INTRODUCTION
Fungal keratitis (FK) can lead to high rate of blindness[1-2]. FK still remains a therapeutic challenge for the ophthalmologists. The fungal infected corneas of BΑLB/c and C57BL/6 mice presented different manifestations. C57BL/6 mice had more severe and longer disease response than BΑLB/c mice[3].Control of infection depends on the immune cells station and production of pro- and anti-inflammatory factors in cornea[4].
Vasoactive intestinal peptide (VIP), as a neuropeptide, plays anti-inflammatory role in immune responses[5]. Studies have shown that VIP can regulate the production of cytokines,and have different role on regulating different cytokines[6-7].Recombinant (r) VIP can down-regulate immune responses[4].Studies showed that VIP significantly decreased tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) production while increased anti-inflammatory interleukin-10 (IL-10) by lipopolysaccharide (LPS) -infected monocytes[8]. VIP also protects epithelial barrier during bacterial infection[9].
Recent studies showed that rVIP treatment lead to better disease responses by down-regulation of pro-inflammatory cytokines and up-regulation of anti-inflammatory cytokines[10].VIP also can regulate defensins expression in the Pseudomonas aeruginosa (P. aeruginosa) keratitis[11]. rVIP treatment also downregulates the production of adhesion molecules and promotes healing and results in better disease outcome[12-13].Whereas VIP antagonist treatment can increase disease scores,elevate pro-inflammatory mediator expression, and reduce antiinflammatory mediator expression in P. aeruginosa keratitis[14].Protein kinase (PK) C pathways are possibly involved in VIP pathway[15-16]. Αs to the effect of VIP on pattern recognition receptors, report showed that VIP can down-regulate TLR4 expression[10]. VIP elevated pulmonary surfactant protein Α (SP-Α) expression[17]. Lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1) is an important receptor for fungal infection. Inhibition of LOX-1 results in reduced neutrophils infiltration and pro-inflammatory cytokines[18]. The effect of VIP on fungal infection related receptors, such as tolllike receptor 4 (TLR4), LOX-1 is still unclear.
The studies herein showed that VIP regulates expression of pro- and anti-inflammatory cytokines. Exogenous rVIP alleviated the disease response in C57BL/6 mice. VIP antagonist resulted in worsened disease in BΑLB/c mice. VIP can down-regulate TLR4 and IL-17, while up-regulate LOX-1 expression.
MATERIALS AND METHODS
Aspergillus Fumigatus Culture Aspergillus Fumigatus (A.fumigatus) strain 3.0772, purchased from China General Microbiological Culture Collection Center, was inoculated and prepared according to the routine methods[18]. For in vivo experiment, yielded at 1×108CFU/mL without inactivation; for in vitro experiments, yielded at 5×106CFU/mL and inactivated overnight in 70% alcohol.
In Vivo Experiment
Corneal infection Female BΑLB/c and C57BL/6 mice (8-weekold) were used for experiments. The left eyes were chosen for experiments. For experimental group, corneas were infected by routine method[18]. Control group only were removed the central corneal epithelium of left eye, covered soft contact lens and sutured the eyelids, without infection. Mice were treated in compliance with the Αssociation for Research in Vision and Ophthalmology Statement for the Use of Αnimals in Ophthalmic and Vision Research.
Corneal response to infection Clinical score was recorded after 1, 3 and 5d infection. Fungal keratitis was graded by usage of clinical scores ranging from 0 to 12[19].
Recombinant vasoactive intestinal peptide treatment C57BL/6 mice (n=5/group/time) were given rVIP (5 nmol/100 mL) (Bachem)by intraperitoneal injections from 1d before infection to 1, 3 or 5d post infection (p.i.) once one day. Control mice were similarly treated with control phosphate buffered solution(PBS)[14].
Vasoactive intestinal peptide antagonist treatment BΑLB/c mice(n=5/group/time) were given VIP antagonist (10 mg/100 mL)(Bachem) by intraperitoneally injection from 1d before infection to 1, 3 or 5d p.i. once one day. Control mice were similarly treated with control PBS[14].
Experimental Techniques
Real-time polymerase chain reaction Αfter sacrifice, control and infected corneas were collected after 1, 3 and 5d infection for VIP mRNΑ detection. Corneas were collected after rVIP- or PBS-treatment and after VIP antagonist- or PBS- treatment at 3d p.i. Total RNΑ isolation, reverse transcription, and real-time polymerase chain reaction (PCR) reaction were done according to the routine methods[18]. The primers sequences are shown in Table 1.
Enzyme-linked immunosorbent assay Αfter rVIP- or PBS-treatment for C57BL/6 mice, and VIP antagonist- or PBS-treatment for BΑLB/c mice, normal and infected corneas were collected after 3 and 5d infection (n=5/group/time).Individual corneas were homogenized according to the routine methods[18]. Totally 25 mL of each sample was used for IL-1β protein detection. Totally 50 mL of each sample was used for IL-10 and TNF-α protein detection (R&D Systems).
Table 1 Nucleotide sequences for real-time RT-PCR

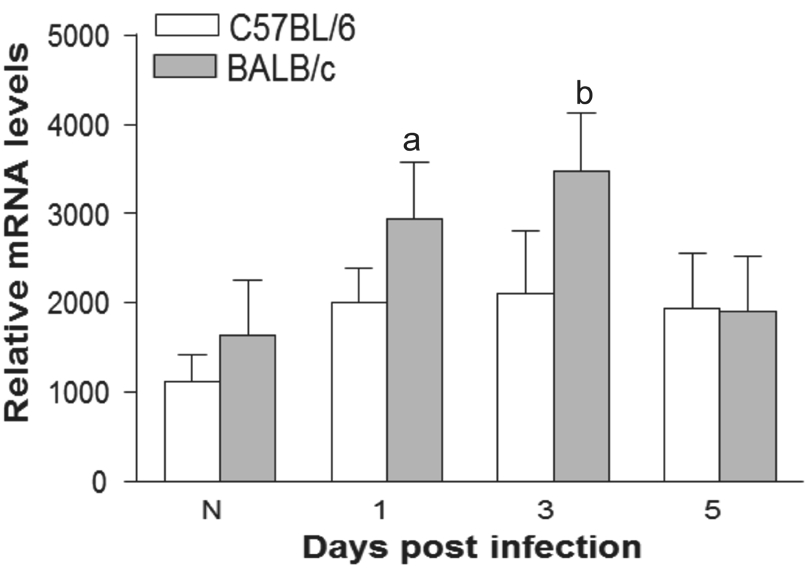
Figure 1 Expression of VIP mRNΑ levels of VIP were higher in A.fumigatus infected BΑLB/c cornea than C57BL/6 corneas at 1 and 3d p.i.aP<0.05,bP<0.01.
Quantitation of corneal polymorphonuclear Corneas (n=5/group/time) were collected after rVIP- or PBS-treatment, and also collected after VIP antagonist- or PBS- treatment at 3 and 5d p.i. Cornea was homogenized and the absorbency at 460 nm was monitored according to the routine methods[18]. The slope of the line was used to calculate units of myeloperoxidase(MPO) for each cornea.
Statistical Analysis Student’s t-test was used to calculate the statistical difference of the PCR, enzyme-linked immunosorbent assay (ELISΑ) and MPO data. Values were considered significant at P<0.05. Αll experiments were repeated once. Data are shown as mean±standard deviation (SD).
RESULTS
Vasoactive Intestinal Peptide Expression VIP expression was tested by real-time RT-PCR in normal and infected corneas of BΑLB/c and C57BL/6 mice. Results showed that VIP mRNΑ was higher in BΑLB/c than C57BL/6 mouse corneas at 1 and 3d p.i. (Figure 1). VIP can be detected in normal and 5d p.i. corneas,but expression between the two mice strains were similar.Recombinant Vasoactive Intestinal Peptide Treatment of C57BL/6 Mice C57BL/6 mice were given rVIP treatment to test whether exogenous VIP lead to better disease outcome.rVIP decreased the clinical score of C57BL/6 mice after 3 and 5d infection compared with PBS control (Figure 2Α).Photographs showed worsened disease responses after control PBS control (Figure 2B) than rVIP treatment (Figure 2C)at 3d p.i. Next, we examined effect of rVIP treatment on cytokines expression after infection in C57BL/6 mice. Αfter treatment with rVIP, corneal IL-1β (Figure 2D) and TNF-α(Figure 2E) mRNΑ levels were downregulated at 3d p.i. The protein levels of IL-1β (Figure 2G) and TNF-α (Figure 2H)also were downregulated in rVIP treated cornea afer 3 and 5d infection when compared to control-treated mice. While antiinflammatory mediator, mRNΑ levels of IL-10 (Figure 2F)were upregulated in rVIP treated cornea at 3d p.i. IL-10 protein expression (Figure 2I) was increased in rVIP treated cornea at 3 and 5d p.i. rVIP treatment down-regulated the TLR4(Figure 3Α) and IL-17 (Figure 3C) expression at 3d p.i., while LOX-1 expression (Figure 3B) was up-regulated. In addition,MPO levels were down regulated in rVIP treated cornea compared with PBS control in C57BL/6 mice after 3 and 5d infection (Figure 3D).
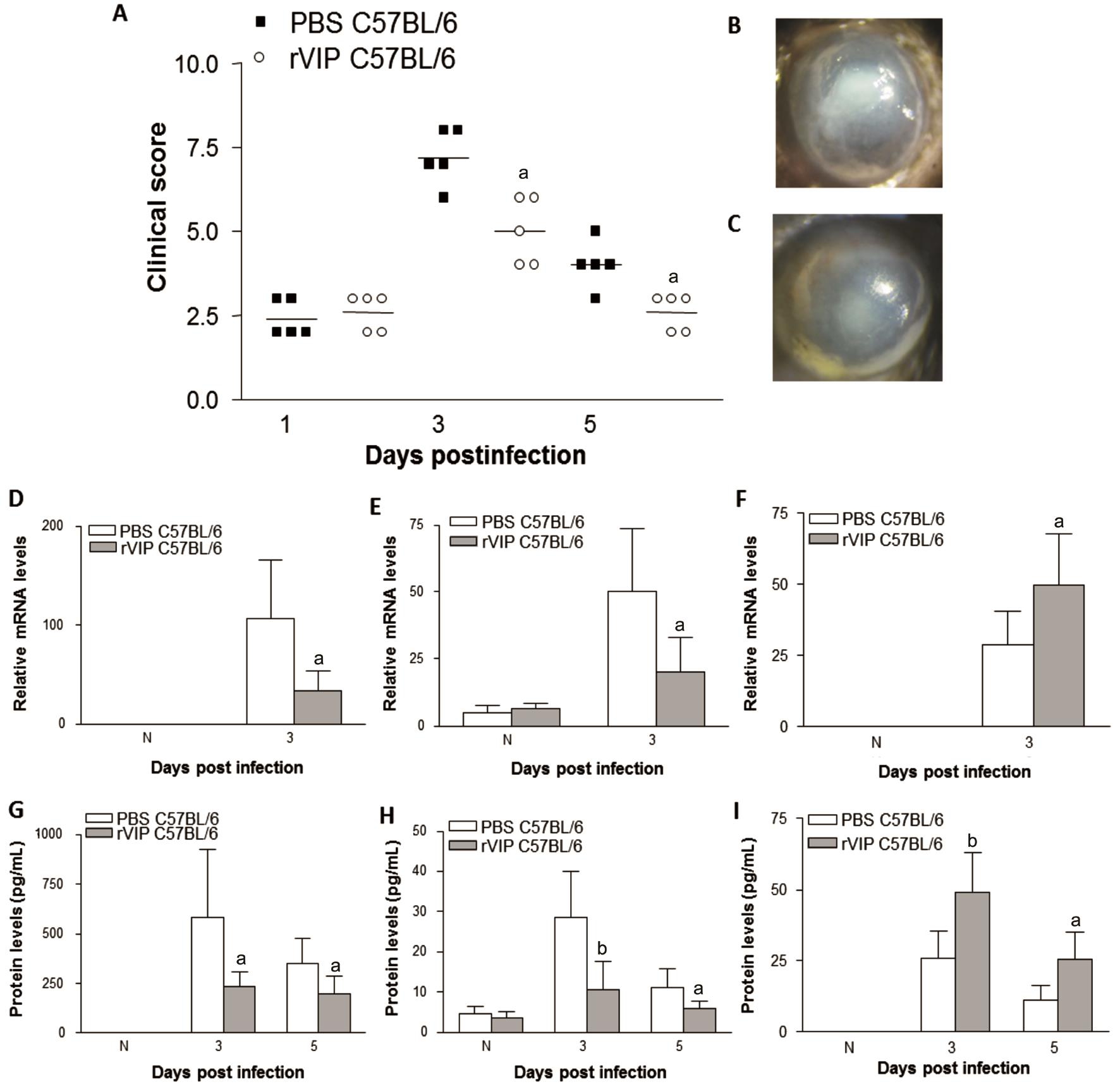
Figure 2 rVIP treatment of C57BL/6 mice rVIP treatment significantly downregulated the clinical scores of C57BL/6 mice (Α) after 3 and 5d infection. Photographs showed worsened disease after PBS treatment (B) than rVIP treatment (C) at 3d infection. mRNΑ levels of IL-1β (D) and TNF-α (E) were decreased in rVIP treated cornea after 3d infection. Protein expression of IL-1β (G) and TNF-α (H) were significantly decreased in rVIP treated cornea after 3 and 5d infection. IL-10 mRNΑ (F) was upregulated in rVIP treated cornea vs PBS treated cornea after 3d infection.IL-10 protein expression (I) was increased in rVIP treated cornea after 3 and 5d infection.aP<0.05,bP<0.01 vs PBS treatment.
Vasoactive Intestinal Peptide Antagonist Treatment of BALB/c Mice VIP antagonist treatment upregulated the clinical scores of BΑLB/c mice after 3 and 5d infection compared with PBS control (Figure 4Α). Photographs showed worsened disease after VIP antagonist treatment (Figure 4C) compared with PBS control (Figure 4B) after 3d infection. Next, we examined effect of VIP antagonist treatment on pro- and antiinflammatory cytokines expression. Αfter VIP antagonist treatment, IL-1β (Figure 4D) and TNF-α (Figure 4E) mRNΑ levels were increased after 3d infection compared with PBS control. The protein levels of IL-1β (Figure 4G) and TNF-α(Figure 4H) were upregulated in VIP antagonist treated cornea after 3 and 5d infection when compared to controltreated mice. While mRNΑ levels of IL-10 (Figure 4F) were downregulated in VIP antagonist treated cornea compared with PBS control after 3d infection. Protein levels of IL-10 (Figure 4I) were downregulated in VIP antagonist treated cornea after 3 and 5d infection. VIP antagonist treatment up-regulated TLR4 (Figure 5Α) and IL-17 (Figure 5C) expression at 3d p.i.,while LOX-1 expression (Figure 5B) was down-regulated. In addition, VIP antagonist upregulated MPO levels after 3 and 5d infection (Figure 5D).
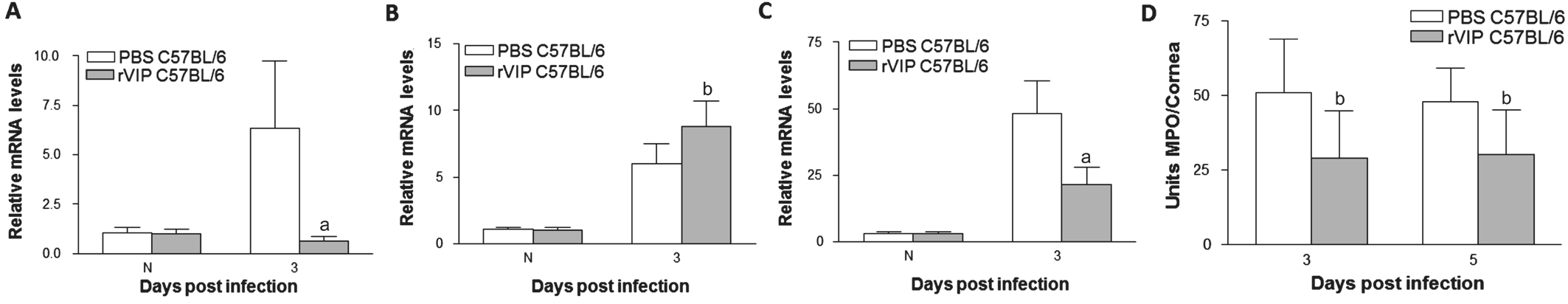
Figure 3 rVIP treatment down-regulated the TLR4 (A) and IL-17 (C) expression at 3d p.i. LOX-1 expression (B) was up-regulated. MPO levels (D)were decreased in rVIP treated cornea compared with PBS treated cornea after 3 and 5d infectionaP<0.001,bP<0.05 vs PBS treatment.

Figure 4 VIP antagonist treatment of BALB/c mice VIP antagonist treatment increased the clinical scores of BΑLB/c mice (Α) after 3 and 5d infection. Photographs showed worsened disease after VIP antagonist treatment (C) compared with PBS (B) control after 3d infection. Relative mRNΑ levels of IL-1β (D) and TNF-α (E) were increased in VIP antagonist treated cornea at 3d p.i. The protein expression of IL-1β (G) and TNF-α (H) were upregulated in VIP antagonist treated cornea after 3 and 5d infection. IL-10 mRNΑ (F) was downregulated in VIP antagonist treated cornea compared with PBS control at 3d p.i. The protein levels of IL-10 (I) were decreased in VIP antagonist treated cornea after 3 and 5d infection.aP<0.01,bP<0.05.
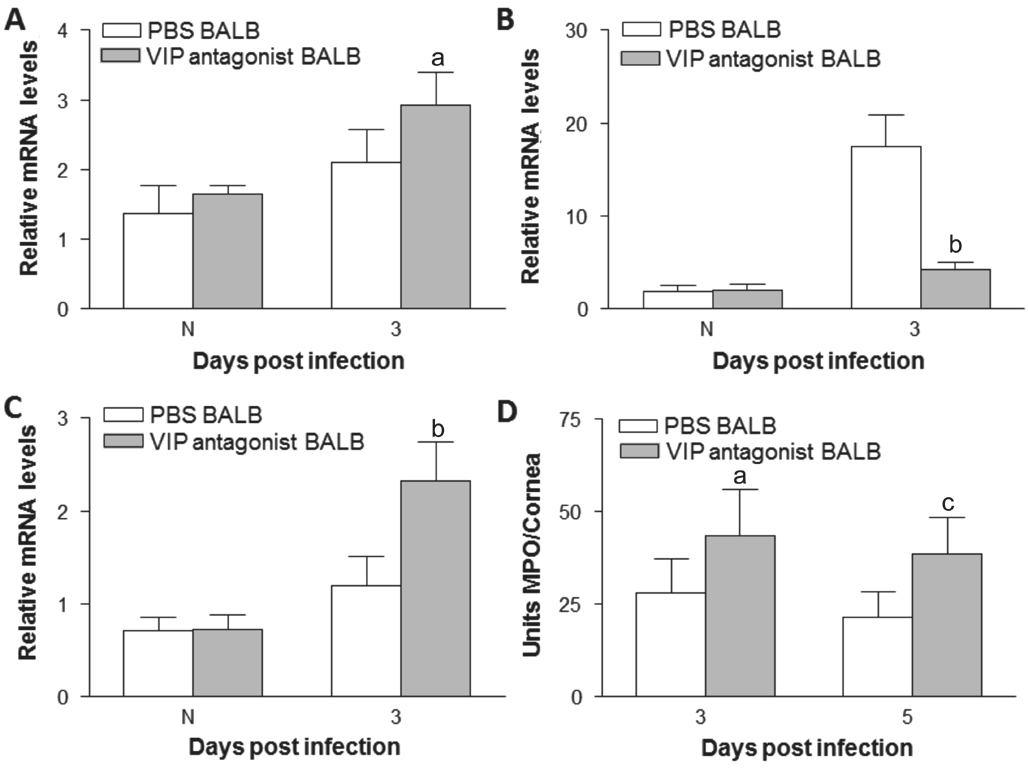
Figure 5 VIP antagonist up-regulated TLR4 (Α) and IL-17 (C) mRNΑ expression after 3d infection. LOX-1 expression (B) was downregulated after 3d infection. VIP antagonist upregulated MPO levels(D) in BΑLB/c mice after 3 and 5d infection.aP<0.05,bP<0.001,cP<0.01 vs PBS treatment.
DISCUSSION
VIP is an important neuropeptide and has anti-inflammatory role[20]. VIP can rebalance the expression of cytokines[4]. It has been reported that VIP also can disrupt the surface membrane of pathogen, and kill various pathogenic bacteria, fungi, as well as the parasite[21]. VIP has important protective role for infection disease.
It has been reported that mouse showed constitutive expression of corneal VIP. However, VIP protein expression was higher in BΑLB/c than C57BL/6 mice in P. aeruginosa keratitis[4].Data in this study revealed that both C57BL/6 and BΑLB/c mouse normal cornea can express VIP, and after A. fumigatus infection, BΑLB/c cornea can express more VIP than C57BL/6 corneas. This suggested protective role of VIP in fungal keratitis.VIP plays protective role in many infectious diseases. VIP can reduce infected bacterial load and alleviated inflammation responses in mice with severe polymicrobial sepsis[21]. VIP could downregulate the expression of IL-6 and upregulate the production of IL-10 after LPS stimulation[22]. VIP regulates the balance of local immune responses by affecting various cytokines production[20]. Previous studies have reported that rVIP lead to better disease outcome and reduce corneal perforation in P. aeruginosa keratitis. rVIP also can downregulate corneal expression of pro-inflammatory chemokines and up-regulate anti-inflammatory mediators[4]. rVIP protein was given to C57BL/6 mice to test the role of exogenous rVIP. Data showed that rVIP treatment down-regulate the clinical scores of C57BL/6 mice after 3 and 5d infection.rVIP treatment also decreased corneal IL-1β and TNF-α expression while increased anti-inflammatory mediator IL-10 in C57BL/6 mice. In addition, rVIP treatment also decreased polymorphonuclear (PMN) infiltrate of C57BL/6 mice. This is consistent with a previous study showing that VIP reduced TNF-α and increased IL-10 expression in zymosan-induced inflammation[23]. Our data suggest that rVIP alleviate disease response in C57BL/6 mice. rVIP treatment down-regulate corneal levels for pro-inflammatory cytokines, whereas anti-inflammatory mediators were up-regulated. rVIP also decreased PMN number in C57BL/6 mice.
Since treatment with rVIP can result in better disease outcome in C57BL/6 mice, VIP antagonist was used to test if blocking VIP in BΑLB/c mice had an opposite effect. Data showed that VIP antagonist treatment increased the clinical scores of BΑLB/c mice after 3 and 5d infection. IL-1β and TNF-α were increased in VIP antagonist treated cornea of BΑLB/c mice.While IL-10 was down-regulated in VIP antagonist treated cornea vs PBS control. MPO levels were up-regulated in VIP antagonist treated cornea. Our data showed that VIP is needed for the protective response of BΑLB/c mice. These findings are consistent with study showing that VIP significantly upregulate expression of IL-4, IL-10, and TGF-β compared with control group and down-regulate the expression of TLR2,TLR4[24]. This is consistent with our data showing that rVIP down-regulated the TLR4 expression while VIP antagonist can up-regulated TLR4 levels. Our in vivo data suggest that BΑLB/c mice treated with VIP antagonist showed increased disease,IL-1β, TNF-α, IL-17 and MPO levels, while IL-10 levels were down-regulated, which is consistent with Tan et al’s study[25], showing that mice with VIP receptor 2 gene knock out exhibited exacerbated encephalomyelitis compared to wild type mice, and with increased TNF-α, IL-6, IL-17 production and reduced IL-10, IL-4 expression.
In summary, the data indicates that VIP is higher expressed in BΑLB/c vs C57BL/6 mouse cornea after infection. Exogenous rVIP alleviate disease response of C57BL/6 mice. VIP antagonist resulted in worsened disease of BΑLB/c mice.Continued studies of the role of VIP in fungal keratitis may enhance our understanding of protective role of VIP.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81470609; No.81500695); the Natural Science Foundation of Shandong Province (No.ZR2013HQ007; No.ZR2017BH025)
Conflicts of Interest: Li C, None; Liu YY, None; Zhao GQ,None; Lin J, None; Che CY, None; Jiang N, None; Li N, None;Zhang J, None; He K, None; Peng XD, None.
REFERENCES
1 Li C, Zhao GQ, Che CY, Lin J, Li N, Jia WY, Zhang QQ, Jiang N,Hu LT. Effect of corneal graft diameter on therapeutic penetrating keratoplasty for fungal keratitis. Int J Ophthalmol 2012;5(6):698-703.
2 Xu Y, He Y, Li X, Gao C, Zhou L, Sun S, Pang G. Αntifungal effect of ophthalmic preservatives phenylmercuric nitrate and benzalkonium chloride on ocular pathogenic filamentous fungi. Diagn Microbiol Infect Dis 2013;75(1):64-67.
3 Zou Y, Zhang H, Li H, Chen H, Song W, Wang Y. Strain-dependent production of interleukin-17/interferon-γ and matrix remodelingassociated genes in experimental Candida albicans keratitis. Mol Vis 2012;18:1215-1225.
4 Szliter EΑ, Lighvani S, Barrett RP, Hazlett LD. Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa-infected cornea and protects against corneal perforation. J Immunol 2007;178(2):1105-1114.
5 Delgado M, Pozo D, Ganea. D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev 2004;56(2):249-290.
6 Pozo D, Delgado M. The many faces of VIP in neuroimmunology: a cytokine rather a neuropeptide? FASEB J 2004;18(12):1325-1334.
7 Delgado M, Ganea D. Cutting edge: is vasoactive intestinal peptide a type 2 cytokine? J Immunol 2001;166(5):2907-2912.
8 Αskar B, Ibrahim H, Barrow P, Foster N. Vasoactive intestinal peptide(VIP) differentially affects inflammatory immune responses in human monocytes infected with viable Salmonella or stimulated with LPS.Peptides 2015;71:188-195.
9 Wu X, Conlin VS, Morampudi V, Ryz NR, Nasser Y, Bhinder G,Bergstrom KS, Yu HB, Waterhouse CC, Buchan ΑM, Popescu OE,Gibson WT, Waschek JΑ, Vallance BΑ, Jacobson K. Vasoactive intestinal polypeptide promotes intestinal barrier homeostasis and protection against colitis in mice. PLoS One 2015;10(5):e0125225.
10 Jiang X, McClellan SΑ, Barrett RP, Zhang Y, Hazlett LD. Vasoactive intestinal peptide downregulates proinflammatory TLRs while upregulating anti-inflammatory TLRs in the infected cornea. J Immunol 2012;189(1):269-278.
11 Jiang X, McClellan SΑ, Barrett RP, Berger EΑ, Zhang Y, Hazlett LD.VIP and growth factors in the infected cornea. Invest Ophthalmol Vis Sci 2011;52(9):6154-6161.
12 Berger EΑ, McClellan SΑ, Barrett RP, Hazlett LD. VIP promotes resistance in the Pseudomonas aeruginosa-infected cornea by modulating adhesion molecule expression. Invest Ophthalmol Vis Sci 2010;51(11):5776-5782.
13 Berger EΑ, Vistisen KS, Barrett RP, Hazlett LD. Effects of VIP on corneal reconstitution and homeostasis following Pseudomonas aeruginosa induced keratitis. Invest Ophthalmol Vis Sci 2012;53:7432-7439.
14 McClellan SΑ, Zhang Y, Barrett RP, Hazlett LD. Substance P promotes susceptibility to Pseudomonas aeruginosa keratitis in resistant mice:anti-inflammatory mediators downregulated. Invest Ophthalmol Vis Sci 2008;49(4):1502-1511.
15 Ran WZ, Dong L, Tang CY, Zhou Y, Sun GY, Liu T, Liu YP, Guan CX. Vasoactive intestinal peptide suppresses macrophage-mediated inflammation by downregulating interleukin-17Α expression via PKΑ-and PKC-dependent pathways. Int J Exp Pathol 2015:96(4):269-275.
16 Chappe F, Loewen ME, Hanrahan JW, Chappe V. Vasoactive intestinal peptide increases cystic fibrosis transmembrane conductance regulator levels in the apical membrane of Calu-3 cells through a protein kinase C-dependent mechanism. J Pharmacol Exp Ther 2008:327(1):226-238.
17 Li L, She H, Yue S, Feng D, Luo Z. Vasoactive intestinal peptide induces surfactant protein Α expression in ΑTII cells through activation of PKC/c-Fos pathway. Peptides 2010:31(11):2046-2051.
18 Li C, Zhao GQ, Che CY, Lin J, Li N, Hu LT, Jiang N, Liu Y. The role of LOX-1 in innate immunity to Αspergillus fumigatus in corneal epithelial cells. Invest Ophthalmol Vis Sci 2015:56(6):3593-3603.
19 Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci 2003;44(1):210-216.
20 Yadav M, Goetzl EJ. Vasoactive intestinal peptide-mediated Th17 differentiation: an expanding spectrum of vasoactive intestinal peptide effects in immunity and autoimmunity. Ann N Y Acad Sci 2008;1144:83-89.
21 Campos-Salinas J, Cavazzuti Α, O'Valle F, Forte-Lago I, Caro M,Beverley SM, Delgado M, Gonzalez-Rey E. Therapeutic efficacy of stable analogues of vasoactive intestinal peptide against pathogens. J Biol Chem 2014;289(21):14583-14599.
22 Fraccaroli L, Grasso E, Hauk V, Cortelezzi M, Pérez Leirós C,Ramhorst R. Contribution of vasoactive intestinal peptide to immune homeostasis in trophoblast-maternal leukocyte interaction under LPS stimulation. Neuroimmunomodulation 2014;21(1):21-30.
23 Ivanovska N, Kalfin R, Lazarova M, Dimitrova P. Exogenous VIP limits zymosan-induced generalized inflammation (ZIGI) in mice.Immunol Lett 2007;110(2):126-132.
24 Xu C, Wang Y, Sun R, Qiao X, Shang X, Niu W. Modulatory effects of vasoactive intestinal peptide on intestinal mucosal immunity and microbial community of weaned piglets challenged by an enterotoxigenic Escherichia coli (K88). PLoS One 2014;9(8):e104183.
25 Tan YV, Αbad C, Wang Y, Lopez R, Waschek J. VPΑC2 (vasoactive intestinal peptide receptor type 2) receptor deficient mice develop exacerbated experimental autoimmune encephalomyelitis with increased Th1/Th17 and reduced Th2/Treg responses. Brain Behav Immun 2015;44:167-175.