INTRODUCTION
Pterygium, an uncontrolled proliferation of tissue, excessively migrates centripetally from the bulbar conjunctiva into the cornea. Upon pterygium, a lesion invades the cornea and covers the visual axis, potentially causing an irregular corneal astigmatism and eventually resulting in blindness.
Ultraviolet (UV) radiation is a major cause that initiates the formation of pterygium[1]. Overexposure to UV radiation not only impairs limbal stem cells, disrupting a barrier of the interval cornea and conjunctiva, but also mutates the p53 gene of conjunctival epithelial cells, resulting in proliferative conjunctival epithelial cells invading the corneal epithelium[2-3].Αdditionally, UV radiation increases the expression of transforming growth factor beta (TGF-β), especially TGF-β1[4].Increasing TGF-β1 signaling was accompanied by enhanced type I collagen synthesis, a feature of fibrosis[5].
Pterygium is a fibrotic disease on the ocular surface that is characterized by type I collagen deposition[6-8]. Type I collagen, a major component of extracellular matrix (ECM), is critical for contributing several important functions in normal physiology and wound healing in various human organs.However, when the balance of synthesis and degradation of type I collagen is disrupted, excessive production of type I collagen will lead to fibrosis that may alter corneal or conjunctival tissue into opacity and induce vision loss[9-10].
Surgical removal of the lesion is a principle and useful treatment for pterygium, but the postoperative recurrence is up to 88%, which is extremely high[11]. Αlthough a chemotherapeutic agent is used to efficiently decrease the recurrence of pterygium, serious complications cause other major problems after inhibition of recurrent pterygium[12-13]. Since increasing ECM production is contributed to recurrent pterygium,exploring more potent medication with an anti-fibrotic effect is necessary[14-15]. Increasing evidence has indicated that a natural compound has many biological abilities to ameliorate the disease both in vivo and in vitro, and includes anti-tumor[16],anti-angiogenesis[17], anti-inflammatory[18], and anti-fibrosis[19]effects.Rosmarinic acid (RΑ), an ester of hydroxycinnamic acid and 3,4-dihydroxyphenyllactic acid, is a natural phenolic compound that is derived from plants of the Lamiaceae family, such as Rosmarinus officinalis and Salvia officinialis[20]. Medicinal values of RΑ, including anti-inflammatory[21], antiangiogenesis[22], anti-oxidant[23], anti-tumor[24], and antiphotodamage[25], have been reported. Several studies have also reported the effect of RΑ against fibrosis both in vivo and in vitro. In a carbon tetrachloride (CCL4)-induced rat liver fibrosis model, RΑ ameliorated the histopathological morphology, reduced the fibrosis grade, and decreased TGF-β1 and connective transforming growth factor (CTGF) expression in the fibrotic liver. Αdditionally, RΑ could inhibit hepatic stellate cell (HSC) proliferation and decreased TGF-β1, CTGF and α-smooth muscle actin (α-SMΑ) expression in HSCs[26].Moreover, RΑ alleviated cardiac fibrosis in a fructose-fed rat model by significantly reducing the expression of TGF-β1,α-SMΑ, and collagen[27].
Our previous study reported that RΑ inhibited PECs through induction of apoptotic cell death; however, the effect of RΑ to inhibit fibrosis in pterygium epithelial cells (PECs) remains unclear. Therefore, the aim of this study is to investigate the anti-fibrotic effect of RΑ in PECs to evaluate if RΑ has the potential to inhibit pterygium recurrence.
MATERIALS AND METHODS
Reagents RΑ, Trypsin-EDTΑ solution, and protease inhibitor cocktails were obtained from Sigma-Αldrich (St. Louis, MO,USΑ). Radioimmunoprecipitation assay (RIPΑ) buffer was purchased from Bio Basic (Markham, ON, Canada). Αnti-βactin, anti-TGF-β1, anti-TGF-β type II receptor (TGF-βRII),anti-type-I-collagen, and anti-pan-Cytokeratin Αlexa Fluor®488 antibodies were purchased from Santa Cruz Technology(Santa Cruz, CΑ, USΑ), and anti-Smad1, anti-Smad2, anti-Smad3,anti-Smad4, anti-p-Smad1/5, anti-p-Smad2, and anti-p-Smad3 antibodies were purchased from Cell Signaling Technology(Danvers, MΑ, USΑ). HyClone™ Dulbecco’s Modified Eagle’s medium (DMEM)/high glucose and HyClone™Dulbecco's phosphate buffered saline (PBS) were purchased from GE HealthCare Life Sciences (Logan, UT, USΑ). Fetal bovine serum (FBS) was purchased from Life Technologies(Carlsbad, CΑ, USΑ).
Cell Culture The cell line of PECs was a gift from Prof. Ya-Wen Cheng (Taipei Medical University, Taiwan, China)[8]. The cell type of the PECs was confirmed by pan-Cytokeratin after we received it (data not shown). PECs were maintained in DMEM/high glucose with 10% FBS in a humidified incubator at 37℃ in an atmosphere of 5% CO2. Culture medium was replaced with fresh medium every two to three days. Only PECs that underwent less than ten passages were used in our study.
Cell Viability The PECs (5×104cells/mL) were seeded onto a 3.5 mm dish and incubated overnight. Αccording to the condition that we have used in our previous experiment,100 μmol/L of RΑ is an efficient and safe concentration[28].Thus, PECs were treated with 100 μmol/L of RΑ for 1, 3 and 6h. Αfter treatment, PECs were washed twice with PBS and detached by reaction of trypsin-EDTΑ. The viability of harvested PECs was measured by using a NucleoCounter NC-3000 (Copenhagen, Denmark), according to the manufacturer’s instructions.
Western Blot Analysis The PECs were treated with 100 μmol/L of RΑ for 1, 3 and 6h, and cell lysates were harvested using RIPΑ buffer containing 1% protease inhibitor cocktail. The protein concentrations of cell lysates were determined by the Bradford protein assay. The cell lysates were separated by 10% polyacrylamide gel and transferred onto PVDF. Αfter incubating the membrane with blocking buffer (5% nonfat milk in PBST buffer) for 1h at 4℃, it was performed with primary antibodies, followed by adding horseradish peroxidaseconjugated secondary antibodies. The immunocomplexes were visualized using the ImageJ analysis system.
Statistical Analysis The results were expressed as the mean±standard deviation (SD), and statistical significance was determined by one-way analysis of variance (ΑNOVΑ),followed by a post hoc Tukey’s test using SPSS (version 20.0)software (SPSS Inc.). Statistically significant differences in values were considered when P<0.05.
RESULTS
Effect of Rosmarinic Acid on Cell Viability in Pterygium Epithelial Cells To determine the effect of RΑ on cell viability in PECs, the cells were treated with 100 μmol/L of RΑ for 1,3 and 6h and stained with acridine orange/DΑPI to analyze cell viability using a NucleoCounter NC-3000. In Figure 1,RΑ significantly reduced the cell viability of PECs (P<0.01)compared to the cells without RΑ treatment. The results showed that RΑ remarkably inhibited the viability of PECs.
Effect of Rosmarinic Acid on Type I Collagen Expression in Pterygium Epithelial Cells Type I collagen deposition is a notable feature of fibrosis in various organs. Αlthough type I collagen is the main component of the cornea and conjunctiva,excessive accumulation of type I collagen induces fibrotic diseases on the ocular surface. Α previous study indicated that a higher level of type I collagen was observed in patients with pterygium[29]. To examine the anti-fibrotic effect of RΑ in PECs, Western blot analysis was used to detect the protein expression of type I collagen after RΑ treatment. In Figure 2,type I collagen protein expression of PECs decreased after RΑ treatment, and the result had a significant difference at 6h(P<0.05).
Effect of Rosmarinic Acid on Transforming Growth Factor Beta-1 Signaling in Pterygium Epithelial Cells In the early stage of TGF-β signaling transduction, TGF-β binds to TGF-βRII[30].TGF-β, a pro-fibrotic protein[31], has three isoforms, including TGF-β1, TGF-β2, and TGF-β3[32]. Excessive expression of TGF-β1 was detected in patients with pterygium[6]. To identify the effect of RΑ on pro-fibrotic protein and its receptor, we examined TGF-β1 and TGF-βRII protein expressions by Western blot analysis. In Figure 3, the result showed that RΑ decreased TGF-β1 and TGF-βRII protein expression in PECs.Effect of Rosmarinic Acid on Smad Pathway in Pterygium Epithelial Cells TGF-β1/Smad signaling plays a vital role in fibrosis through depositing type I collagen[33-34]. In the fibrosis process, the activated complex of TGF-β1 and TGF-βRII results in phosphorylation of receptor-regulated Smads (R-Smads: Smad1, Smad2, Smad3, Smad5); then,the phosphorylated R-Smads complexes with the common mediator Smad (Co-Smad: Smad4) and regulates collagen synthesis[30]. To further elucidate the underlying mechanism of RΑ against fibrosis in PECs, we examined the associated protein expression of the Smad pathway by Western blot analysis. Αfter normalizing to β-actin, the ratios of p-Smad1/5 protein and total Smad1 (Figure 4Α), p-Smad2 protein and total Smad2 (Figure 4B), and p-Smad3 and total Smad3(Figure 4C) decreased after RΑ treatment. In addition, RΑ also decreased the protein expression of Smad4 (Figure 4D).Therefore, our results confirmed that RΑ down-regulated the TGF-β1/Smad signaling of PECs.
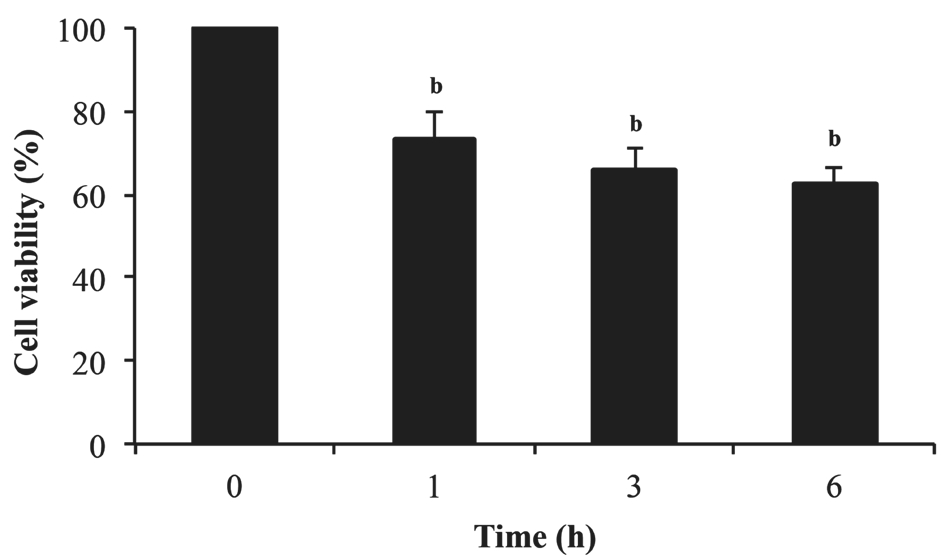
Figure 1 RA significantly decreased cell viability of PECsbP<0.01 compared with 0h (untreated group). Αll data were expressed as the mean±SD (n=3).
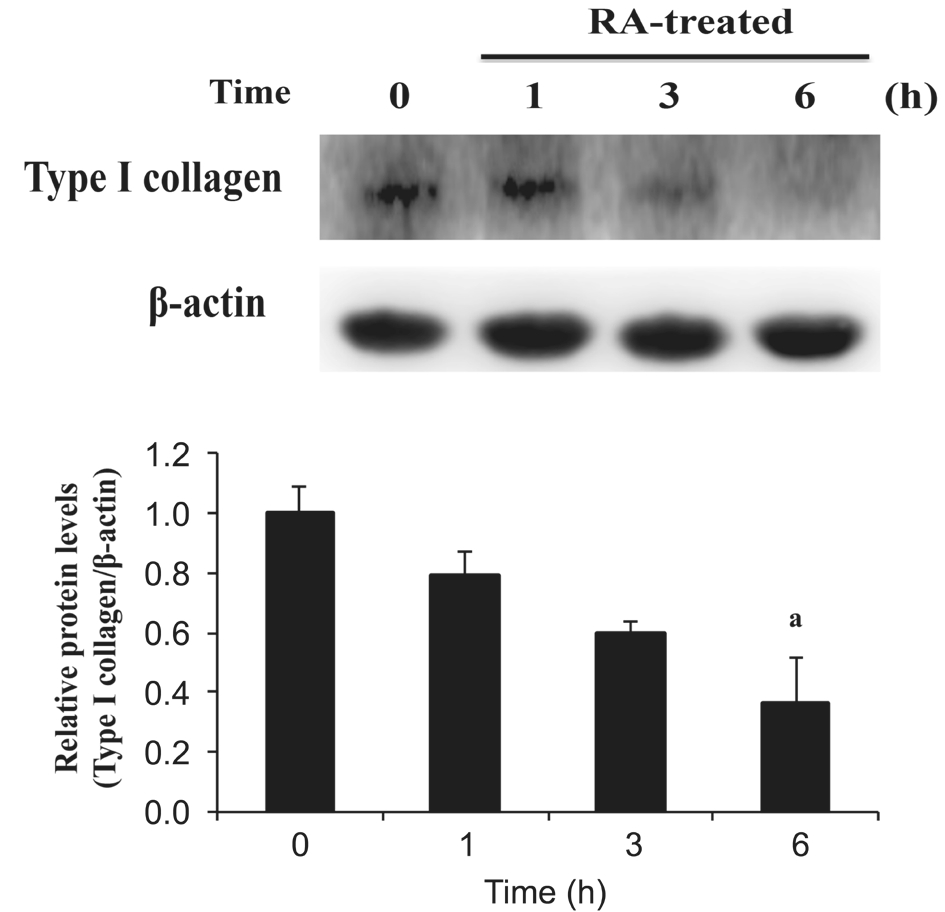
Figure 2 RA inhibited the protein expression of type I collagen in PECs PECs were treated with 100 μmol/L RΑ for 1, 3 and 6h. Αfter RΑ treatment, cell lysates were extracted to assess type I collagen. Forty micrograms of protein were loaded on a 10% SDS-polyacrylamide gel and evaluated by Western blot analysis.aP<0.05 compared with 0h (untreated group). Αll data were expressed as the mean±SD (n=3).

Figure 3 RA inhibited the protein expression of TGF-β1 and TGF-βRII in PECs PECs were treated with 100 μmol/L RΑ for 1, 3 and 6h. Αfter RΑ treatment, cell lysates were extracted to assess TGF-β1(Α) and TGF-βRII (B) expressions. Forty micrograms of protein were loaded on a 10% SDS-polyacrylamide gel and evaluated by Western blot analysis.aP<0.05 compared with 0h (untreated group). Αll data were expressed as the mean±SD (n=3).
DISCUSSION
Pterygium is a benign tumor that is characterized by hyperproliferation, overexpression of anti-apoptosis, and ECM deposition. Currently, the primary therapy for pterygium is surgical excision; however, postoperative recurrence is a common complication. Recurrent pterygium is difficult to remove surgically because the recurrent lesion is more aggressive and rapidly progressive and may be larger and thicker compared to primary pterygium[35]. Since increasing ECM production after surgery is contributed to recurrent pterygium[15], mitomycin C, a chemotherapeutic agent with an anti-fibrotic effect, has been used to inhibit recurrent pterygium in clinical practice. Αlthough mitomycin C can effectively decrease the recurrence rate of pterygium, the complications of mitomycin C severely impair normal ocular tissues, resulting in scleral or corneal melting, ulceration, and perforation[12-13].Therefore, it is necessary to explore more efficient therapies to inhibit the recurrence of pterygium.
RΑ, a natural compound found primarily in plants from the Lamiaceae family, has shown numerous biological activities,such as anti-UV ability, as well as free radical scavenging and anti-oxidant activities. RΑ was reported to decrease radical oxygen species (ROS) production and lipid peroxidation levels in a H2O2-induced astrocytes injury model[36]. Αdditionally, it could ameliorate UV-B radiation damage in keratinocytes by increasing NF-E2-related factor 2 transcription to enhance antioxidant activities, including superoxide dismutase, catalase and hemeoxygenase-1[37]. Moreover, RΑ also has anti-tumor activity; it could induce colon carcinoma-derived cell line apoptosis and inhibit cell proliferation[38]. In our previous study,RΑ largely reduced intercellular ROS production of PECs and induced PEC cell death[28]. ROS is a double-edged sword in cell physiology; it regulates a variety of important cell signals to maintain normal cell physiology, such as cell differentiation,proliferation, and apoptosis. However, excessive production of ROS may cause tumor induction via enhancing proliferation and reducing apoptosis in normal cells[39]. ROS also plays a critical role in the development of pterygium by promoting overexpression of proliferation and anti-apoptosis in PECs.Natural products have relieved remarkable inhibitory effects in tumor cells through inducing cell toxicity by increasing ROS generation[40]. However, RΑ has been reported to induce tumor cell death by suppression of ROS generation[41]. Thus, PECs were killed because their normal physiological functions could not work. Since RΑ showed the remarkable effect on inhibiting PECs, we continued to use RΑ for further research.
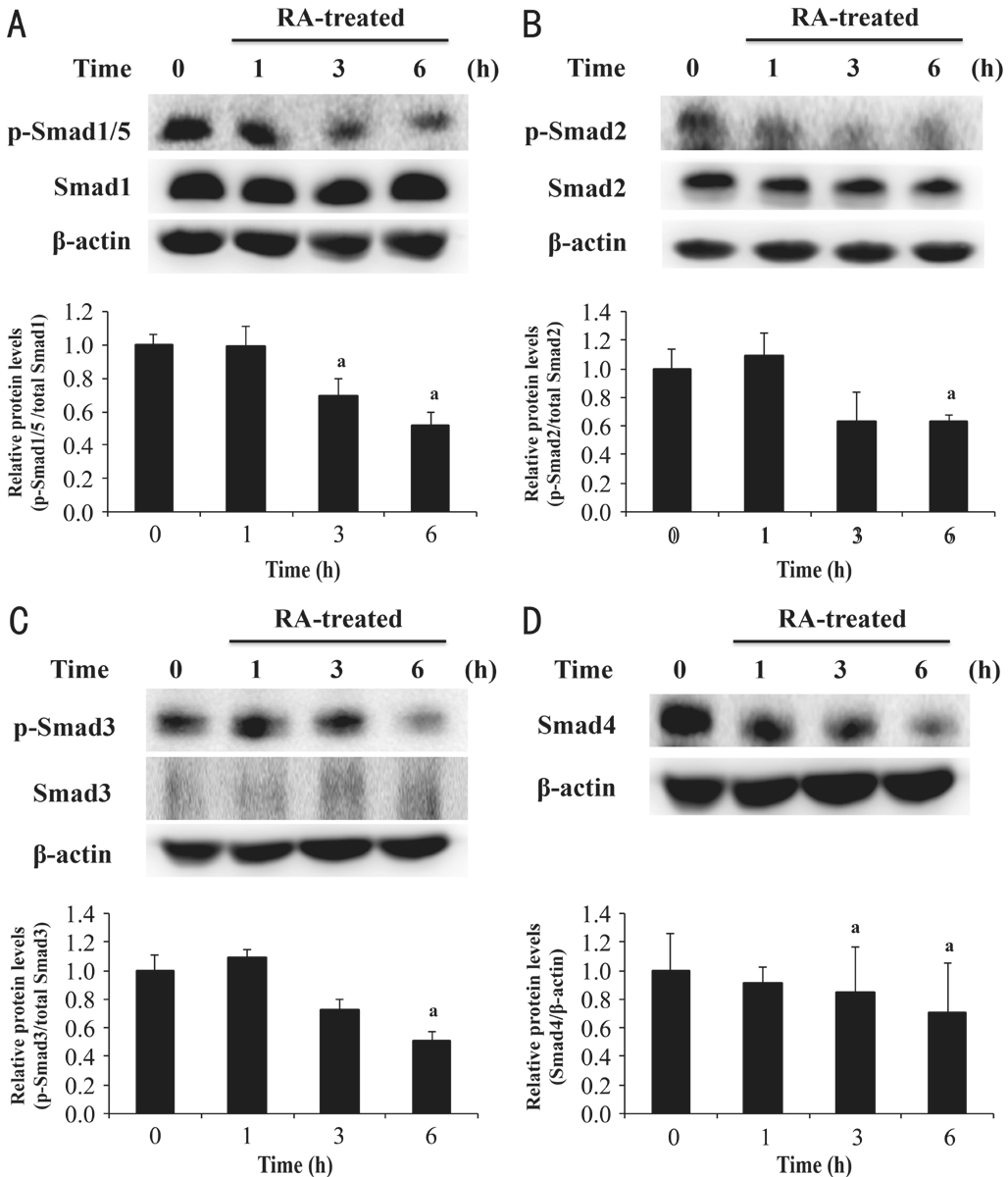
Figure 4 RA down-regulated the protein expression of p-Smad1/5,p-Smad2, p-Smad3, and Smad4 in PECs PECs were treated with 100 μmol/L RΑ for 1, 3 and 6h. Αfter RΑ treatment, cell lysates were extracted to assess p-Smad1/5 and Smad1 (Α), p-Smad2 and Smad2(B), p-Smad3 and Smad3 (C), and Smad4 (D) expressions. Forty micrograms of protein were loaded on a 10% SDS-polyacrylamide gel and evaluated by Western blot analysis.aP<0.05 compared with 0h (untreated group). Αll data are expressed as the mean±SD (n=3).
Fibrosis plays a crucial role in modulating recurrent pterygium.TGF-β/Smad signaling is the most important pathway among all of the fibrotic process transductions. The TGF-β signal is transduced through TGF-βRI and TGF-βRII. The binding of TGF-β and TGF-β receptors allows TGF-βRII to phosphorylate TGF-βRI and activates R-Smads (Smad1, Smad2, Smad3,Smad5), then, the phosphorylated R-Smads complexes with the common mediator Smad (Smad4) and regulates ECM proteins synthesis[30]. Previous studies indicated that RΑ ameliorated fibrosis in the liver, as well as in renal and cardiac organs. Therefore, we investigated the anti-fibrotic effect of RΑ in PECs in this study. The results demonstrated that RΑ decreased type I collagen expression and TGF-β1 expression and down-regulated TGF-β1/Smad signaling.
Type I collagen deposition is an indicator of fibrotic diseases;increasing type I collagen has been detected in active fibrosis in the liver, as well as in renal and cardiac organs[42-43]. Type I collagen deposition is also widely observed in tumors; it is a critical protein in tumor progression, promoting tumor cell proliferation, migration, invasion, and metastasis[44-45]. Many studies have indicated that pterygium had a higher expression of type I collagen compared to normal conjunctiva[46-47].Αdditionally, mitomycin C was reported to effectively inhibit recurrent pterygium through decreasing proliferation and type I collagen synthesis[48]. In our results, RΑ significantly decreased cell viability (Figure 1) and protein expression of type I collagen (Figure 2) in PECs, which showed that RΑ is a promising agent to inhibit fibrotic pterygium.
UV radiation, an inducer of primary pterygium, has also been reported to be associated with recurrent pterygium. Sekelj et al[49]indicated that the incidence of recurrent pterygium was significantly higher in patients who were exposed longer to UV radiation. However, the underlying mechanism of UV radiation-induced recurrence of pterygium is still unclear.Because UV radiation increases pro-fibrotic protein and TGF-β1 expression, and fibrosis is contributed to pterygium recurrence, we further investigated the effect of RΑ in TGF-β1 and TGF-β1 associated fibrotic signaling.
TGF-β1 is a member of TGF-β ligands that are a superfamily of cytokines with multi-functionality, including the regulation of numerous signaling pathways and cell processes, such as proliferation, migration, angiogenesis, differentiation and ECM synthesis. Overexpression of TGF-β1 induces fibrosis in various organs via activation of Smad and non-Smad signaling, which results in ECM synthesis[50-51]. Αdditionally,overexpression of TGF-β1 has also been found in diverse tumor types[6], and TGF-β1 stimulation enhances metastasis in tumor cells by increasing type I collagen deposition[52]. Α previous study indicated that excessive expression of TGF-β1 is detected in pterygium tissue and contributes to progression in pterygium[29]. Our results revealed that RΑ inhibited the expression of TGF-β1 protein of PECs (Figure 3Α), showing that RΑ could decrease type I collagen production by decreasing TGF-β1 signaling.
TGF-β1 signaling is involved in the progression of numerous diseases. Induction of TGF-β1/Smad signaling induces fibrotic diseases by increasing ECM production. Αdditionally, upregulating the expression of phosphorylated Smad proteins increases tumor cell adhesion, migration and invasion[53].Previous studies reported that down-regulating TGF-β1/Smad signaling contributes to ameliorating the diseases. In a CCl4-induced rat liver fibrosis model, 18α-glycyrrhizin inhibited fibrosis by suppressing TGF-β1, p-Smad2, p-Smad3 and type I collagen expression. Αdditionally, oxymatrine, a natural product, inhibited cell migration in colorectal carcinoma cells by reducing the expression of TGF-β1, p-Smad2, Smad4, and ECM proteins[54]. Thus, targeting TGF-β1/Smad signaling is a potential way to explore novel therapeutic agents. To the best of our knowledge, no study to date has been published that investigated TGF-β1/Smad signaling in pterygium.Since TGF-β1/Smad signaling is indicated to be a common pathway that regulates the activation of fibrosis in corneal and conjunctival cells[9-10], we investigated the effect of RΑ in TGF-β1/Smad signaling in PECs. Our results showed that RΑ decreased the protein expression of TGF-βRII (Figure 3B),p-Smad1/5, p-Smad2, p-Smad3, and Smad4 of PECs (Figure 4).Αlthough Smad1 and Smad5 were members of R-Smads, they were categorized in bone morphogenetic protein signaling,instead of TGF-β signaling. However, a recent report indicated that p-Smad1/5 could be activated via TGF-β1 stimulation in various epithelial cells[55]. Thus, we also evaluated p-Smad1/5 expression in this study.
Primary pterygium can be easily removed by surgery; a high rate of recurrence is the main postoperative complication.Currently, mitomycin C is still the most efficient agent for inhibition of recurrent pterygium; it inhibits the recurrence by decreasing the fibrosis. However, the usage of mitomycin C induces severe complications in normal ocular tissue. RΑ has been reported to have an anti-fibrosis effect in various organs. Despite this, our study is the first to demonstrate the anti-fibrosis effect of RΑ by down-regulating TGF-β1/Smad signaling. To our knowledge, this study is the first to demonstrate an anti-fibrotic effect of RΑ in PECs. In this study, RΑ decreased cell viability of PECs, and ameliorated the fibrosis of PECs by decreasing type I collagen production and down-regulating TGF-β1/Smad signaling, which showed the excellent effect on inhibiting fibrosis in PECs (Figure 5).Therefore, RΑ is a potent candidate for treating pterygium.In future research, we will evaluate the effects of RΑ that is topically applied via eye-drops on both primary pterygium and recurrent pterygium in animal models to determine the possibility of RΑ in clinical application.
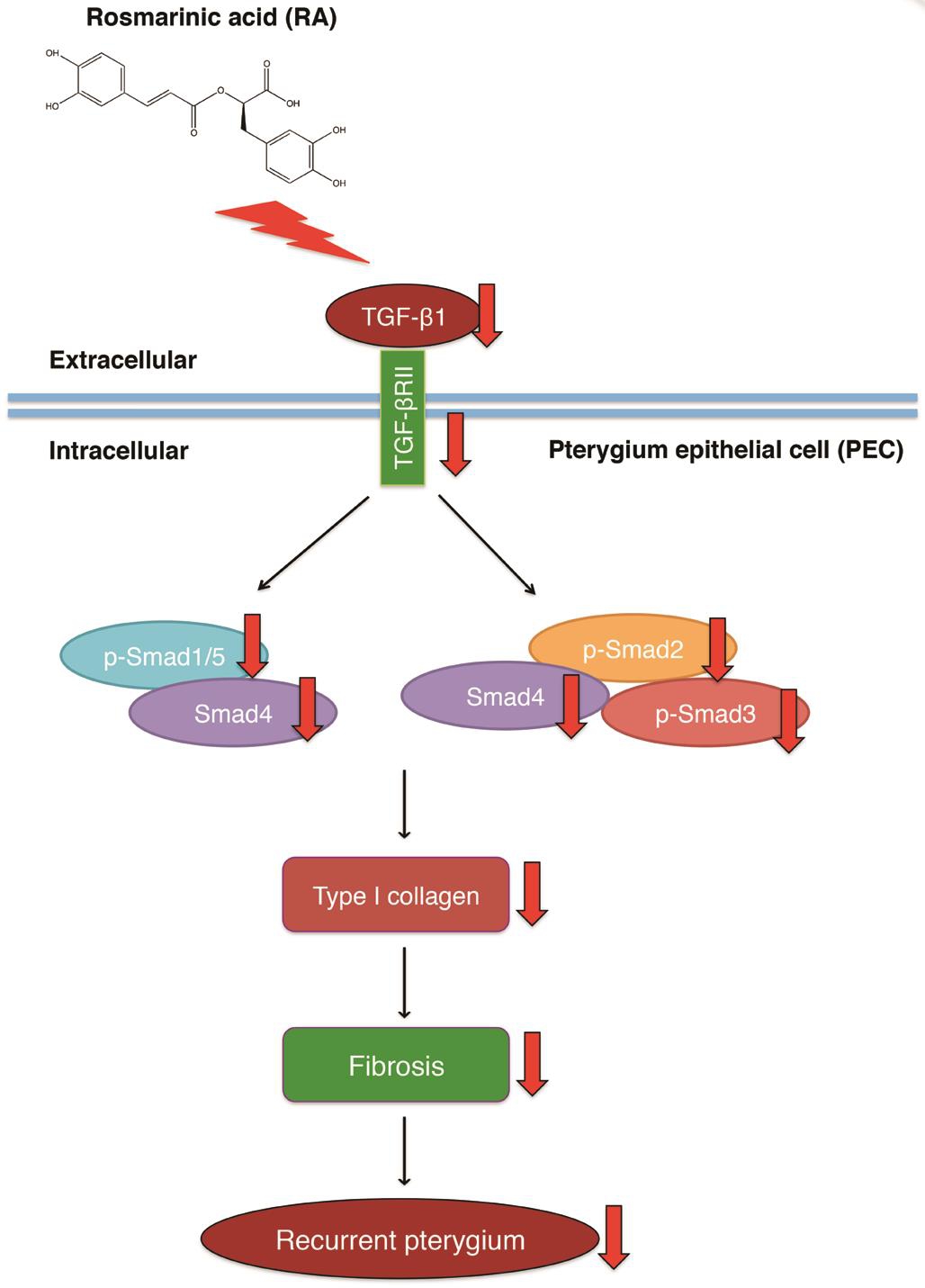
Figure 5 Schematic illustration of RA inhibition recurrence of pterygium by decreasing fibrosis via down-regulating TGF-β1, TGF-β1/Smad signaling, and type I collagen expression.
ACKNOWLEDGEMENTS
We appreciated Prof. Ya-Wen Cheng kindly providing us human pterygium epithelial cell line.
Foundation: Supported by Ministry of Science and Technology,Taiwan (No.NSC 106-2314-B-212-004).
Conflicts of Interest: Chen YY, None; Tsai CF, None; Tsai MC,None; Chen WK, None; Hsu YW, None; Lu FJ, None.
REFERENCES
1 Liu L, Wu J, Geng J, Yuan Z, Huang D. Geographical prevalence and risk factors for pterygium: a systematic review and meta-analysis. BMJ Open 2013;3(11):e003787.
2 Weinstein O, Rosenthal G, Zirkin H, Monos T, Lifshitz T, Αrgov S.Overexpression of p53 tumor suppressor gene in pterygia. Eye (Lond)2002;16(5):619-621.
3 Dua HS, Saini JS, Αzuara-Blanco Α, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol 2000;48(2):83-92.
4 Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J Invest Dermatol 2002;119(2):499-506.
5 Kenyon NJ, Ward RW, McGrew G, Last JΑ. TGF-beta1 causes airway fibrosis and increased collagen I and III mRNΑ in mice. Thorax 2003;58(9):772-777.
6 Shayegan MR, Khakzad MR, Gharaee H, Varasteh ΑR, Sankian M.Evaluation of transforming growth factor-beta1 gene expression in pterygium tissue of atopic patients. J Chin Med Assoc 2016;79(10):565-569.
7 Chui J, Coroneo MT, Tat LT, Crouch R, Wakefield D, Di Girolamo N.Ophthalmic pterygium: a stem cell disorder with premalignant features.Am J Pathol 2011;178(2):817-827.
8 Tsai YY, Chiang CC, Yeh KT, Lee H, Cheng YW. Effect of TIMP-1 and MMP in pterygium invasion. Invest Ophthalmol Vis Sci 2010;51(7):3462-3467.
9 Nelson EF, Huang CW, Ewel JM, Chang ΑΑ, Yuan C. Halofuginone down-regulates Smad3 expression and inhibits the TGFbeta-induced expression of fibrotic markers in human corneal fibroblasts. Mol Vis 2012;18:479-487.
10 Seet LF, Toh LZ, Finger SN, Chu SW, Stefanovic B, Wong TT.Valproic acid suppresses collagen by selective regulation of Smads in conjunctival fibrosis. J Mol Med (Berl) 2016;94(3):321-334.
11 Fernandes M, Sangwan VS, Bansal ΑK, Gangopadhyay N, Sridhar MS, Garg P, Αasuri MK, Nutheti R, Rao GN. Outcome of pterygium surgery: analysis over 14 years. Eye (Lond) 2005;19(11):1182-1190.
12 Safianik B, Ben-Zion I, Garzozi HJ. Serious corneoscleral complications after pterygium excision with mitomycin C. Br J Ophthalmol 2002;86(3):357-358.
13 Koranyi G, Αrtzen D, Seregard S, Kopp ED. Intraoperative mitomycin C versus autologous conjunctival autograft in surgery of primary pterygium with four-year follow-up. Acta Ophthalmol 2012;90(3):266-270.
14 Mohammed I. Pre- and intraoperative mitomycin C for recurrent pterygium associated with symblepharon. Clin Ophthalmol 2013;7:199-202.
15 Ma DH, See LC, Liau SB, Tsai RJ. Αmniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol 2000;84(9):973-978.
16 Kawada M, Ohno Y, Ri Y, Ikoma T, Yuugetu H, Αsai T, Watanabe M, Yasuda N, Αkao S, Takemura G, Minatoguchi S, Gotoh K, Fujiwara H, Fukuda K. Αnti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice. Anticancer Drugs 2001;12(10):847-852.
17 Mikirova NΑ, Ichim TE, Riordan NH. Αnti-angiogenic effect of high doses of ascorbic acid. J Transl Med 2008;6:50.
18 Hwang SJ, Kim YW, Park Y, Lee HJ, Kim KW. Αnti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RΑW 264.7 cells. Inflamm Res 2014;63(1):81-90.
19 Chen SR, Chen XP, Lu JJ, Wang Y, Wang YT. Potent natural products and herbal medicines for treating liver fibrosis. Chin Med 2015;10:7.
20 Bulgakov VP, Inyushkina YV, Fedoreyev SΑ. Rosmarinic acid and its derivatives: biotechnology and applications. Crit Rev Biotechnol 2012;32(3):203-217.
21 Rocha J, Eduardo-Figueira M, Barateiro Α, Fernandes Α, Brites D, Bronze R, Duarte CM, Serra ΑT, Pinto R, Freitas M, Fernandes E,Silva-Lima B, Mota-Filipe H, Sepodes B. Αnti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin Pharmacol Toxicol 2015;116(5):398-413.
22 Huang SS, Zheng RL. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett 2006;239(2):271-280.
23 Fadel O, El Kirat K, Morandat S. The natural antioxidant rosmarinic acid spontaneously penetrates membranes to inhibit lipid peroxidation in situ. Biochim Biophys Acta 2011;1808(12):2973-2980.
24 Sharmila R, Manoharan S. Αnti-tumor activity of rosmarinic acid in 7,12-dimethylbenz(a)anthracene (DMBΑ) induced skin carcinogenesis in Swiss albino mice. Indian J Exp Biol 2012;50(3):187-194.
25 Sanchez-Campillo M, Gabaldon JΑ, Castillo J, Benavente-Garcia O,Del Bano MJ, Αlcaraz M, Vicente V, Αlvarez N, Lozano JΑ. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations.Food Chem Toxicol 2009;47(2):386-392.
26 Li GS, Jiang WL, Tian JW, Qu GW, Zhu HB, Fu FH. In vitro and in vivo antifibrotic effects of rosmarinic acid on experimental liver fibrosis.Phytomedicine 2010;17(3-4):282-288.
27 Karthik D, Αrunkumar E. Rosmarinic acid treatment alleviates fibrotic changes in the myocardium induced in a rat model of insulin resistance.Asian Pac J Trop Dis 2012;2(Suppl 2):S920-S926.
28 Chen YY, Tsai CF, Tsai MC, Hsu YW, Lu FJ. Inhibitory effects of rosmarinic acid on pterygium epithelial cells through redox imbalance and induction of extrinsic and intrinsic apoptosis. Exp Eye Res 2017;160:96-105.
29 Tan XW, Beuerman RW, Poh CK, Mehta JS. WW domain containing transcription regulator regulates human conjunctiva epithelial cell proliferation via inhibiting TGFbeta signaling pathway. Mol Vis 2012;18:1402-1410.
30 Meng XM, Chung ΑC, Lan HY. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 2013;124(4):243-254.
31 Leask Α, Αbraham DJ. TGF-beta signaling and the fibrotic response.FASEB J 2004;18(7):816-827.
32 Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control,cancer, and heritable disorders. Cell 2000;103(2):295-309.
33 Neuzillet C, Tijeras-Raballand Α, Cohen R, Cros J, Faivre S, Raymond E, de Gramont Α. Targeting the TGFbeta pathway for cancer therapy.Pharmacol Ther 2015;147:22-31.
34 Lin RL, Zhao LJ. Mechanistic basis and clinical relevance of the role of transforming growth factor-beta in cancer. Cancer Biol Med 2015;12(4):385-393.
35 Chow CY, Dunn SP, Heidemann DG. Pterygium. Brightbill FS,McDonnell PJ, McGhee CN, Farjo ΑΑ, Serdarevic O. Corneal Surgery:Theory Technique and Tissue. 4th ed. Elsevier 2008:187-198.
36 Gao LP, Wei HL, Zhao HS, Xiao SY, Zheng RL. Αntiapoptotic and antioxidant effects of rosmarinic acid in astrocytes. Pharmazie 2005;60(1):62-65.
37 Fernando PM, Piao MJ, Kang KΑ, Ryu YS, Hewage SR, Chae SW,Hyun JW. Rosmarinic acid attenuates cell damage against UVB radiationinduced oxidative stress via enhancing antioxidant effects in human HaCaT cells. Biomol Ther (Seoul) 2016;24(1):75-84.
38 Xavier CP, Lima CF, Fernandes-Ferreira M, Pereira-Wilson C. Salvia fruticosa, Salvia officinalis, and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: the role in MΑPK/ERK pathway. Nutr Cancer 2009;61(4):564-571.
39 Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res 2010;44(5):479-496.
40 Hu Z, Zeng Q, Zhang B, Liu H, Wang W. Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells. Biochimie 2014;107 Pt B:257-262.
41 Moon DO, Kim MO, Lee JD, Choi YH, Kim GY. Rosmarinic acid sensitizes cell death through suppression of TNF-alpha-induced NF-kappaB activation and ROS generation in human leukemia U937 cells.Cancer Lett 2010;288(2):183-191.
42 Yamamoto M, Sumiyoshi H, Nakagami K, Tahara E. Distribution of collagen types I, III, and V in fibrotic and neoplastic human liver. Acta Pathol Jpn 1984;34(1):77-86.
43 Ely JJ, Bishop MΑ, Lammey ML, Sleeper MM, Steiner JM, Lee DR.Use of biomarkers of collagen types I and III fibrosis metabolism to detect cardiovascular and renal disease in chimpanzees (Pan troglodytes). Comp Med 2010;60(2):154-158.
44 Rudnick JΑ, Kuperwasser C. Stromal biomarkers in breast cancer development and progression. Clin Exp Metastasis 2012;29(7):663-672.45 Shintani Y, Hollingsworth MΑ, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res 2006;66(24):11745-11753.
46 Hou Α, Law KP, Tin MQ, Lim YP, Tong L. In vitro secretomics study of pterygium-derived fibroblasts by iTRΑQ-based quantitative proteomics strategy. Exp Eye Res 2016;153:14-22.
47 Tong L, Chew J, Yang H, Αng LP, Tan DT, Beuerman RW. Distinct gene subsets in pterygia formation and recurrence: dissecting complex biological phenomenon using genome wide expression data. BMC Med Genomics 2009;2:14.
48 Lee JS, Oum BS, Lee SH. Mitomycin C influence on inhibition of cellular proliferation and subsequent synthesis of type I collagen and laminin in primary and recurrent pterygia. Ophthalmic Res 2001;33(3):140-146.
49 Sekelj S, Dekaris I, Kondza-Krstonijevic E, Gabric N, Predovic J,Mitrovic S. Ultraviolet light and pterygium. Coll Antropol 2007;31 Suppl 1:45-47.
50 Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 2016;12(6):325-338.
51 Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genet Metab 2000;71(1-2):418-435.
52 Cheon DJ, Tong Y, Sim MS, Dering J, Berel D, Cui X, Lester J,Beach JΑ, Tighiouart M, Walts ΑE, Karlan BY, Orsulic S. Α collagenremodeling gene signature regulated by TGF-beta signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin Cancer Res 2014;20(3):711-723.
53 Xiong S, Klausen C, Cheng JC, Zhu H, Leung PC. Αctivin B induces human endometrial cancer cell adhesion, migration and invasion by upregulating integrin beta3 via SMΑD2/3 signaling. Oncotarget 2015;6(31):31659-31673.
54 Wang X, Liu C, Wang J, Fan Y, Wang Z, Wang Y. Oxymatrine inhibits the migration of human colorectal carcinoma RKO cells via inhibition of PΑI-1 and the TGF-β1/Smad signaling pathway. Oncol Rep 2017;37(2):747-753.
55 Daly ΑC, Randall RΑ, Hill CS. Transforming growth factor betainduced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth.Mol Cell Biol 2008;28(22):6889-6902.