Effectiveness of 0.1% topical salicylic acid on blepharoconjunctivitis affecting glaucoma patients treated with topical prostaglandin analogues: a prospective randomized trial
Aloisa Librando, Sandra Cinzia Carlesimo, Giorgio Albanese, Giuseppe Maria Albanese, Raffaele Migliorini,Elena Pacella
Department of Sense Organs, Faculty of Medicine and Dentistry, Sapienza University of Rome, Rome 00161, Italy
Abstract● AlM: To evaluate the ef ficacy of 0.1% topical salicylic acid(TSA) to treat iatrogenic chronic blepharoconjunctivitis in patients with primary open angle glaucoma (POAG),treated with topical prostaglandin analogues (TPAs).● METHODS: Totally 60 patients were randomly distributed into 3 equal size groups, two of which treated with 0.1%TSA (OMKASA®) and 0.1% topical clobetasone butyrate(TCB; VlSUCLOBEN®) respectively, and one consisting of untreated controls. The parameters taken into account at baseline (T0) and after 30d (T1) of therapy were: conjunctival hyperemia, lacrimal function tests [Schirmer l test and break up time (BUT)] and intraocular pressure (lOP).● RESULTS: Conjunctival hyperemia showed a substantial improvement in both treated groups (P<0.001) but not among controls. Similarly, lacrimal function tests displayed an improvement of Schirmer l test in both treated groups(P<0.05) and an extension of BUT only in the group treated with 0.1% TSA (P<0.05). The lOP increase was statistically significant only in those patients treated with 0.1% TCB(P<0.001).● CONCLUSlON: The 0.1% TSA has proved to be an effective anti-inflammatory treatment of blepharoconjunctivitis affecting glaucoma patients on therapy with TPAs, leading to a sizeable decrease of inflammation as well as both quantitative and qualitative improvement of tear film.Furthermore, differently from 0.1% TCB, it does not induce any signi ficant lOP increase.
INTRODUCTION
The blepharoconjunctivitis represents a multifactorial and very common condition[1], characterized by in flammation of conjunctiva as well as anterior and/or posterior eyelid margin,accompanied by irritative symptoms of different degree and high tendency to become chronic. We can distinguish anterior and posterior blepharitis, the former affecting the lid margin anterior to the gray line and involving skin and eyelashes, and the latter involving the ori fices of the meibomian glands. There are mixed forms also, which affect the whole eyelid margin.The major symptoms reported from patients are: burning,foreign body sensations, grittiness, frequent soreness, light photofobia and itching. A typical feature is the exacerbation of symptoms in the morning. Hyperemia of the eyelid margin,edema, madarosis and poliosis are speci fic signs to be detected.Moreover, it is also possible to find hard scales and crusting around the bases of the eyelashes. The tear film instability occurring in blepharitis may be accompanied by chronic conjunctival in flammation that, if not treated appropriately, can exacerbate the dysfunction of lacrimal glands and, therefore,promote patients discomfort, creating a vicious circle. When the disease becomes chronic, symptoms and signs worsen enough to cause irregular blinking[2-3].
Frequently the subjective complaints do not match the clinical objective picture, and this contributes to complicate the management of this condition by the clinician. With regards to the aetiology, the anterior blepharitis is often caused byStaphilococcus aureus, Streptococcus, Demodex folliculorum(parasite),Helycobacter pylori,Malasessia furfur.Among the common factors that contribute to make the disease worse there are: seborrheic dermatitis, refractive uncorrected errors, immune diseases (i.e.rosacea, scleroderma, Sjögren syndrome), digestive disorders and metabolic disorders[4],such as hypercholesterolemia and diabetesetc. Meibomian glands dysfunction and alteration of their secretion are crucial factors in causing posterior blepharitis[5]. In these cases, the alteration of the lipid layer of tear film implies an increased evaporation of the aqueous component and, as a result, patients usually complain subjective discomfort and ocular dryness. Blepharoconjunctivitis can be also iatrogenic whenever induced by protracted use of topical drugs as those instilled by glaucoma patients[6]. Speci fically, the use of topical prostaglandin analogues (TPAs), in addition to determining conjunctivitis and/or blepharoconjunctivitis, can determine increased pigmentation of the lashes and distichiasis[7].
In all literature there is no consensus about blepharonconjunctivitis treatment[8]. Therapies proposed to improve both the objective and subjective issues of this condition are numerous, but none of them is widely recognized as the ideal. An effective treatment involves common measures of eyelid hygiene combined, when needed, with pharmacological therapies including the administration of topical and/or systemic antibiotics[3-6], topical corticosteroids and tear substitutes[8].These measures would decrease the bacterial overgrowth locally and, on the other hand, would contribute to improve the inflammation and restore the normal balance among the components of tear film. One of the most widely used topical antibiotics is azithromycin 1%, a second-generation macrolide,while tetracyclines (doxycycline) are the most popular as oral antibiotics. As to topical corticosteroids, dexamethasone 0.1%,loteprednol etabonate 0.5% and clobetasone butyrate (TCB)0.1% are the most common steroidal agents in use. Antiseptic agents, such as povidone iodine 10%, can also be employed in the treatment of blepharoconjunctivitis. Several clinical studies revealed that merging antibiotics and corticosteroids turns out to be signi ficantly useful as well[9-10].
However, the use of steroid eye drops in glaucoma patients could potentially result in intraocular pressure (IOP) increase with deleterious effect on the underlying optic neuropathy[2,11-13].Therefore, the use of a non-steroidal anti-in flammatory drugs(NSAID) would represent a valid alternative to improve the in flammatory process occurring on lid margin and conjunctiva,and the accompanying irritative symptoms[14].
Presently, clinical trials on the ef ficacy of 0.1% topical salicylic acid (TSA) in chronic blepharoconjunctivitis due to TPAs are not available in literature.
The aim of our study is to evaluate the effects caused by salicylic acid eye drops on glaucoma patients affected by chronic blepharoconjunctivitis and treated with TPAs.
SUBJECTS AND METHODS
The randomised clinical trial included 60 patients referred to the Glaucoma Center of the Policlinico Umberto I, from 1stto 30thMay 2016. The study was approved by the Ethics Committee of the University of Rome “La Sapienza” and was performed in accordance with the ethical standards of the Declaration of Helsinki. A written informed consent was obtained from each patient.
All patients were affected by primary open angle glaucoma(POAG), treated with TPAs and suffering from chronic blepharoconjunctivitis. According to the Declaration of Helsinki, all the patients and healthy controls were carefully informed about the use of their data and have signed the relative informed consent form.
Patients (n=60) were randomly assigned to 3 equal size groups (n=20 for each group): the first group was treated with 0.1% TSA (OMKASA®) instilled 3 times a day for 30d, the second one (n=20) treated with 0.1% TCB (VISUCLOBEN®)administered 2 times a day for 30d and the third one (n=20)was made up of untreated patients. Clinical evaluation was performed on the whole sample at baseline (T0) and at a 30-day follow up (T1). Each patient underwent the following investigations: visual acuity (expressed in logMAR);biomicroscopic examination at slit lamp; photograph of the anterior segment; schirmer I test; break up time (BUT);applanation tonometry.
Patients affected by POAG treated with TPAs, with best corrected visual acuity (BCVA) between 0 and 0.05 logMAR,IOP below 21 mm Hg and blepharoconjunctivitis for more than 30d, have been included in the clinical trial. Patients who underwent any surgical procedures to lower IOP, with seborrhoeic dermatitis or rosacea and with known history of prior dry eye were excluded.
Results of clinical examination were indicated in terms of conjunctival hyperemia intensity, from 1 (+) to 3 (+++).Schirmer I test results were expressed in mm of wetting of a No.41 Whatman filter paper strip. BUT was expressed in seconds and IOP was measured in mm Hg using Goldmann applanation tonometer.
Statistical AnalysisWith regards to statistical analysis, the Chi-square test or the Fisher’s exact test were used to compare the distribution of objective examination results at baseline among the 3 groups. The one way analysis of variation(Oneway ANOVA) was used to compare BUT, Schirmer I test and IOP among the 3 groups at baseline. Thet-test for paired data was used to evaluate variations between T0 and T1, separately for each group. For each quantitative parameter,the average change of each group and the relative confident intervals (95%CI) were calculated. The Wilcoxon Signed-Rank test was employed to evaluate the change of the outcomes over time, separately in the 3 groups and the Kruskal Wallis test was used to compare such evaluation in all the groups. AP-value <0.05 was considered statistically signi ficant. The whole analysis was performed through STATA 14.1.
Table 1 Baseline data of glaucoma patients
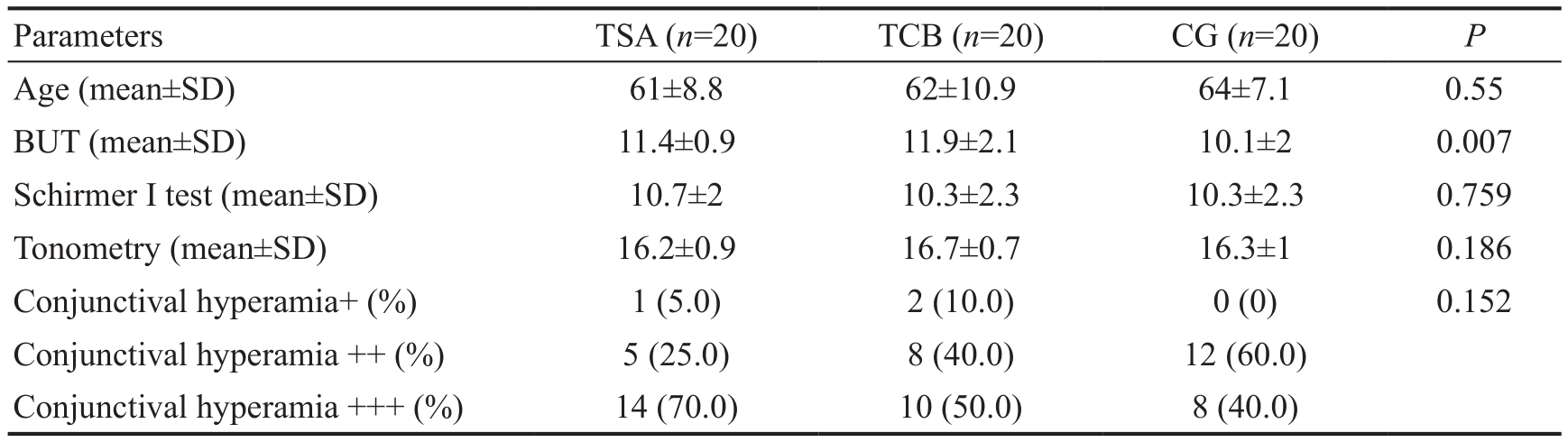
TCB: 0.1% topical clobetasone butyrate; TSA: 0.1% topical salicylic acid; CG: Control group; BUT: Break up time.
 Age (mean±SD) 61±8.8 62±10.9 64±7.1 0.55 BUT (mean±SD) 11.4±0.9 11.9±2.1 10.1±2 0.007 Schirmer I test (mean±SD) 10.7±2 10.3±2.3 10.3±2.3 0.759 Tonometry (mean±SD) 16.2±0.9 16.7±0.7 16.3±1 0.186 Conjunctival hyperamia+ (%) 1 (5.0) 2 (10.0) 0 (0) 0.152 Conjunctival hyperamia ++ (%) 5 (25.0) 8 (40.0) 12 (60.0)Conjunctival hyperamia +++ (%) 14 (70.0) 10 (50.0) 8 (40.0)
Age (mean±SD) 61±8.8 62±10.9 64±7.1 0.55 BUT (mean±SD) 11.4±0.9 11.9±2.1 10.1±2 0.007 Schirmer I test (mean±SD) 10.7±2 10.3±2.3 10.3±2.3 0.759 Tonometry (mean±SD) 16.2±0.9 16.7±0.7 16.3±1 0.186 Conjunctival hyperamia+ (%) 1 (5.0) 2 (10.0) 0 (0) 0.152 Conjunctival hyperamia ++ (%) 5 (25.0) 8 (40.0) 12 (60.0)Conjunctival hyperamia +++ (%) 14 (70.0) 10 (50.0) 8 (40.0)
RESULTS
Data from 60 patients were collected, 20 for each group,aged between 27 and 82, with a mean age of 63 and standard deviation (SD) of 9.0.
Baseline ComparisonAt T0 the 3 groups did not signi ficantly differ in terms of age, Schirmer I test, applanation tonometry and objective examination, while differences related to BUT (P=0.007) were unveiled. In particular, the post hoc analysis with Bonferroni correction showed a significant difference between the group treated with 0.1% TCB and the control group (P=0.007) and a difference, close to statistical signi ficance, was found between 0.1% TSA and control group(P=0.063), while no differences were recognized between 0.1%TCB and 0.1% TSA (P=1.00; Table 1).
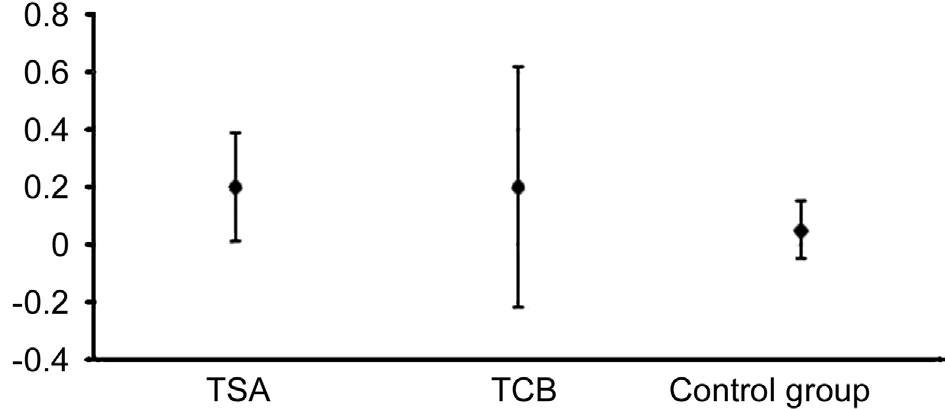
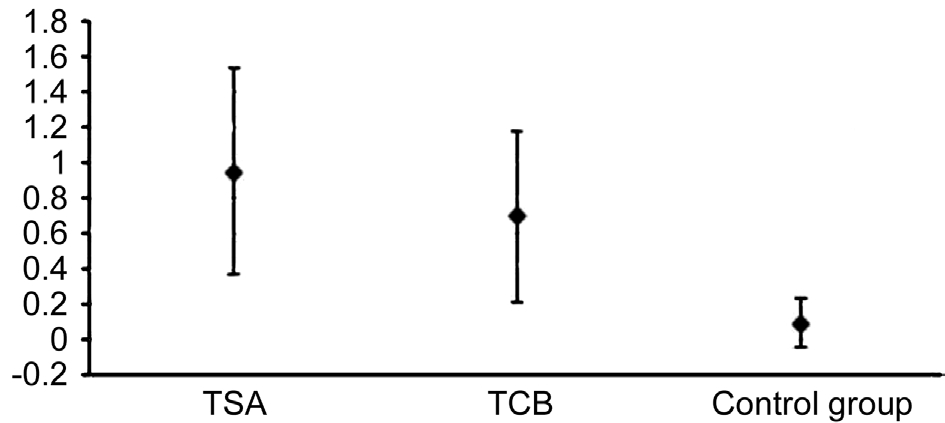
BUT experienced a slight increase in all the three groups, but the only one which showed a statistically signi ficant increase was that one treated with 0.1% TSA (P=0.042). The Schirmer I test displayed a signi ficant increase of acqueous tear production in all the groups but in the control one. The variations among the three groups differed significantly (oneway ANOVAP=0.0019). Particularly, the Bonferroni correction revealed a significant difference between 0.1% TSA group and controls(P=0.019), whereas the differences between TCB group and controls (P=0.149) and between TCB and TSA (P=1.00) were not relevant.
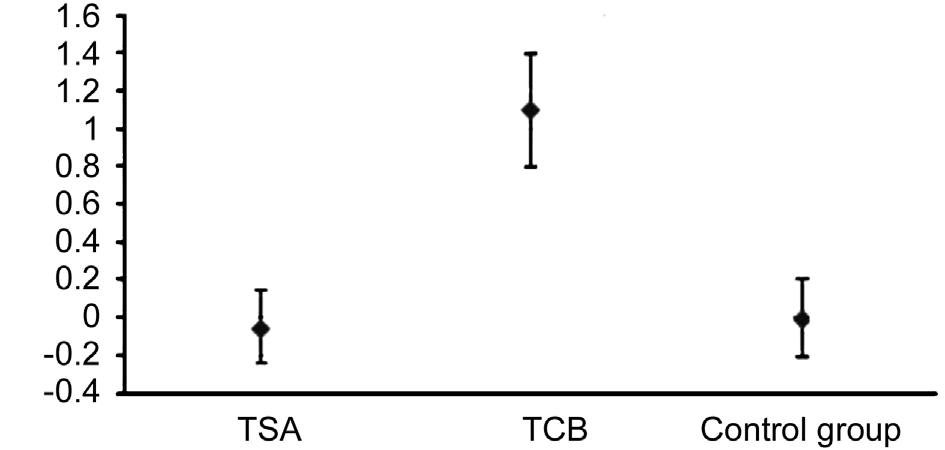
The IOP significantly increased just in the TCB group.The variation found among patients treated with TCB was significantly greater than that observed in those treated with TSA and in the control group (P>0.001 with Bonferroni correction).
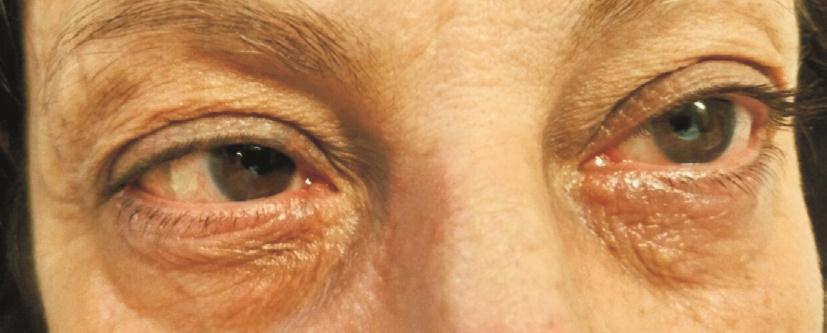
The objective examination signi ficantly changed in the treated groups but not in the control group. Among those patients treated with 0.1% TSA, the only one who showed hyperemia+ at T0, 3 out of 5 patients with hyperemia ++ and 1 out of 15 who presented with hyperemia +++ underwent a reduction to level - (hyperemia -). All the other patients decreased to hyperemia +. Therefore, all the 20 patients showed an improvement of conjunctival hyperemia by at least one class, while 17 out of 20 (85%) by at least 2 classes (Table 2;Figures 1, 2).
In the TCB group, 1 out of 2 patients with hyperemia + recovered to hyperemia -, all the other 8 hyperemia ++ and 5 out of 6 with hyperemia +++ decreased to level +, the other 5 with hyperemia +++ improved to ++. Only 1 patient out of 20 did not bene fit at all, while 14 out of 20 improved by one level.
In the control group, just 1 patient underwent an increase of hyperemia from ++ to +++ and 2 patients with hyperemia+++ spontaneously improved to ++. In conclusion, 1 patient worsened by one class, 2 patients improved by one class and the other 17 remained steady. The variations at the objective examination differed significantly among the three groups(P<0.001). Each group significantly differed from the others(P=0.001; Table 2).
Figures 3-5 illustrate the variations between T1 and T0 and the relative confidence intervals. If confidence interval does not include zero, the variation over time turns out to be statistically signi ficant. Moreover, the difference in variation among the 3 groups can also be noticed.
DISCUSSION
The purpose of our study was to evaluate the possible therapeutic effects induced by 0.1% salicylic acid eye drops on POAG patients affected by chronic blepharoconjunctivitis who are on ocular hypotensive therapy with TPA.
Blepharoconjunctivitis affecting these particular patients represents, indeed, a challenging issue that ophthalmologists frequently have to deal with[14]. TPAs, which often triggeriatrogenic blepharoconjunctivitis, represent a chronic in flammatory stimulus that causes even severe consequences on lid margin, eyelashes, meibomian glands function and,in turn, tear film stability. All the available therapies aim at obtaining an anti-inflammatory effect with a consequent improvement of both the objective and subjective aspects[5-6].Furthermore, a decrease of inflammation of the posterior lid margin leads to a restoration of normal lipid composition of the tear film, with decrease of the aqueous component evaporation and reduction of symptoms due to secondary dry eye. What is really paramount in the management of patients on ocular hypotensive therapy is the attempt to minimise the iatrogenic side effects. First of all, we should avoid any rises of IOP,which is widely illustrated in the literature to be a feature of topical steroids[10-11].
Table 2 The variations at the objective examination differed signi ficantly among the three groups

TCB: 0.1% topical clobetasone butyrate; TSA: 0.1% topical salicylic acid; CG: Control group; BUT: Break up time; T0: Time 0; T1: After 30d of therapy.
 BUT (mean±SD) 11.4±0.9 11.6±0.8 0.2 0.4 0.01 to 0.39 0.042 Schirmer I test (mean±SD) 10.7±2 11.7±1 0.95 1.2 0.37 to 1.53 0.003 Tonometry (mean±SD) 16.2±0.9 16.1±0.9 -0.05 0.4 -0.25 to 0.14 0.578 Conjunctival hyperemia - (%) 0 (0) 5 (25.0) <0.001 Conjunctival hyperemia + (%) 1 (5.0) 15 (75.0)Conjunctival hyperemia ++ (%) 5 (25.0) 0 (0)Conjunctival hyperemia +++ (%) 14 (70.0) 0 (0)Patients (n=20) TCB (T0) TCB (T1) Mean SD 95%CIPBUT (mean±SD) 11.9±2.1 12.1±1.7 0.2 0.9 -0.22 to 0.62 0.33 Schirmer I test (mean±SD) 10.3±2.3 11±1.7 0.7 1 0.22 to 1.18 0.007 Tonometry (mean±SD) 16.7±0.7 17.8±1 1.1 0.6 0.80 to 1.40 <0.001 Conjunctival hyperemia - (%) 0 (0) 1 (5.0) <0.001 Conjunctival hyperemia + (%) 2 (10.0) 14 (70.0)Conjunctival hyperemia ++ (%) 8 (40.0) 5 (25.0)Conjunctival hyperemia +++ (%) 10 (50.0) 0 (0)Patients (n=20) CG (T0) CG (T1) Mean SD 95%CIPBUT (mean±SD) 10.1±2 10.2±2 0.05 0.2 -0.05 to 0.15 0.3299 Schirmer I test (mean±SD) 10.3±2.3 10.4±2.3 0.1 0.3 -0.04 to 0.24 0.163 Tonometry (mean±SD) 16.3±1 16.3±0.8 0 0.5 -0.21 to 0.21 1 Conjunctival hyperemia - (%) 0 (0) 0 (0) 0.564 Conjunctival hyperemia + (%) 0 (0) 0 (0)Conjunctival hyperemia ++ (%) 12 (60.0) 13 (65.0)Conjunctival hyperemia +++ (%) 8 (40.0) 7 (35.0)
BUT (mean±SD) 11.4±0.9 11.6±0.8 0.2 0.4 0.01 to 0.39 0.042 Schirmer I test (mean±SD) 10.7±2 11.7±1 0.95 1.2 0.37 to 1.53 0.003 Tonometry (mean±SD) 16.2±0.9 16.1±0.9 -0.05 0.4 -0.25 to 0.14 0.578 Conjunctival hyperemia - (%) 0 (0) 5 (25.0) <0.001 Conjunctival hyperemia + (%) 1 (5.0) 15 (75.0)Conjunctival hyperemia ++ (%) 5 (25.0) 0 (0)Conjunctival hyperemia +++ (%) 14 (70.0) 0 (0)Patients (n=20) TCB (T0) TCB (T1) Mean SD 95%CIPBUT (mean±SD) 11.9±2.1 12.1±1.7 0.2 0.9 -0.22 to 0.62 0.33 Schirmer I test (mean±SD) 10.3±2.3 11±1.7 0.7 1 0.22 to 1.18 0.007 Tonometry (mean±SD) 16.7±0.7 17.8±1 1.1 0.6 0.80 to 1.40 <0.001 Conjunctival hyperemia - (%) 0 (0) 1 (5.0) <0.001 Conjunctival hyperemia + (%) 2 (10.0) 14 (70.0)Conjunctival hyperemia ++ (%) 8 (40.0) 5 (25.0)Conjunctival hyperemia +++ (%) 10 (50.0) 0 (0)Patients (n=20) CG (T0) CG (T1) Mean SD 95%CIPBUT (mean±SD) 10.1±2 10.2±2 0.05 0.2 -0.05 to 0.15 0.3299 Schirmer I test (mean±SD) 10.3±2.3 10.4±2.3 0.1 0.3 -0.04 to 0.24 0.163 Tonometry (mean±SD) 16.3±1 16.3±0.8 0 0.5 -0.21 to 0.21 1 Conjunctival hyperemia - (%) 0 (0) 0 (0) 0.564 Conjunctival hyperemia + (%) 0 (0) 0 (0)Conjunctival hyperemia ++ (%) 12 (60.0) 13 (65.0)Conjunctival hyperemia +++ (%) 8 (40.0) 7 (35.0)
In this context, our study aims to demonstrate for the first time the effectiveness of TSA to treat glaucoma patients affected by iatrogenic chronic blepharoconjunctivitis.
The comparison of TSA with a topical low-potency steroid,such as clobetasone butyrate, has been adopted in order to better support our findings.
The outcomes indicate that, although both drugs exhibit statistically significant efficacy in reducing inflammation,TCB may cause a statistically signi ficant IOP rise compared to patients treated with TSA. This aspect ends up with being even more critical if we consider that all the treated patients have a glaucomatous optic neuropathy that can worsen owing to a prolonged rise of IOP over the entire course of steroid drops.In this respect, it would have been useful to include intermediate follow up between T0 and T1 to exactly determine at what time the iatrogenic increase in IOP started. However, the evidences have demonstrated that topical clobetasone can cause slighter IOP increase than topical dexamethasone, prednisolone,hydrocortisone and betamethasone[9-14].
With regards to tear film, if on one hand the Schirmer I test highlights a quantitative improvement of the aqueous component in both the treated groups, on the other hand a significant stabilisation of the tear film was only obtained in patients treated with salicylic acid. The restoration of meibomian gland function in these patients led to a decrease in the evaporation of the aqueous component with following improvement of the irritative symptoms related to eye dryness.Studies evaluating the effectiveness of salicylic acid in the treatment of blepharoconjunctivitis caused by prostaglandin analogues as well as a comparison with topical steroids are not currently available in literature.
In conclusion, the introduction of 0.1% salicylic acid eye drops represents a valid therapeutic alternative for the treatment of chronic blepharoconjunctivitis affecting glaucoma patients on medication with TPAs therapy. The improvement has been strongly shown both in terms of anti-inflammatory effectiveness and tear film stability, without any secondary IOP increases. We intend to extend our research to a larger sample in order to increase the statistical validity of the present clinical trial.
ACKNOWLEDGEMENTS
Conflicts of Interest: Librando A,None;Carlesimo SC,None;Albanese G,None;Albanese GM,None;Migliorini R,None;Pacella E,None.
REFERENCES
1 Lemp MA, Nichols KK. Blepharitis in the United States 2009: a surveybased perspective on prevalence and treatment. Ocul Surf2009;7(2 Suppl):S1-14.
2 Cunniffe MG, Medel-Jiménez R, González-Candial M. Topical antiglaucoma treatment with prostaglandin analogues may precipitate meibomian gland disease.Ophthal Plast Reconstr Surg2011;27(5):e128-129.
3 Pflugfelder SC, Karpecki PM, Perez VL. Treatment of blepharitis:recent clinical trials.Ocul Surf2014;12(4):273-284.
4 Feher J, Kovacs I, Pacella E, Keresz S, Spagnardi N, Balacco Gabrieli C. Pigment epithelium-derived factor (PEDF) attenuated capsaicin-induced neurotrophic keratouveitis.Invest Ophthalmol Vis Sci2009;50(11):5173-5180.
5 Pelletier JS, Stewart KP, Capriotti K, Capriotti JA. Rosacea blepharoconjunctivitis treated with a novel preparation of dilute povidone iodine and dimethylsulfoxide: a case report and review of the literature.Ophthalmol Ther2015;4(2):143-150.
6 Hamann KU, Grob D. Therapy of blepharoconjunctivitis with eye drops contaning salicylic acid.J Prakt Augenheilkd1995;16:97-100.
7 Pacella E, Pacella F, Cavallotti C, Librando A, Feher J, Pecori-Giraldi J. The combination latanoprost-timolol versus twice daily 0.50% timolol administration either associated or not with latanoprost: efficacy and tolerability in the primary open-angle glaucoma.Eur Rev Med Pharmacol Sci2010;14(5):477-480.
8 Hosseini K, Lindstrom RL, Foulks G, Nichols KK. A randomized,double-masked, parallel-group, comparative study to evaluate the clinical ef ficacy and safety of 1% azithromycin-0.1% dexamethasone combination compared to 1% azithromycin alone, 0.1% dexamethasone alone, and vehicle in the treatment of subjects with blepharitis.Clin Ophthalmol2016;10:1495-1503.
9 Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroidsIndian Dermatol Online J2011;2(2):67-69.
10 Dunne JA, Travers JP. Double-blind clinical trial of topical steroids in anterior uveitis.Br J Ophthalmol1979;63(11):762-767.
11 Williamson J, Eilon LA, Walker SR. Clobetasone butyrate eye drops.Effect on ocular in flammation and intraocular pressure.Trans Ophthalmol Soc UK1981;101(1):27-29.
12 Cavallotti C, Artico M, Pescosolido N, Tranquilli Leali FM, Pacella E. Distribution of peptidergic nerve fibres in the guinea pig trabecular meshwork.Anat Histol Embryol2000;29(6):387-391.
13 Pacella F, Turchetti P, Santamaria V, Impallara D, Smaldone G,Brillante C, Librando A, Damiano A, Pecori-Giraldi J, Pacella E.Differential activity and clinical utility of latanoprost in glaucoma and ocular hypertension.Clin Ophthalmol2012;6:811-815.
14 Ramsell TG, Bartholomew RS, Walker SR. Clinical evaluation of clobetasone butyrate: a comparative study of its effects in postoperative in flammation and on intraocular pressure.Br J Ophthalmol1980;64(1):43-45.
● KEYWORDS:blepharoconjunctivitis; glaucoma; intraocular pressure; lacrimal function tests; prostaglandin analogues;topical clobetasone butyrate; topical salicylic acid
Citation:Librando A, Carlesimo SC, Albanese G, Albanese GM,Migliorini R, Pacella E. Effectiveness of 0.1% topical salicylic acid on blepharoconjunctivitis affecting glaucoma patients treated with topical prostaglandin analogues: a prospective randomized trial.Int J Ophthalmol2018;11(12):1936-1940
DOl:10.18240/ijo.2018.12.10
Received:2017-02-05 Accepted: 2018-05-17
Correspondence to:Elena Pacella. Department of Sense Organs, Faculty of Medicine and Dentistry, Sapienza University of Rome, Viale del Policlinico, Rome 00161, Italy.elena.pacella@uniroma1.it

 Age (mean±SD) 61±8.8 62±10.9 64±7.1 0.55 BUT (mean±SD) 11.4±0.9 11.9±2.1 10.1±2 0.007 Schirmer I test (mean±SD) 10.7±2 10.3±2.3 10.3±2.3 0.759 Tonometry (mean±SD) 16.2±0.9 16.7±0.7 16.3±1 0.186 Conjunctival hyperamia+ (%) 1 (5.0) 2 (10.0) 0 (0) 0.152 Conjunctival hyperamia ++ (%) 5 (25.0) 8 (40.0) 12 (60.0)Conjunctival hyperamia +++ (%) 14 (70.0) 10 (50.0) 8 (40.0)
Age (mean±SD) 61±8.8 62±10.9 64±7.1 0.55 BUT (mean±SD) 11.4±0.9 11.9±2.1 10.1±2 0.007 Schirmer I test (mean±SD) 10.7±2 10.3±2.3 10.3±2.3 0.759 Tonometry (mean±SD) 16.2±0.9 16.7±0.7 16.3±1 0.186 Conjunctival hyperamia+ (%) 1 (5.0) 2 (10.0) 0 (0) 0.152 Conjunctival hyperamia ++ (%) 5 (25.0) 8 (40.0) 12 (60.0)Conjunctival hyperamia +++ (%) 14 (70.0) 10 (50.0) 8 (40.0)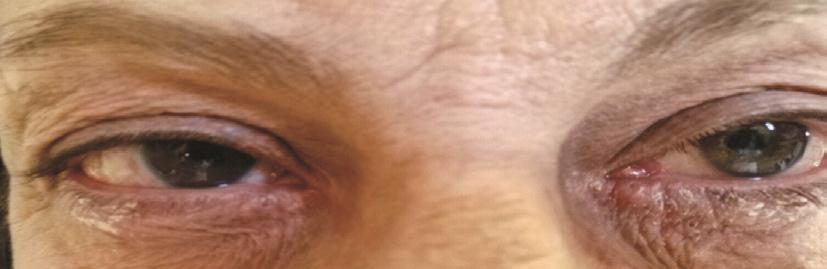

 BUT (mean±SD) 11.4±0.9 11.6±0.8 0.2 0.4 0.01 to 0.39 0.042 Schirmer I test (mean±SD) 10.7±2 11.7±1 0.95 1.2 0.37 to 1.53 0.003 Tonometry (mean±SD) 16.2±0.9 16.1±0.9 -0.05 0.4 -0.25 to 0.14 0.578 Conjunctival hyperemia - (%) 0 (0) 5 (25.0) <0.001 Conjunctival hyperemia + (%) 1 (5.0) 15 (75.0)Conjunctival hyperemia ++ (%) 5 (25.0) 0 (0)Conjunctival hyperemia +++ (%) 14 (70.0) 0 (0)Patients (
BUT (mean±SD) 11.4±0.9 11.6±0.8 0.2 0.4 0.01 to 0.39 0.042 Schirmer I test (mean±SD) 10.7±2 11.7±1 0.95 1.2 0.37 to 1.53 0.003 Tonometry (mean±SD) 16.2±0.9 16.1±0.9 -0.05 0.4 -0.25 to 0.14 0.578 Conjunctival hyperemia - (%) 0 (0) 5 (25.0) <0.001 Conjunctival hyperemia + (%) 1 (5.0) 15 (75.0)Conjunctival hyperemia ++ (%) 5 (25.0) 0 (0)Conjunctival hyperemia +++ (%) 14 (70.0) 0 (0)Patients (