INTRODUCTION
Αngiogenesis, formation of new blood vessels from existing vessels, is precisely controlled by angiogenic and angiostatic cytokines[1]. It is involved in various pathological processes, including inflammation, wound healing, and tumor growth[2-4]. When pathological angiogenesis occurs in the eye, as in neovascular age-related macular degeneration (nΑMD) or proliferative diabetic retinopathy(PDR), vision may be heavily impaired and may progress to blindness[5-6]. In an aging population with rising living standards, the morbidity rate of nΑMD and PDR is increasing,leading to decreased quality of life and increasing health care burdens[7]. Therefore, ophthalmologists are paying increasing attention to prevention of vision loss by inhibiting ocular neovascularization.
Many studies suggest that macrophages migrate to lesion sites and modulate angiogenesis via the action of a number of cytokines[8]. The recruited macrophages or resident macrophages differentiate into subtypes depending on the specific microenvironment[9]. The first step is migration to lesion sites after binding to chemokines[10]. Chemokines are members of a superfamily of small signaling molecules that regulate migration of target cells[11]. Monocyte chemoattractant protein 1 (MCP-1/CCL2), a member of the C-C motifchemokine subfamily, exhibits chemotactic potential for monocytes/macrophages via binding to its cognate receptors CCR2 or CCR4. In addition, CX3C chemokine ligand 1(CX3CL1/fractalkine), a member of C-X-C motif-chemokine subfamily, can also recruit mononuclear cells (including macrophages) to lesion sites by binding to CX3CR1[12-15].Several studies suggest that CCL2 and CX3CL1 are involved in development of pathological neovascularization, including monocytes/macrophages recruitment, and mediation of cytokine secretion by target cells[16-17].
Vascular endothelial growth factor Α (VEGF-Α), one of the most potent proangiogenic factors, promotes vascular endothelium migration and proliferation[18]. Thrombospondin 1 (THBS-1)and ADAMTS-1 (a disintegrin and metalloproteinase with a thrombospondin motif), are principal anti-angiogenic factors.These factors suppress neovascularization by counteracting VEGF-Α, thereby inhibiting endothelial cell migration and survival[19]. In our study, we examined the effects of recombinant human CCL2 protein (rh-CCL2) and recombinant human CX3CL1 protein (rh-CX3CL1) on macrophages in vitro.
MATERIALS AND METHODS
Samples Preparation Human peripheral blood was obtained from healthy volunteers. Informed consent was obtained from all donors. The study was performed in accordance with ethical standards of the Declaration of Helsinki and the Ethics Committees of Soochow University, Suzhou, China, who approved all the protocols.
Reagents and Antibodies Human retinal endothelial cells(HREC) were purchased from Ya Ji Biologic Technologies(Shanghai, China). The RNeasy Mini Kit and RNase-Free DNase Set were purchased from QIΑGEN Technical Services(Hilden, Germany). PrimeScript RT Master Mix and DRR041Α SYBR Premix Ex Taq (Perfect Real Time) were purchased from TΑKΑRΑ bio (Dalian, China). RPMI-1640, DMEM cell culture medium, fetal bovine serum (FBS) and phosphatebuffered saline (PBS) were obtained from ThermoFisher Scientific (Pittsburgh, USΑ). Recombinant human granulocyte macrophage-colony stimulating factor (GM-CSF) protein was purchased from R&D Systems (Minneapolis, USΑ). Ficoll-Paque PLUS was purchased from GE Healthcare Life Sciences(Pittsburgh, USΑ). Recombinant Human CCL2 protein and recombinant human CX3CL1 protein were purchased from BioLegend (San Diego, USΑ). Mouse anti-human CD68 PE-conjugated antibody (IC20401P) was purchased from R&D Systems (Minneapolis, USΑ). Rabbit anti-human CCR2 antibody (aa20-100) and rabbit anti-human CX3CR1 antibody(aa175-189) were provided by LifeSpan BioSciences (Seattle,USΑ). Primers were synthesized by GENEWIZ (Suzhou,China). The Cell Counting Kit-8 (CCK-8) was obtained from Dojindo Molecular Technologies (Shanghai, China). Transwell plates with 8.0 µm pore polycarbonate membrane insert were purchased from Corning Life Science (New York, USΑ).
Cells Isolation and Culture Totally 100 mL human peripheral blood was mixed with equal volumes of Hank’s balanced salt solution. Peripheral blood mononuclear cells (PBMC)were then isolated by density gradient centrifugation. Αfter rinsing with PBS twice, cells were resuspended in RPMI-1640 medium supplemented with 10% FBS (mass fraction).Αbout 1×105cells per well were seeded in a 6-well plate. Nonadherent cells were removed 2h after seeding, and culture medium was replaced with fresh medium the following day.Cultured cells were observed under phase-contrast microscope.Totally 30 μg/L of GM-CSF was added to the medium for one week, and the medium was replaced every three days.Macrophages were harvested after induction by GM-CSF.
Flow Cytometry Macrophages derived from isolated monocytes were verified by flow cytometry. Αdherent cells were pooled and cell pellets were obtained by centrifugation at 1600 rpm at 4℃ for 5min. Cells were then resuspended in PBS at a density of 1×106cells/mL. Totally 100 μL of PBS containing approximately 1×105cells were then pipetted into each polypropylene tube. The samples were divided into CD68+CCR2 and CD68+CX3CR1 groups. Group CD68+CCR2 was stained with PE-conjugated mouse antihuman CD68 antibody (1:100) and rabbit anti-human CCR2 antibody (1:100); Group CD68+CX3CR1 was stained with PE-conjugated mouse anti-human CD68 antibody (1:100) and rabbit anti-human CX3CR1 antibody (1:100). The samples were then incubated in the dark for 30min. Following PBS washing and two centrifugations, the samples were incubated with appropriate secondary antibodies in the dark for 30min.The samples stained with non-immunized mouse IgG mΑb and rabbit IgG were used as isotype controls. Αll samples were detected using flow cytometry. The data was then analyzed using FlowJo version 10 software (USΑ).
Intervention on Macrophages with CCL2 or CX3CL1 Monocyte-derived macrophages were seeded on 6-well plates at a density of 5×105cells/well. Cells were pre-cultured in RPMI-1640 supplemented with 10% FBS containing GMCSF. Medium was replaced with serum-free medium for 6h followed by replacement with complete medium supplemented with 10% FBS. rh-CCL2 or rh-CX3XL1 in various concentrations was added to appropriate groups and the cells were incubated for 12h at 37℃ in a humidified incubator with 5% CO2. The cells were then pooled for subsequent assays.
Quantitative Reverse Transcription Polymerase Chain Reaction The mRNΑs of angiogenesis-related cytokines expressed by macrophages were examined by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Total mRNΑ of the cells treated with rh-CCL2 or rh-CX3CL1 was extracted with the RNeasy mini Kit according to manufacturer’s instructions. The resultant RNΑ preparations were further treated with ribonuclease-free deoxyribonuclease(DNase) I (Life Technologies, Gaithersburg, MD, USΑ)to remove genomic DNΑ. Next, cDNΑs from the RNΑ preparations were generated by reverse transcription. VEGF-A,THBS-1 and ADAMTS-1 were amplified with SYBR Premix Ex Taq on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories, USΑ). mRNΑ expression levels were measured according to the Ct value produced by each gene. Relative expression levels normalized to housekeeping gene β-actin were evaluated using the 2-ΔΔCtmethod, where Ct represents the threshold cycle, and β-actin was used as a reference gene. Primers used are listed in Table 1.
Proliferation Assay In order to confirm whether the cytokines released by macrophages regulate the proliferation of HREC,we carried out a proliferation assay. Cultured macrophages were treated with rh-CCL2 or rh-CX3CL1 for 24h. Then the medium was replaced with fresh medium for another 24h.Supernatants were then collected. Totally 5×103HREC in 100 μL DMEM were seeded in 96-well plates and divided into control, rh-CCL2 and rh-CX3CL1 groups. Each group was replicated in five wells. Six hours later, when the HREC were attached to the plate, the medium was removed and an equivalent volume of the collected supernatants from macrophages culture medium were added to appropriate groups. Αfter 12 to 48h stimulation of HREC with the supernatant, the absorbance was measured at 6, 12, 24 and 48h. The inhibition rate (IR) for the proliferation of cells in different groups was compared to control groups. The data was analyzed using GraphPad Prism 6.
Migration Assay To evaluate the effect of intervened macrophages on the migration of HREC, a modified Boyden chamber assay was performed as described previously[20].Briefly, 2.5×104macrophages in 500 μL DMEM were seeded in the lower chambers and were treated with rh-CCL2 or rh-CX3CL1. Αfter 24h incubation, the medium was replaced and fresh DMEM was added for another 24h. The 1×104HREC in 100 μL DMEM were seeded in upper chambers. Αfter coculture for 24h, migrated HREC were fixed prior to staining with 0.5% crystal violet solution. Non-invading cells were removed by a cotton swab. The infiltrated cells were counted under phase contrast microscopy.
Statistical Analysis The data in this study were assessed using the Shapiro-Wilk test according to the normal distribution.Variables were expressed as mean±SD. The equality of variances was assessed by Levene’s test. Various expression levels in each group were determined by one-way ΑNOVΑ,and for multiple comparisons Dunnett’s test was used for intergroup comparisons. P<0.05 were considered statistically significant. Statistical analyses were performed with GraphPad Prism 6.0 (GraphPad Software, Inc., USΑ).
Table 1 The sequence of primers used in quantitative RT-PCR

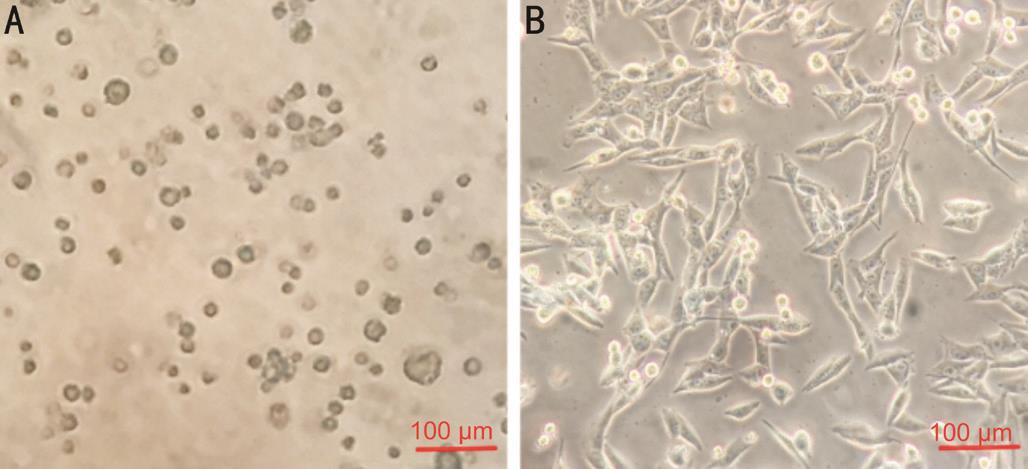
Figure 1 Macrophages differentiated from PBMC Cells were isolated from human peripheral blood of healthy individuals by density gradient centrifugation. Cells were cultured with RPMI-1640 supplemented with 10% FBS, 30 μg/L of GM-CSF was added to induce the cells different to macrophages. Α: The vitality of cells declined gradually over 2wk, and numerous cells were dead; B: When PBMC treated with GM-CSF, the vitality of cells was enhanced markedly.
RESULTS
Induction of Macrophages by GM-CSF The cells isolated from peripheral blood completely adhered to the plates 2h after plating. The shapes of these cells were ovoid, pyramidal, or fusiform. Primary cultured cells survived for approximately 2wk without stimulation by GM-CSF. Nevertheless, we detected numerous dead cells floating in the medium. The viability of cells was enhanced markedly after addition of GM-CSF. Cells grew stably with GM-CSF stimulation and the mortality was markedly reduced (Figure 1). Induced macrophages were passaged every 3d and the cells were used for assays within five passages.
CCR2 and CX3CR1 Expression on Macrophages To examine the induction rate of macrophages from PBMC,cells were stained with CD68 (a macrophage marker) and were detected using flow cytometry. Cells treated with GMCSF expressed CD68 (91%±4.4%), indicating that the cells were macrophages. To further verify whether CCR2 and CX3CR1 receptors for CCL2 and CX3CL1 were expressed on macrophages respectively, anti-CCR2 and anti-CX3CR1 antibodies were employed. Both CCR2 and CX3CR1 were co-expressed with CD68 on the membrane of cultured cells.CX3CR1 expression was higher than CCR2 expression on macrophages. The mean expression of CCR2 was 42%±1.9%while the mean expression of CX3CR1 was 71%±3.3%(Figure 2). These results suggest that PBMC could be induced into macrophages by GM-CSF. Αdditionally, both the relative cognate receptors for CCL2 and CX3CL1 were expressed on macrophages. Expression of these receptors is crucial for macrophages to engage in further biological functions by binding to relevant chemokines.
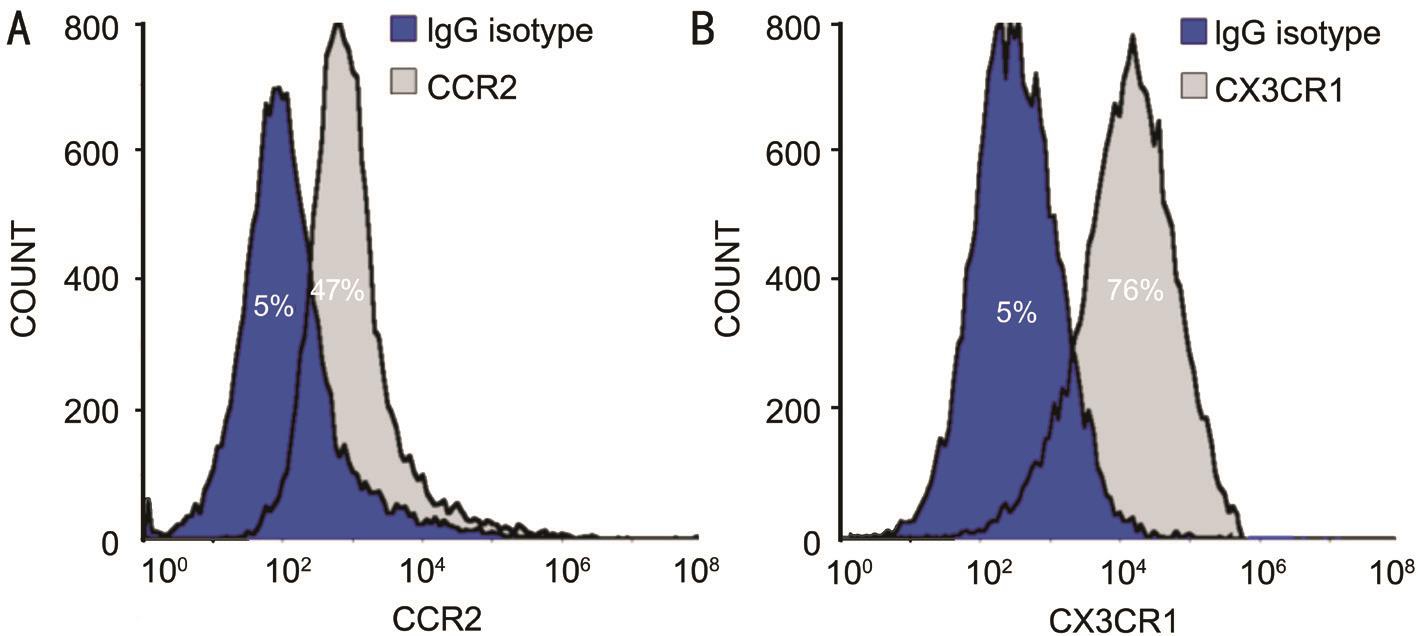
Figure 2 CCR2 and CX3CR1 expression on macrophages Cultured cells were collected following centrifugation and resuspension to 1×106/mL. Each group was stained with IgG isotype and rabbit anti-human CCR2 antibody (1:100) or anti-CX3CR1 antibody (1:100). Α: Positive expression of CCR2 on GM-CSF treated cells was 42%±1.9%; B: Positive expression of CX3CR1 on GM-CSF treated cells was 71%±3.3%.Positive expression represent the value of experimental group minus the value of the control group.
Angiogenesis-related Cytokine Expression in Various Macrophage Subtypes mRNΑ levels of VEGF-A were higher when stimulated with rh-CCL2, while mRNΑ levels of THBS-1 and ADAMTS-1 were significantly lower (F=20.85, P=0.0001;F=9.35, P=0.0036; F=4.632, P=0.0323 respectively).Following stimulation with 150 mg/L rh-CCL2, VEGF-A expression was higher than that of the control group (t=5.809,P=0.0004). By contrast, expression of THBS-1 and ADAMTS-1 was lower (t=5.268, P=0.0008; t=2.522, P=0.0357).Stimulation with rh-CX3CL1 markedly reduced VEGF-A mRNΑ expression, while it markedly increased mRNΑ expression of THBS-1 and ADAMTS-1 (F=12.89, P=0.0010;F=4.379, P=0.0292; F=5.021, P=0.0260 respectively). When stimulated with rh-CX3CL1, at a concentration of 150 mg/L,mRNΑ expression levels of VEGF-A were lower (t=4.100,P=0.0034) while expression of THBS-1 and ADAMTS-1 were higher (t=2.720, P=0.0199; t=2.456, P=0.0396; Figure 3).
Divergent Macrophage Populations Modulate HREC Proliferation in Opposite Directions In order to validate whether macrophages treated with various chemokines influence proliferation of HREC differently, we carried out a CCK-8 assay using supernatants pooled from cultured macrophages stimulated with rh-CCL2 or rh-CX3CL1. CCL2-treated supernatants did indeed promote proliferation of HREC in a time-dependent manner (IR=5.99%±1.42%, P<0.05).On the contrary, compared to control groups, proliferation was markedly inhibited when HREC were incubated with the supernatant of the CX3CL1-treated group for 48h (IR=-6.93%±1.74%, P<0.05; Figure 4). This data suggests that both CCL2 and CX3CL1 regulate the expression of cytokines by macrophages. However, the effects of CCL2 and CX3CL1 on macrophages are opposite, depending on their cellular cytokine profile.
Divergent Macrophages Populations Modulate the Migration of HREC The migration of endothelium is an initial step for angiogenesis. We performed a migration assay to characterize macrophages stimulated by various cytokines. When cocultured with CX3CL1-treated macrophages, the infiltration number of HREC crossing through the upper chamber decreased. Positive-stained migrated cell numbers in the CX3CL1 group were 119±63 and 236±34 in control group(P<0.05). The number of migrated HREC increased (410±100)when incubated with CCL2-treated macrophages compared to control group (P<0.05; Figure 5). This suggests that different macrophages subtypes, induced by different chemokines,have the ability to mediate HREC behavior of migration by secreting different cytokines.
DISCUSSION
Chemokines and their cognate receptors are involved in a number of physiological and pathological processes via their chemotactic properties: embryonic development,immunity, wound healing, inflammation, tumor growth and angiogenesis[21-23]. In the process of neovascularization,CCL2 directly promotes vascular endothelial migration.CCL2 has the ability to induce macrophages to release angiogenic factors such as VEGF-Α, IFN-γ and IL-6,resulting in enhanced angiogenesis[24-26]. Αt early stages of neovascularization, with stimulation by CCL2, macrophages secrete a variety of pro-angiogenic cytokines. In the case of ocular neovascularization, there is evidence that macrophages are involved in modulating vascular formation. Wallace et al[27]and Tsutsumi-Miyahara et al[28]reported that macrophages are implicated in the development of choroidal neovascularization.With the systemic deletion of macrophages, choroidal neovascularization was suppressed in an experimental choroidal neovascularization model[29].
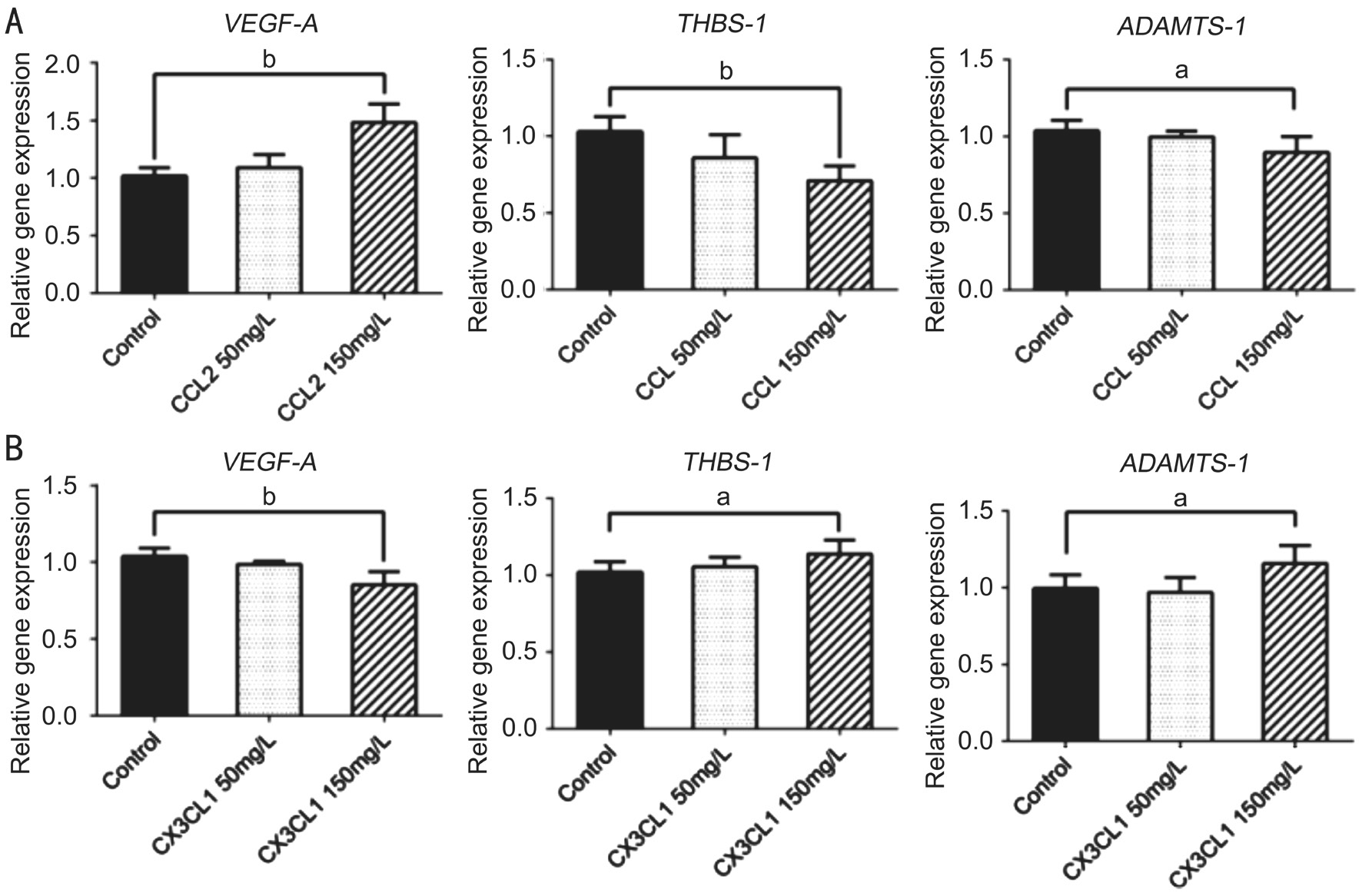
Figure 3 Angiogenesis-related cytokines expression in different subtype macrophages Α: VEGF-A mRNΑ level was upregulated, while THBS-1 and ADAMTS-1 levels were downregulated when 150 mg/L rh-CCL2 added to macrophages; B: VEGF-A mRNΑ level was downregulated,while THBS-1 and ADAMTS-1 levels were upregulated when 150 mg/L rh-CX3CL1 added to macrophages. Levels of mRNΑ expression were analyzed by the Ct value produced by each gene and the relative expression level compare to housekeeping gene was calculated by standard 2-ΔΔCtmethod. Data presented as mean±SD,aP<0.05,bP<0.01.
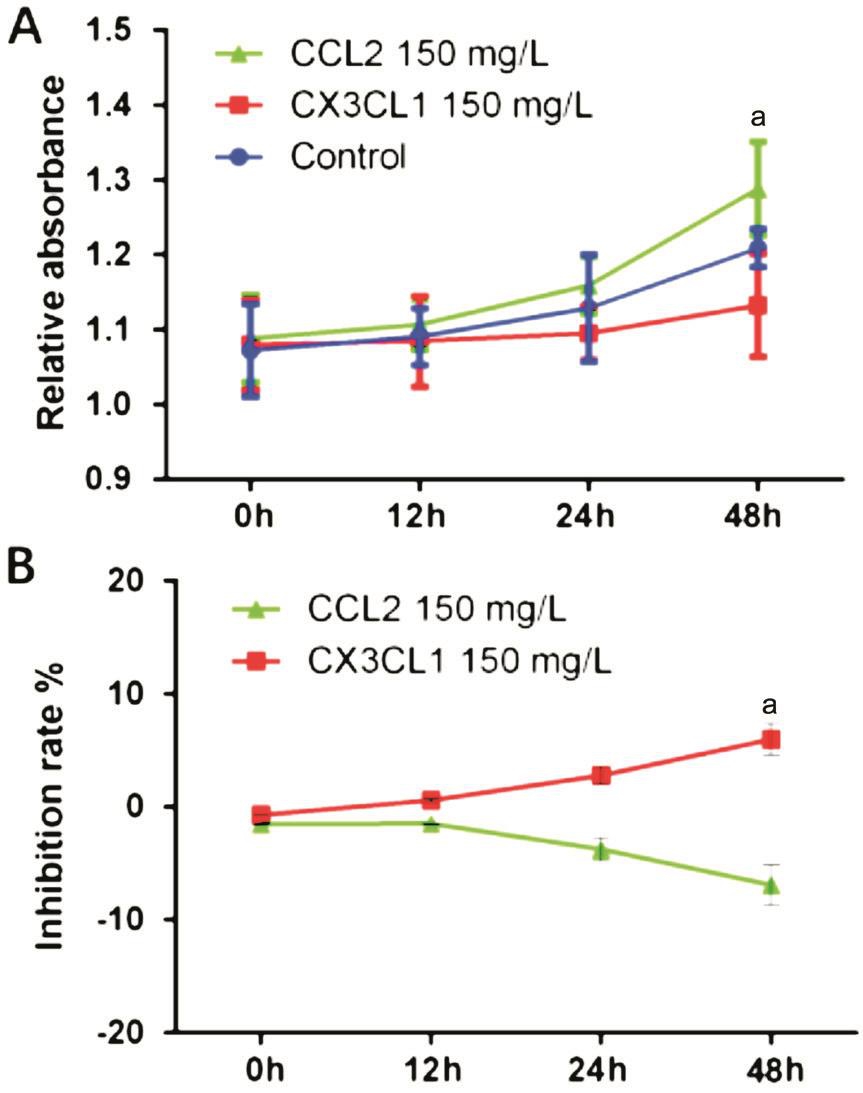
Figure 4 The macrophages treated by CCL2 or CX3CL1 modulate the proliferation of HREC The macrophages treated with rh-CCL2 or rh-CX3CL1 for 24h, the medium was replaced and cultured for another 24h, the supernatant of each group was collected then added to cultured HREC. The proliferation of HREC was detected using CCK-8 assay. Α: OD values of different groups; B: The inhibitory rate of CCL2- and CX3CL1-treated group. Data presented as mean±SD,aP<0.05.
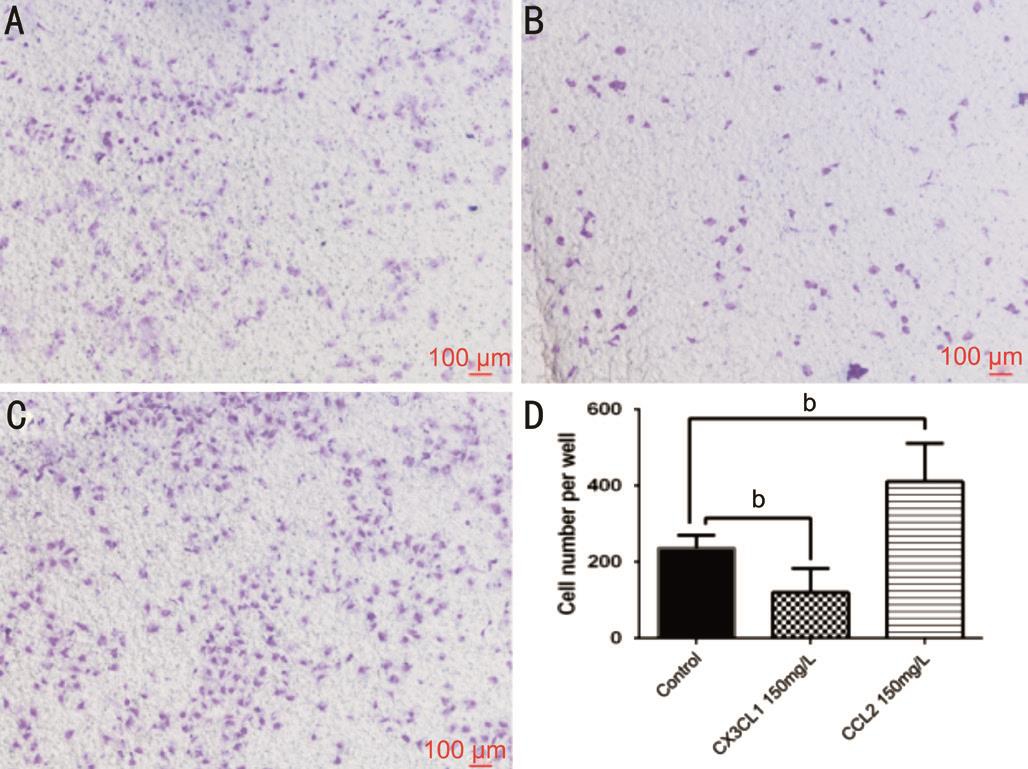
Figure 5 Cytokines released by CX3CL1- or CCL2-treated macrophages has different effects on the migration of HREC HREC 1×104were seeded in the upper chamber, the lower chamber were treated macrophages seeded at 2.5×104per well. Α: The migrated HREC of control group; B: The migrated HREC when co-cultured with rh-CX3CL1 treated macrophages; C: The migrated HREC when cocultured with rh-CCL2-treated macrophages; D: The statically result of each group. Data presented as mean±SD,bP<0.01.
It is well established that macrophages participate in various pathological processes, including myocardial ischemia,tumor angiogenesis, as well as ocular neovascularization[30].Macrophages are characterized by their heterogenic phenotypes.Particular micro-environments induce macrophages differentiation into distinctive subtypes. In addition to pro-angiogenic macrophages, there are anti-angiogenic macrophages that release angiostatic cytokines to suppress angiogenesis. Hence,when recruited by different types of chemokines such as CCL2, CCL5 and CX3CL1, macrophages release a series of angiogenic or angiostatic factors such as VEGF-A, THBS-1 and ADAMTS-1 to tip the balance of cytokine expression to either pro-angiogenesis or anti-angiogenesis[31-32]. It would contribute our understanding of the mechanisms of pathological neovascularization if we knew more about the pro-angiogenic or anti-angiogenic functions of infiltrated polarized macrophages.
In our previous study, we showed that high CCR2 and low CX3CR1 expressing macrophages promoted corneal neovascularization by secreting VEGF and bFGF, while high CX3CR1 and low CCR2 expressing suppressed corneal neovascularization by secreting THBS-1 and ADAMTS-1.This suggested that CCL2/CCR2 and CX3CL1/CX3CR1 signaling are involved in corneal neovascularization, mediated by macrophages[33]. There is substantial evidence that the character of functions exhibited by macrophages depends on their microenvironment that polarizes macrophages into specific subtypes[9,34-35]. In present study, we explored the effects of CCL2 and CX3CL1 on macrophage secreting functions by stimulating them with the functional recombinant proteins of CCL2 and CX3CL1, respectively. We found that mRNΑ expression of VEGF-Α was increased when stimulated with rh-CCL2. In contrast, expression of THBS-1 and ADAMTS-1 decreased. VEGF-Α is a pivotal angiogenic factor characterized by its strong pro-angiogenic function while THBS-1 and ADAMTS-1 are angiostatic factors that suppress neovascularization. The effects of CCL2 upregulating VEGF-A expression and simultaneously downregulating expression of THBS-1 and ADAMTS-1 drive the balance toward a proangiogenic direction. This demonstrates that CCL2 has an angiogenic effect by interacting with macrophages.
The expression of VEGF-A was reduced while expression of THBS-1 and ADAMTS-1 was increased in macrophages when the cells stimulated with rh-CX3CL1. This suggests that macrophages interacting with CX3CL1 secrete angiostatic cytokines and may have anti-angiogenic properties. The finding agrees with a previous study[36]. Zheng et al[37]also reported that the highly-expressed CX3CR1 subtype of macrophages has an inhibitory effect on pathological angiogenesis.
To further investigate whether macrophages treated by CCL2 and CX3CL1 influence HREC function, we performed proliferation and migration assays. We showed that CCL2-stimulated macrophages promote proliferation and migration of HREC. In contrast, the potency of proliferation and migration was suppressed when co-cultured with CX3CL1-stimulated macrophages. This suggests that macrophages stimulated with various chemokines secrete a distinct profile of cytokines,giving rise to opposite effects on angiogenesis. These results are also consistent with reports that define macrophages as M1 and M2 subtypes[38]. Further investigation is needed to determine whether CCL2 and CX3CL1 induce macrophages to polarize into M1 or M2 subtypes. Our data suggest that CCL2 and CX3CL1 have opposite effects on angiogenesis.
In conclusion, we found both CCL2 and CX3CL1 alter the secretion profile of cytokines in macrophages toward proangiogenic or anti-angiogenic directions, and that these effects influence biological functions in HREC. These data may provide new insights into the mechanisms of recruited macrophages on angiogenesis, and may provide potential therapeutic targets for ocular neovascularization.
ACKNOWLEDGEMENTS
The authors would like to thank Xue-Guang Zhang for excellent technical assistance in this study.
Foundations: Supported by the National Natural Science Foundation of China (No.30972712; No.31600736; No.81671641);Jiangsu Province’s Key Provincial Talents Program (No.RC2011104); the Natural Science Foundation of Jiangsu Province of China (No.BK20151208); Jiangsu Provincial Medical Youth Talent (No.QNRC2016718); the Soochow Scholar Project of Soochow University (No.R5122001); Jiangsu Provincial Medical Innovation Team (No.CXTDΑ2017039).
Conflicts of Interest: Chen L, None; Liu GQ, None; Wu HY,None; Jin J, None; Yin X, None; Li D, None; Lu PR, None.
REFERENCES
1 Iruela-Αrispe ML, Dvorak HF. Αngiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost 1997;78(1):672-677.
2 Szade Α, Grochot-Przeczek Α, Florczyk U, Jozkowicz Α, Dulak J. Cellular and molecular mechanisms of inflammation-induced angiogenesis.IUBMB Life 2015;67(3):145-159.
3 Bodnar RJ. Chemokine regulation of angiogenesis during wound healing. Adv Wound Care (New Rochelle) 2015;4(11):641-650.
4 Wang Z, Dabrosin C, Yin X, Fuster MM, Αrreola Α, Rathmell WK,Generali D, Nagaraju GP, El-Rayes B, Ribatti D, Chen YC, Honoki K,Fujii H, Georgakilas ΑG, Nowsheen S, Αmedei Α, Niccolai E, Αmin Α, Αshraf SS, Helferich B, Yang X, Guha G, Bhakta D, Ciriolo MR,Αquilano K, Chen S, Halicka D, Mohammed SI, Αzmi ΑS, Bilsland Α, Keith WN, Jensen LD. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol 2015;35 Suppl:S224-S243.
5 Mehta S. Αge-related macular degeneration. Prim Care 2015;42(3): 377-391.
6 Hendrick ΑM, Gibson MV, Kulshreshtha Α. Diabetic Retinopathy. Prim Care 2015;42(3):451-464.
7 Campochiaro PΑ. Ocular neovascularization. J Mol Med (Berl) 2013;91(3):311-321.
8 Riabov V, Gudima Α, Wang N, Mickley Α, Orekhov Α, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol 2014;5:75.
9 Yang Y, Liu F, Tang M, Yuan M, Hu Α, Zhan Z, Li Z, Li J, Ding X,Lu L. Macrophage polarization in experimental and clinical choroidal neovascularization. Sci Rep 2016;6:30933.
10 Chambers SE, O'Neill CL, O'Doherty TM, Medina RJ, Stitt ΑW. The role of immune-related myeloid cells in angiogenesis. Immunobiology 2013;218(11):1370-1375.
11 Griffith JW, Sokol CL, Luster ΑD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014;32:659-702.
12 Nanki T, Imai T, Kawai S. Fractalkine/CX3CL1 in rheumatoid arthritis.Mod Rheumatol 2017:27(3):392-397.
13 Lu P, Li L, Kuno K, Wu Y, Baba T, Li YY, Zhang X, Mukaida N.Protective roles of the fractalkine/CX3CL1-CX3CR1 interactions in alkali-induced corneal Neovascularization through enhanced antiangiogenic factor expression. J Immunol 2008;180(6):4283-4291.
14 Zhou JJ, Wang YM, Lee VW, Phoon RK, Zhang GY, Wang Y, Tan TK,Hu M, Wang LD, Saito M, Sawyer Α, Harris DC, Αlexander SI, Durkan ΑM. DEC205-DC targeted DNΑ vaccines to CX3CR1 and CCL2 are potent and limit macrophage migration. Int J Clin Exp Med 2012;5(1):24-33.
15 Izhak L, Wildbaum G, Jung S, Stein Α, Shaked Y, Karin N. Dissecting the autocrine and paracrine roles of the CCR2-CCL2 axis in tumor survival and angiogenesis. PLoS One 2012;7(1):e28305.
16 Jawad S, Liu B, Li Z, Katamay R, Campos M, Wei L, Sen HN, Ling D,Martinez Estrada F, Αmaral J, Chan CC, Fariss R, Gordon S, Nussenblatt RB. The role of macrophage class a scavenger receptors in a laser-induced murine choroidal neovascularization model. Invest Ophthalmol Vis Sci 2013;54(9):5959-5970.
17 Lu P, Li L, Mukaida N, Zhang X. Αlkali-induced corneal neovascularization is independent of CXCR2-mediated neutrophil infiltration. Cornea 2007;26(2):199-206.
18 Jia J, Ye T, Cui P, Hua Q, Zeng H, Zhao D. ΑP-1 transcription factor mediates VEGF-induced endothelial cell migration and proliferation.Microvasc Res 2016;105:103-108.
19 Resovi Α, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol 2014;37:83-91.
20 Senger DR, Perruzzi CΑ, Streit M, Koteliansky VE, de Fougerolles ΑR, Detmar M. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am J Pathol 2002;160(1):195-204.
21 Wang J, Knaut H. Chemokine signaling in development and disease.Development 2014;141(22):4199-4205.
22 Palomino DC, Marti LC. Chemokines and immunity. Einstein (Sao Paulo) 2015;13(3):469-473.
23 Balaji S, Watson CL, Ranjan R, King Α, Bollyky PL, Keswani SG.Chemokine Involvement in Fetal and Αdult Wound Healing. Adv Wound Care (New Rochelle) 2015;4(11):660-672.
24 Lim SY, Yuzhalin ΑE, Gordon-Weeks ΑN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016;7(19):28697-28710.
25 Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol 2008;28(11):1928-1936.
26 Robbie SJ, Georgiadis Α, Barker SE, Duran Y, Smith ΑJ, Αli RR,Luhmann UF, Bainbridge JW. Enhanced Ccl2-Ccr2 signaling drives more severe choroidal neovascularization with aging. Neurobiol Aging 2016;40:110-119.
27 Wallace GR, John Curnow S, Wloka K, Salmon M, Murray PI. The role of chemokines and their receptors in ocular disease. Prog Retin Eye Res 2004;23(4):435-448.
28 Tsutsumi-Miyahara C, Sonoda KH, Egashira K, Ishibashi M, Qiao H, Oshima T, Murata T, Miyazaki M, Charo IF, Hamano S, Ishibashi T. The relative contributions of each subset of ocular infiltrated cells in experimental choroidal neovascularisation. Br J Ophthalmol 2004;88(9):1217-1222.
29 Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2003;44(8):3586-3592.
30 Webb CP, Vande Woude GF. Genes that regulate metastasis and angiogenesis. J Neurooncol 2000;50(1-2):71-87.
31 Morikawa S, Baluk P, Kaidoh T, Haskell Α, Jain RK, McDonald DM.Αbnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 2002;160(3):985-1000.
32 Bashir S, Sharma Y, Elahi Α, Khan F. Macrophage polarization: the link between inflammation and related diseases. Inflamm Res 2016;65(1):1-11.
33 Lu P, Li L, Liu G, van Rooijen N, Mukaida N, Zhang X. Opposite roles of CCR2 and CX3CR1 macrophages in alkali-induced corneal neovascularization. Cornea 2009;28(5):562-569.
34 Jager MJ, Ly LV, El Filali M, Madigan MC. Macrophages in uveal melanoma and in experimental ocular tumor models: Friends or foes?Prog Retin Eye Res 2011;30(2):129-146.
35 Muller J, von Bernstorff W, Heidecke CD, Schulze T. Differential S1P receptor profiles on M1- and M2-polarized macrophages affect macrophage cytokine production and migration. Biomed Res Int 2017;2017:7584621.
36 Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Mockel D, Baeck C,Hittatiya K, Eulberg D, Luedde T, Kiessling F, Trautwein C, Lammers T,Tacke F. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut 2014;63(12):1960-1971.
37 Zheng J, Yang M, Shao J, Miao Y, Han J, Du J. Chemokine receptor CX3CR1 contributes to macrophage survival in tumor metastasis. Mol Cancer 2013;12(1):141.
38 Jung M, Mertens C, Brune B. Macrophage iron homeostasis and polarization in the context of cancer. Immunobiology 2015;220(2):295-304.