 Anti-histamine (%) 16.0 - 12.0 - 0.99 BDI 16.9±13.7 (0.0-54.0) 13.7 7.8±6.7 (0.0-24.3) 6.7 0.001
Age (y) 61.8±14.9 (31-87) 67.0 61.3±13.6 (31-79) 67.0 0.97 Gender (female%) 88.0 - 88.0 - 0.99 TBUT (s) 3.8±1.1 (2.0-6.0) 4.0 10.6±2.3 (10.0-14.0) 11.0 <0.001 Fluorescein score 3.4±1.7 (0.0-6.0) 3.0 0.1±0.3 (0.0-1.0) 0.00 <0.001 Schirmer I test (mm) 12.2±2.5 (2.0-16.0) 12.0 21.7±3.9 (12.0-27.0) 23.0 <0.001 DEQS score 36.1±25.0 (8.3-90.0) 25.0 10.4±9.0 (0.0-38.3) 10.0 <0.001 In flammaDry positive (%) 72.0 - 20.0 - 0.001 Tear osmolarity (mOsm/L) 315.0±6.0 (299.3-322.7) 314.2 302.4±2.7 (294.7-307.8) 302.2 <0.001 Current smoking (%) 4.0 - 4.0 - 0.99 Medications Hypotensive drug (%) 20.0 - 16.0 - 0.99 Anti-anxiety (%) 8.0 - 12.0 - 0.99 Anti-depressant (%) 32.0 - 4.0 - 0.02 Anti-histamine (%) 16.0 - 12.0 - 0.99 BDI 16.9±13.7 (0.0-54.0) 13.7 7.8±6.7 (0.0-24.3) 6.7 0.001
Table 3 IVCM parameters in patients with DED compared with the control group
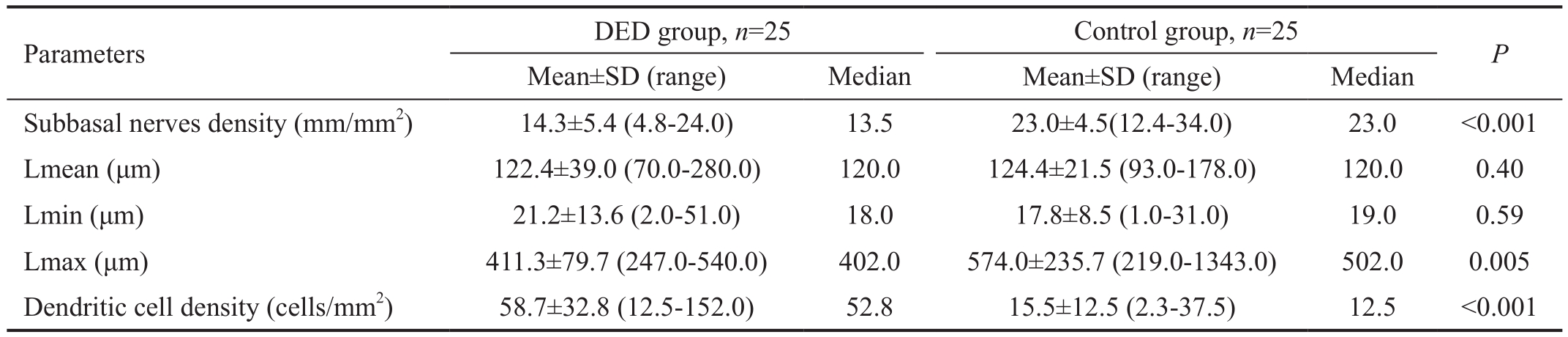
IVCM:In vivoconfocal microscopy; DED: Dry eye disease; SD: Standard deviation.
?
In the present study, the results demonstrated that compared with age and gender matched control group, corneal subbasal nerves in non-Sjögren dry eye disease patients showed lower density and dendritic cell did higher density. Subbasal corneal nerves have been previously evaluated in DED using IVCM with conflicting results. When evaluating subbasal nerve density, some authors observed a decrease[24-26], while others found no change[22,27-29]or even an increase in density in patients with DED[30]. However, most of these studies were conducted on Sjögren syndrome patients, who were different from our non-Sjögren dry eye disease patients. Such conflicting results may be due to associated confounding factors, individual variations, or the degree of disease severity.Because DED is a heterogeneous disease with multiple underlying etiologies, we need to distinguish among subtypes in terms of in flammation. In flammation has been found to play a central role in the pathogenesis of dry eye[31-32]. Dendritic cells have been demonstrated to have an association with the DED pathogenesis[33-34]. In our study, compared with the control group, the patients with non-Sjögren dry eye disease had significantly higher density of dendritic cell, which demonstrating that dendritic cells play a significant role in corneal immune homeostasis in even cases with non-Sjögren dry eye disease. Kheirkhahet al[5]reported that when aqueousdeficient DED with underlying systemic immune disease,such as Sjögren syndrome and graft versus host disease, were compared with nonimmune conditions, the immunologic subgroup demonstrated significantly higher dendritic cell density (239.6±52.9 cell/mm2), dendritic cell size, and number of dendrites. Their dendritic cell density (58.9±9.4 cell/mm2) in patients with evaporative DED was similar to that in our current non-Sjögren dry eye disease patients (58.7±32.8 cell/mm2). We believe that it is attributed to similar patient’s characteristics and dry eye de finition.
There are at least three limitations of this study. First, the sample size in this study was relatively small for detecting the correlation between DEQS and dendritic cell density.However, this study confirmed that the sample size in this study offered >90% statistical power at the 5% level. A further study with greater numbers of patients is required to con firm these preliminary findings. Second, the current study did not include Sjögren dry eye patients. A new study on the IVCM measurements in these patients is currently being conducted.Third, other dendritic cell parameters such as cell size, number of dendrites, and cell field, were not included in this study. It is also unclear if that DED patients had normal morphological corneal nerves and only their number was lower than that of normal subjects. Further studies are required to determine several IVCM parameters such as tortuosity and re flectivity of corneal nerves using an image analysis in non-Sjögren dry eye disease patients.
In conclusion, in patients with non-Sjögren dry eye disease,anti-depressant medications and depression assessment scale affect dry eye symptoms. Our findings suggest that dry eye symptoms associate with higher depressive symptoms and its medications, although our patients were not followed longitudinally. No association between IVCM parameters and dry eye symptoms can be seen. Further studies with large number of patients are necessary to confirm our preliminary findings.
Conflicts of Interest: Kobashi H, None;Kamiya K,None;Sambe T,None;Nakagawa R,None.
1 The definition and classification of dry eye disease: report of the De finition and Classi fication Subcommittee of the International Dry Eye WorkShop (2007).Ocul Surf2007;5(2):75-92.
2 Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation.Semin Ophthalmol2010;25(5-6):171-177.
3 Labbé A, Alalwani H, Van Went C, Brasnu E, Georgescu D,Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease.Invest Ophthalmol Vis Sci2012;53(8):4926-4931.
4 Marsovszky L, Németh J, Resch MD, Toldi G, Legány N, Kovács L,Balog A. Corneal Langerhans cell and dry eye examinations in ankylosing spondylitis.Innate Immun2014;20(5):471-477.
5 Kheirkhah A, Rahimi Darabad R, Cruzat A, Hajrasouliha AR, Witkin D, Wong N, Dana RZ, Hamrah P. Corneal epithelial immune dendritic cell alterations in subtypes of dry eye disease: a pilot in vivo confocal microscopic study.Invest Ophthalmol Vis Sci2015;56(12):7179-7185.
6 Kheirkhah A, Qazi Y, Arnoldner MA, Suri K, Dana RZ. In vivo confocal microscopy in dry eye disease associated with chronic graft-versus-host disease.Invest Ophthalmol Vis Sci2016;57(11):4686-4691.
7 Wang TJ, Wang IJ, Hu CC, Lin HC. Comorbidities of dry eye disease: a nationwide population-based study.Acta Ophthalmol2012;90(7):663-668.
8 Li MY, Gong L, Chapin WJ, Zhu M. Assessment of vision-related quality of life in dry eye patients.Invest Ophthalmol Vis Sci2012;53(9):5722-5727.
9 Labbé A, Wang YX, Jie Y, Baudouin C, Jonas JB, Xu L. Dry eye disease, dry eye symptoms and depression: the Beijing Eye Study.Br J Ophthalmol2013;97(11):1399-1403.
10 Labbé A, Liang Q, Wang Z, , Zhang Y, Xu L, Baudouin C, Sun X.Corneal nerve structure and function in patients with non-sjogren dry eye:clinical correlations.Invest Ophthalmol Vis Sci2013;54(8):5144-5150.
11 Szakáts I, Sebestyén M, Németh J, Birkás E, Purebl G. The role of health anxiety and depressive symptoms in dry eye disease.Curr Eye Res2016;41(8):1044-1049.
12 Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007).Ocul Surf2007;5(2):108-152.
13 Shimmura S, Ono M, Shinozaki K, Toda I, Takamura E, Mashima Y,Tsubota K. Sodium hyaluronate eyedrops in the treatment of dry eyes.Br J Ophthalmol1995;79(11):1007-1011.
14 Sakane Y, Yamaguchi M, Yokoi N, Uchino M, Dogru M, Oishi T,Ohashi Y, Ohashi Y. Development and validation of the dry eye-related quality-of-life score questionnaire.JAMA Ophthalmol2013;131(10):1331-1338.
15 Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression.Arch Gen Psychiatry1961;4:561-571.
16 Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy.Cornea2001;20(4):374-384.
17 Galor A, Feuer W, Lee DJ, Florez H, Faler AL, Zann KL, Perez VL.Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States veterans affairs administrative database.Am J Ophthalmol2012;154(2):340-346.e2.
18 Wen W, Wu YR, Chen YH, Gong L, Li MY, Chen XL, Yan MN, Xiao ZP, Sun XH. Dry eye disease in patients with depressive and anxiety disorders in Shanghai.Cornea2012;31(6):686-692.
19 Werner FM, Coveñas R. Classical neurotransmitters and neuropeptides involved in major depression: a review.Int J Neurosci2010;120(7):455-470.
20 Maes M, Bosmans E, de Jongh R, Kenis G, Vandoolaeghe E, Neels H.Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression.Cytokine1997;9(11):853-858.
21 Schmidl D, Witkowska KJ, Kaya S, Baar C, Faatz H, Nepp J,Unterhuber A, Werkmeister RM, Garhofer G, Schmetterer L. The association between subjective and objective parameters for the assessment of dry-eye syndrome.Invest Ophthalmol Vis Sci2015;56(3):1467-1472.
22 Tuominen IS, Konttinen YT, Vesaluoma MH, Moilanen JA, Helinto M, Tervo TM. Corneal innervation and morphology in primary Sjögren’s syndrome.Invest Opthalmol Vis Sci2003;44(6):2545-2549.
23 Villani E, Baudouin C, Efron N, Hamrah P, Kojima T, Patel SV,P flugfelder SC, Zhivov A, Dogru M. In VivoConfocal microscopy of the ocular surface: from bench to bedside.Curr Eye Res2014;39(3):213-231.
24 Benítez-Del-Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D,Gegúndez JA, Fernandez C, García-Sánchez J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye.Invest Ophthalmol Vis Sci2007;48(1):173-181.
25 Benítez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye.Invest Ophthalmol Vis Sci2004;45(9):3030-3035.
26 Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjögren’s syndrome: an in vivo confocal study.Invest Opthalmol Vis Sci2007;48(5):2017-2022.
27 Hoşal BM, Örnek N, Zilelioğlu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study.Eye2005;19(12):1276-1279.
28 Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren’s syndrome.Exp Eye Res2008;86(6):879-885.
29 Erdélyi B, Kraak R, Zhivov A, Guthoff R, Németh J. In vivo confocal laser scanning microscopy of the cornea in dry eye.Graefes Arch Clin Exp Ophthalmol2006;245(1):39-44.
30 Zhang M, Chen JQ, Luo LH, Xiao QG, Sun MX, Liu ZG. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy.Cornea2005;24(7):818-824.
31 Nagineni CN, William A, Cherukuri A, Samuel W, Hooks JJ, Detrick B. In flammatory cytokines regulate secretion of VEGF and chemokines by human conjunctival fibroblasts: role in dysfunctional tear syndrome.Cytokine2016;78:16-19.
32 de Paiva CS, Rocha EM. Sjögren syndrome: what and where are we looking for?Curr Opin Ophthalmol2015;26(6):517-525.
33 Barabino S, Chen YH, Chauhan S, Reza D. Ocular surface immunity:homeostatic mechanisms and their disruption in dry eye disease.Prog Retin Eye Res2012;31(3):271-285.
34 Pflugfelder SC, Stern ME. Mucosal environmental sensors in the pathogenesis of dry eye.Expert Rev Clin Immunol2014;10(9):1137-1140.