Table 1 Comparation of average MD between consecutive visual acuity groups in 928 observed eyes at the patients’ first visit

MD: Mean deviation.P: compared with upper line of average MD.
?
Retinitis pigmentosa (RP), characterized by lengthy process and miserable outcome, is presently a leading cause of human blindness all over the world. The primary pathogenesis of RP is degeneration of photoreceptors, which leads to a decline of sensitivity in visual field, and subsequent night blindness as well as deterioration of visual acuity.One of the visual field indexes, mean deviation (MD), is the average elevation or depression of visual sensitivity in the patient’s overall field compared with that of the normal agecorrected reference fieldand can be used to estimate visual field sensitivity and meanwhile to estimate severity of RP[1-4].
The present paper focused on MD data of 928 observed eyes from 928 RP patients, investigated the correlation between MD and visual acuity, as well as that between MD and retinal pigmentation in these eyes. Among the 928 observed eyes,201 were followed up for around 10y (9-11y). The authors observed the MD decreasing rate in the 201 eyes during 10y,and inquired into the influence of gender, age, RP family history and retinal pigmentation at the first visit on the rate, in case to investigate the associations between demographic and clinical factors with the MD decline in RP patients.
ParticipantsAfter informed consent was obtained from the patients in accord with Declaration of Helsinki, the study was carried out in the Department of Ophthalmology with approval from our hospital.
A clinical diagnosis of RP was determined based on the following criteria: 1) rod-predominant degeneration shown on subjective symptoms, that is, night blindness; 2) fundus examination showing typical arteriolar attenuation with or without retinal pigmentation; 3) constriction of visual field defects.Patients were included in this study when meeting all the three criteria at their first visit to our Department of Ophthalmology.At the first visit, exclusion criteria comprised: 1) history of ocular trauma, intraocular surgery, or suffering from other intraocular diseases except ametropia; 2) presence of refractive media opacities that would disturb vision examination,fundus examination and visual field examination; 3) RP syndromes like Usher syndrome, Laurence Moon-Biedl syndrome, Refsum syndrome, Kears-Sayre syndrome, Jeune syndrome and Bassen-Kornzweig syndrome; 4) atypical RP like unilateral pigmentary retinopathy, sectorial pigmentary retinopathy, crystalline retinopathy, and fundus albipunctatus;5) suffering from severe systemic diseases such as cirrhosis and lung cancer; 6) best-corrected visual acuity worse than 0.02 (decimal fraction).
Those, who were followed up for around 10y but underwent intraocular diseases, intraocular surgeries or eye diseases that would induce refractive media opacity in the period of 10y,were excluded from the study of “MD decreasing rate”.
From October 2002 to June 2016, with complete information and eligible for inclusion, 928 RP patients (aged from 9 years old to 63 years old with mean age 37.59±12.30y) were enrolled in this study at their first visit to our Retina Division of Ophthalmology Department. Among the 928 patients,201 (aged from 9 years old to 53 years old with mean age 33.72±10.09y at their first visit) were followed up for around 10y (9-11y).
MethodsFirst of all, some self-created phrases in this paper need explaining. Observed eye means the eye with better visual acuity in a RP patient at the first visit. Data of visual acuity,MD and retinal pigmentation in this paper all originated from the observed eyes. First MD means the MD of an observed eye at the first visit. The 10y MD means the MD of an observed eye in about 10y after the first visit.
At each visit to our department, doctors inquired the RP patient’s eye disease history, family history of RP and general physical condition, and made an ocular examination. The items of the ocular examination included visual field, visual acuity, slit-lamp microscope, ophthalmoscope or fundus photography, and non-contact tonometry. Patients’ gender, age,family history of RP, general physical condition, MD, visual acuity and retinal pigmentation were recorded. Based on the data from the 928 patients’ first visit, the authors analysed the correlation between MD and visual acuity, as well as that between MD and retinal pigmentation in these patients’observed eyes.
Among the 928 RP patients, 201 were followed up for around 10y (9-11y). The authors calculated the MD decreasing rate during 10y in the 201 patients, and inquired into the in fluence of gender, age, RP family history and retinal pigmentation at the first visit on the rate.
Standard Logarithmic/Decimal Visual acuity Chart, made in Guangzhou Yuexiu Medical Instrument Factory according to National Standard GB11533-89 of the People’s Republic of China, was used to check visual acuity by decimal recording.There were no 0.9 and 0.7 visual acuity lines on the chart.Although RP is often bilateral, in most patients the severity is not the same in different eyes. Choosing the eye with less severity as observed eye may facilitate long period fellow up and statistical data processing. We choose the eye with better naked vision in emmetropia or better corrected vision in ametropia as observed eye in this paper. Only the visual acuity of observed eye was brought into statistical analysis.
Binocular indirect ophthalmoscope (Model YZ25, 66 visual Polytron Technologies Inc., China) and fundus camera (Model FF450 plus, Carl Zeiss Meditec©, Germany) were used for fundus examination. Before the observation, pupil was dilated with tropicamide eye drops. The items of observation included retinal vessels and retinal pigmentation. Only the data from observed eyes would be collected for statistics.
Visual field was assessed by a Humphrey Visual Field analyzer(Humphrey Instruments, California, USA) that ran 30-2 SITA Fast Programs to measure 30 degrees temporally and nasally and test 76 points. After understanding operational approach,the patient underwent dark adaption for 5-10min, then one eye (corrected with spectacles if suffering from ametropia)of the patient was selected at random to test firstly and the other non-tested eye was covered with an opaque patch. The patient was positioned appropriately and comfortably against the forehead rest and chin rest. Minor adjustments to the head position were made to centre the pupil on the display screen to allow eye monitoring throughout the test. The patients used a handheld button that they pressed to indicate when they saw a light, in case to assess their retina’s ability to detect a stimulus at speci fic points within the visual field. This ability was called visual field sensitivity and was recorded in decibels(dB). MD, one of visual field indices and derived from the total deviation of visual field and representing the overall mean departure from the age-corrected norm, was used in this paper to represent the visual field sensitivity. A negative value of MD indicates field loss. All measurements were carried out by the same technician. Visual fields data were excluded if fixation loss, false positive response and false negative response rates were greater than 20%.
Statistical AnalysisSPSS 19.0 was used for regression analysis of the correlation between MD and visual acuity of the 928 observed eyes. Independent two-samplet-test was used for difference in the comparation of average MD between consecutive visual acuity groups, and the in fluence of gender,age, RP family history and retinal pigmentation at the first visit on the 10 years’ MD decreasing rate in 201 eyes. And paired samplest-test was used for difference between first MD and 10y MD of 201 observed eyes. All the statistics usedP<0.05 for statistical signi ficance.
Correlation Between MD and Visual Acuity as well as that between MD and Retinal Pigmentation
Mean deviation and visual acuityAverage MD and visual acuity of the 928 observed eyes at the patients’ first visit were-14.44±8.61dB and 0.79±0.35. By regression analysis with SPSS 19.0, the regression coef ficient between MD and visual acuity was 1.247 and the linear regression equation was y (visual acuity)=1.2473+0.0319×MD (R2=0.6168,P<0.001).
When visual acuity was at 1.0 and above, or at 0.3 and below,no signi ficant differences of the average MD existed between consecutive visual acuity groups, but when visual acuity was from 1.0 to 0.3, significant differences of average MD appeared between consecutive visual acuity groups (Table 1). It seemed that average MD decreased more rapidly when visual acuity was among 1.0 to 0.3. On the other hand, when average MD decreased to -9.18 dB, the visual acuity still kept normal.When average MD was lower than -26.72 dB the visual acuity would be lower than 0.3, already in the range of low vision.
Average mean deviation and retinal pigmentationThe medium of average MD between eyes with or without retinal pigmentation in 928 observed eyes at the first visit was-14.82 dB. In 13.25% (123/928) of observed eyes the average MD was lower than -14.82 dB but no retinal pigmentation appeared. On the contrary, in those with average MD higher than -14.82 dB, pigmentation arose on retina in 16.49%(153/928) of observed eyes (Table 2).
Mean Deviation Decreasing Rate and In fluence Factors to the Rate in 201 Observed Eyes
Mean deviation rate during 10y in 201 observed eyesThe average MD (first MD) of 201 observed eyes at first visit was -10.89±5.48 dB, and 10y later their average MD (10y MD) was -18.91±5.52 dB. Signi ficant difference (t=31.0857,P<0.001) existed between the first MD and 10y MD. The average difference value in 10y between first MD and 10y MD was -8.01±3.66 dB, meaning 0.8 dB decreased in MD every year averagely.
Influence of gender, age, RP family history and retinal pigmentationTable 3 showed there were no significant effects of gender, age and RP family history on MD decreasing rate. In observed eyes with retinal pigmentation at first visit, the difference value between first MD and 10y MD was significantly larger than that in eyes without retinal pigmentation.
MD indicates the mean difference between the normal expected retinal sensitivity in terms of age and visual acuity and the measured patient sensitivity[5]. This definition has already indicated the comparation with normal eyes, so there is no need to set a normal comparative group when MD is used for clinical research. In this paper, as MD is the major research index, there is also no need to set normal comparative groups for minor indexes such as visual acuity, gender, age, and so on.Calculating according to the above linear regression equation of MD on visual acuity in 928 observed eyes at the patients’first visit, the authors expected that when visual acuity was lower than 1.0 the MD should be lower than -7.75 dB. As theR2value of the equation was 0.6168, it was possible that the equation couldn’t well express the correlation between MD and visual acuity. Table 1 showed when average MD inobserved eyes was -9.18 dB, the visual acuity was still at 1.0 and above. It indicated that in the early stage of RP the visual acuity of observed eyes would maintain normal. The socalled early stage in this paper was that when average MD was higher than or equal to -9.18 dB, but the authors suggested the clinical early stage of RP should be de fined as the period from the onset of the disease to the time point when patient’s visual acuity was just under 1.0. This definition accorded with the pathologic process of RP. The progressive atrophy of the rod photoreceptor cells leads to the secondary death of the cones in RP, affected individuals often present with night blindness and constricted visual fields, but in the early stage of RP the central vision is normal or nearly normal. Eventually central vision is lost as the cone cells degenerate[6-9]. Clinically, visual field becomes more and more narrow, accordingly visual field sensitivity or MD is lower and lower. When MD is low to a certain extent, visual acuity will be less than 1.0.
Table 1 Comparation of average MD between consecutive visual acuity groups in 928 observed eyes at the patients’ first visit

MD: Mean deviation.P: compared with upper line of average MD.
?
Table 2 Average MD and retinal pigmentation in 928 observed eyes at the patients’ first visit
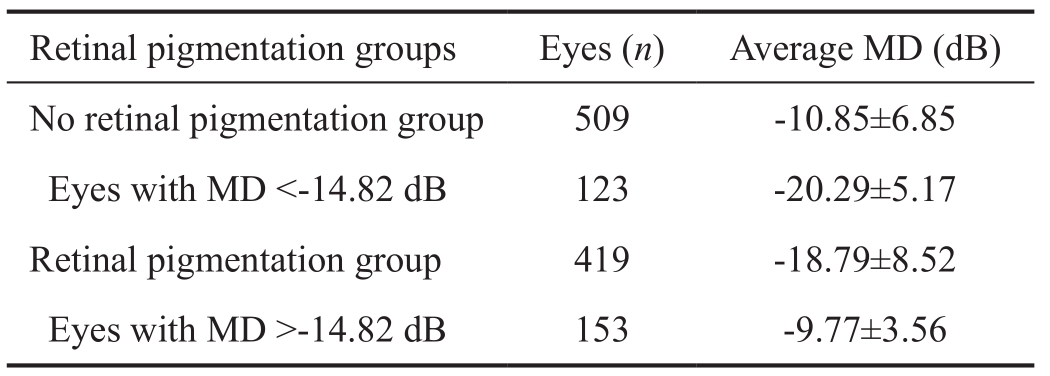
MD: Mean deviation.
?
Table 3 Influence of gender, age, RP family history and retinal pigmentation at the first visit on MD decreasing rate in 201 observed eyes
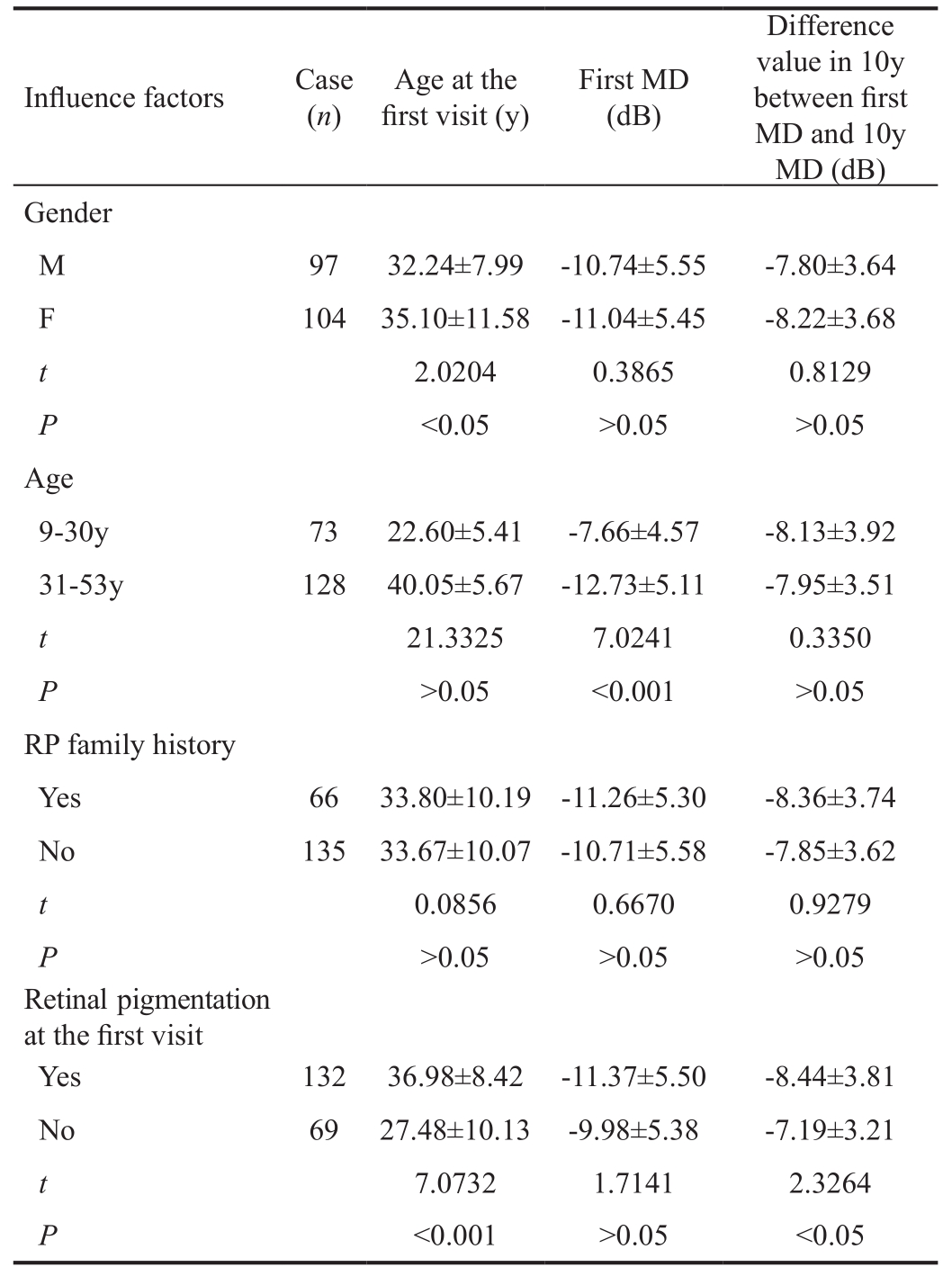
MD: Mean deviation.
?
Retinal pigment epithelium (RPE), composed of a single layer of hexagonal cells with pigment granules and directly on the neuroepithelium (i.e.rods and cones), has several functions such as absorbing scattered light, supplying nutrients to photoreceptors, phagocytosing photoreceptor outer segment membranes, participating in visual cycle, and so on[10]. With the progression of RP, RPE cells often detach from Bruch’s membrane and migrate to perivascular sites in the inner retina,producing so-called bone spicule pigment and surrounding the branching retinal blood vessels[11]. The appearance of retinal pigmentation heralds a cure in estimating the severity of RP,but the amount of pigmentation on retina does not necessarily reflect the severity of RP[12]. In this paper the authors only observed whether retinal pigmentation exited on retina, not emphasizing the amount and site of the pigmentation.
According to our study of 928 observed eyes, median in average MD value of two groups with and without retinal pigmentation was -14.82 dB. It seemed retinal pigmentation should appear when MD was lower than -14.82 dB, but actual situation was not completely like that. From Table 2, 123(13.25% of 928 eyes) observed eyes were non-pigmented even with MD less than -14.82 dB, while in other 153 (16.49%of 928 eyes) eyes, contrary to what one might suppose,pigmentation arose in retina when MD was higher than-14.82 dB. It was worthy of further study.
As for the reasons for inconsistency between MD and retinal pigmentation, error was firstly considered. It was undeniable that some errors in different degrees certainly existed in clinical research, the reasons why the MDs did not correspond with retinal pigmentation in so many cases were not so simple.As to those eyes with MD lower than -14.82 dB but nonpigmented, the most likely reason was that the patients suffered from retinitis pigmentosa sine pigmento (RPSP), a type of atypical RP. Characteristics of RPSP are as same as the typical RP including night blindness, reduced visual acuity,visual field constriction, optic disc pallor, attenuated retinal vessels and typical electroretinography (ERG) changes, but no pigment deposits in the retina, the characteristics constitute the diagnostic criteria of RPSP[13]. Both typical RP and RPSP can maintain a good central visual acuity after symptoms appear for a long time[13-14], the only difference is that in RPSP there is no pigment deposits in the retina. Generally RPSP accounted for about 10% of all RP[15], and the authors believed the majority of the 13.25% of eyes might belong to RPSP. It suggested the possibility of RPSP in RP diagnosis as to those with MD less than -14.82 dB and non-pigmented.
In our study, there were 153 (16.49% of 928 eyes) observed eyes with retinal pigmentation but MD higher than -14.82 dB.It seemed RP in these eyes originated from RPE instead of rod, which explains the late damage of rod function[16]. As RPE takes up nutrients such as glucose, retinol, and fatty acids from capillary of choroid and delivers these nutrients to photoreceptors, the authors suggest that a treatment by dilating blood vessels should be performed in RP patients with retinal pigmentation but MD higher than -14.82 dB, because in the world today there is no really effective clinical therapy for RP.The average MD of 201 observed eyes decreased by -8.01 dB in around 10y, 0.8 dB decreased per year averagely. According to the speed, from the onset of RP, 40y later the patient’s MD will reach -32 dB. By clinical experience, the visual field grayscale with MD -32 dB looks like a black paper, hinting the patient’s low life quality. There are few reports published about visual field loss. Several years ago Bersonet al[17]followed up 94 patients over three years and found visual field (detected with Goldmann perimeter) loss was about 4.6% per year, but Massofet al[18]reported that the visual field loss in 172 RP patients was 9%-10% per year. The patients in this paper were mobile and in around 10y they might go to different hospitals to receive several kinds of treatment, including the treatment with traditional Chinese medicine. Due to the uncertain ef ficacy of present clinical RP treatment, the above parameter,0.8 dB decreased per year in MD, was valuable in the clinical estimation of RP severity.
From the data of Table 3, the average MD decreasing rate was not related to gender, age or RP family history. To our knowledge, very few reports involved the effect of sex and age on RP. Tanabeet al[19]mentioned if an RP patient had affected sibling, the risk for male siblings was about 1.7 times higher than for female siblings. As the onset ages of RP differ from patient to patient and in autosomal dominant forms of RP the onset age can even be as late as 50y of age[6], it is dif ficult to discuss the relationship between age and clinical presentation of RP. RP family history is often relevant to the pattern of inheritance. Pierrottetet al[20]and Anasagastiet al[21]proposed that various forms of RP can be classified according to the pattern of inheritance, including autosomal dominant (15%-40% of cases), autosomal recessive (5%-60% of cases), and X-linked (5%-15%). The remaining cases are currently of unclassi fied inheritance. These unclassi fied RP patients have a negative family history of RP, and are denoted as simplex RP.The majority of simplex RP cases are believed to be autosomal recessive[22]. According to that, the majority of the 153 patients in Table 3 who had not RP family history could be classi fied into some kind of hereditary pattern. The 153 patients would be the first RP patient of their families and from them on the inheritance began. It might be the reason why there was no effect of RP family history on MD decreasing rate in our paper.Table 3 showed the MD decreasing rate in pigmented eyes(-8.44±3.81 dB) was more rapid than that in non-pigmented eyes (-7.19±3.21 dB). The difference was signi ficant. As was mentioned above, with the progression of RP, RPE cells often detach from Bruch’s membrane and migrate to perivascular sites in the inner retina, producing so-called bone spicule pigment and surrounding the branching retinal blood vessels[7].Extracellular matrix formed from RPE is usually deposited between the RPE and endothelial cells of the thin walled venules and capillaries, resulting in an attenuation in retinal vessels and decline in retinal function. As a result the MD decreasing rate in pigmented eyes signi ficantly exceeds that in non-pigmented eyes.
Electrophysiological testing is valuable in the diagnosis of RP because ERG abnormalities occur early and often precede the characteristic fundoscopic signs[23]. It has been estimated that RP patients lose approximately 17% of remaining ERG amplitude per year[24]. As the ERG device was bought only two years ago in our department, there is a lack of ERG data in a large majority of the inclusive patients in this observation, this is a weakness in this paper.
In summary, the authors analysed the correlation between MD of visual field and visual acuity as well as that between MD and retinal pigmentation in 928 RP eyes, calculated MD decreasing rate in 201 RP eyes that were followed up for around 10y, and investigated the effects of gender, age, RP family history and retinal pigmentation on the MD decreasing rate in the 201 RP eyes. Results showed the linear regression equation of the correlation between MD and visual acuity in 928 RP eyes was y (visual acuity)=1.2473+0.0319×MD. When actual MD was lower than -9.18 dB the visual acuity would be below 1.0. As to the correlation between MD and retinal pigmentation, the average MD in pigmented eyes was significantly lower than that in non-pigmented eyes. In 13.25% of 928 eyes the MD was already low but there was no appearance of pigment in retina, possibly the majority of the 13.25% of eyes suffering from RPSP. As for other 16.49% of eyes with high MD as well as retinal pigmentation at the same time, the authors speculated that RP originated from RPE instead of rod in these eyes.
The MD decreasing rate in RP eyes was 0.8 dB every year averagely. There were no signi ficant effects of gender, age and RP family history on the rate, but in pigmented eyes the rate was signi ficantly more rapid than that in non-pigmented eyes.In a word, the value of MD could well re flect the severity of RP.
Conflicts of Interest: Ye H, None; Xia XP, None.
1 Bakbak B, Gedik S, Koktekir BE, Guzel H, Altınyazar HC. Structural and functional assessment in patients treated with systemic isotretinoin using optical coherence tomography and frequency-doubling technology perimetry.Neuroophthalmology2013;37(3):100-103.
2 Kaczorowski K, Mulak M, Szumny D, Baranowska M, Jakubaszko-Jabłońska J, Misiuk-Hojło M. Comparison of visual field measurement with heidelberg edge perimeter and humphrey visual field analyzer in patients with ocular hypertension.Adv Clin Exp Med2016;25(5):937-944.
3 Tawada A, Sugawara T, Ogata K, Hagiwara A, Yamamoto S. Improvement of central retinal sensitivity six months after topical isopropyl unoprostone in patients with retinitis pigmentosa.Indian J Ophthalmol2013;61(3):95-99.
4 Pahor D. Retinal light sensitivity in haemodialysis patients.Eye (Lond)2003;17(2):177-182.
5 Molina-Martín A, Piñero DP and Pérez-Cambrodí RJ. Decreased perifoveal sensitivity detected by microperimetry in patients using hydroxychloroquine and without visual field and fundoscopic anomalies.J Ophthalmol2015;2015:437271
6 Wert KJ, Lin JH, Tsang SH. General pathophysiology in retinal degeneration.Dev Ophthalmol2014;53(1):33-43.
7 Giacalone JC, Wiley LA, Burnight ER, Songstad AE, Mullins RF, Stone EM, Tucker BA. Concise Review: patient-speci fic stem cells to interrogate inherited eye disease.Stem Cells Transl Med2016;5(2):132-140.
8 Punzo C, Xiong W, Cepko CL. Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame?J Biol Chem2012;287(3):1642-1648.
9 Sancho-Pelluz J, Arango-Gonzalez B, Kustermann S, Romero FJ,van Veen T, Zrenner E, Ekström P, Paquet-Durand F. Photoreceptor cell death mechanisms in inherited retinal degeneration.Mol Neurobiol2008;38(3):253-269.
10 Alexander P, Thomson HAJ, Luff AJ, Lotery AJ. Retinal pigment epithelium transplantation: concepts, challenges and future prospects.Eye(Lond)2015;29(8):992-1002.
11 Pach J, Kohl S, Gekeler F, Zobor D. Identi fication of a novel mutation in the PRCD gene causing autosomal recessive retinitis pigmentosa in a Turkish family.Mol Vis2013;19(19):1350-1355.
12 Hamel C. Retinitis pigmentosa.Orphanet J Rare Dis2006;1(1):40-51.
13 Chang S, Vaccarella L, Olatunji S, Cebulla C, Christoforidis J.Diagnostic challenges in retinitis pigmentosa: genotypic multiplicity and phenotypic variability.Curr Genomics2011;12(4):267-275.
14 Ma L, Sheng XL, Li HP, Zhang FX, Liu YN, Rong WN, Zhang JL.Identi fication of a novel p.R1443W mutation in RP1 gene associated with retinitis pigmentosa sine pigmento.Int J Ophthalmol2013;6(4):430-435.
15 Sun XW, Yin XB, Li MD, He T, Cui H, Li G. Bilateral retinitis pigmentosa sine pigmento, one case report.Zhonghua Yan Ke Za Zhi2015;51(5):380-382.
16 Alexander P, Thomson HA, Luff AJ, Lotery AJ. Retinal pigment epithelium transplantation: concepts, challenges, and future prospects.Eye(Lond) 2015;29(8):992-1002.
17 Berson EL, Sandberg MA, Rosner B, Birch DG, Hanson AH. Natural course of retinitis pigmentosa over a three-year interval.Am J Ophthalmol1985;99(3):240-251.
18 Massof RW, Dagnelie G, Benzschawel T, Palmer RW, Stein DF. First order dynamics of visual field loss in retinitis pigmentosa.Clin Vision Sci1990;5(1):1-26
19 Tanabe U, Fujiki K, Hayakawa M, Nakajima A, Kabasawa K. The empirical risk of retinitis pigmentosa in Japan.Nippon Ganka Gakkai Zasshi1992;96(2):231-236.
20 Pierrottet CO, Zuntini M, Digiuni M, Bazzanella I, Ferri P, Paderni R, Rossetti LM, Cecchin S, Orzalesi N, Bertelli M. Syndromic and non-syndromic forms of retinitis pigmentosa: a comprehensive Italian clinical and molecular study reveals new mutations.Genet Mol Res2014;13(4):8815-8833.
21 Anasagasti A, Irigoyen C, Barandika O, López de Munain A, Ruiz-Ederra J. Current mutation discovery approaches in retinitis pigmentosa.Vision Res2012;75(1):117-129.
22 Wert KJ, Davis RJ, Sancho-Pelluz J, Nishina PM, Tsang SH. Gene therapy provides long-term visual function in a pre-clinical model of retinitis pigmentosa.Hum Mol Genet2013;22(3):558-567.
23 Whatham AR, Nguyen V, Zhu Y, Hennessy M, Kalloniatis M. The value of clinical electrophysiology in the assessment of the eye and visual system in the era of advanced imaging.Clin Exp Optom2014;97(2):99-115.
24 Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW,Weigel-DiFranco C, Willett W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa.Arch Ophthalmol1993;111(6):761-772.