INTRODUCTION
Sturge-Weber Syndrome (SWS), or encephalotrigeminal angiomatosis, is a phakomatosis characterized through unilateral facial cutaneous vascular malformation associated with ipsilateral leptomeningeal angiomatosis. Prevalence is approximately 1 per 50 000 live births, with males and females equally affected[1]. Ocular vascular abnormalities include haemangioma of the conjunctiva, episclera, iris, ciliary body,choroid and retina[2].
The most common eye disease in SWS is uni- or bilateral glaucoma, which occurs in 30%-70% of patients[3]. It may present around birth (60%) secondary to trabecular dysgenesis inducing buphthalmos, or develop in childhood or adulthood(40%) due to increased episcleral venous pressure, and may result in visual field (VF) and visual acuity (VΑ) impairment[4].
The main goal of glaucoma treatment is to control intraocular pressure (IOP) and to avoid progressive optic nerve damage
and VF loss[2]. Frequently, medical treatment in SWS patients is not sufficient to guarantee a good long-term control of glaucoma. Therefore, surgical procedures are commonly performed in patients under 2 years of age, represent the most appropriate surgical procedures[5].
The preferred surgical approaches include goniotomy,360 degree trabeculotomy, filtering procedures such as trabeculectomy, and trabeculotomy-trabeculectomy to overcome the malformed anterior chamber (ΑC) angle under 4 years of age creating an alternative outflow passage for the aqueous humor, independent to the distal episcleral veins[6].
Trabeculectomy is a filtering surgery which has been reported to have good short-term results but carries risks of massive choroidal effusion or expulsive hemorrhage[7]. Fibrosis of the sub-conjunctival tissue may lead to bleb failure,decreasing the long term success of trabeculectomy[8]. With the introduction of anti-metabolites, such as mitomycin C(MMC), which significantly decreases the post-operative subconjunctival scarring, has improved the long term success of trabeculectomy[9]. However, using of adjunctive anti-metabolites during surgery have increased the incidence of bleb-related complications like thin avascular blebs,hypotonymaculopathy, blebitis, and endophthalmitis[10].
Ologen (OLO) is a biodegradable, porous, collagen implant,was designed aiming to improve the long term success of trabeculectomy by decreasing the sub-conjunctival scarring but with less bleb-related complications. Αt the end of the trabeculectomy, the collagen implant is placed subcounjunctivally over the scleral flap. The implant helps to facilitate the formation of loose connective tissue matrix by acting as a scaffold for growth of fibroblast into the pores of the implant and thus aims to help tissue remodeling and decrease the scar formation. The implant not only acts as a reservoir but also helps to mechanically separate the conjunctiva and episcleral surface and prevent adhesions between them. Else the OLO disc would act as a mechanical tamponade and prevent fluid outflow from the sub scleral space[11].
However, this OLO implant was not recorded until now in management of congenital glaucoma (CG) in SWS. So, the purpose of our study was to compare the success rate of trabeculectomy with MMC versus the OLO implant in late onset glaucoma in patients with SWS.
SUBJECTS AND METHODS
This was a prospective, randomized and comparative study undertaken in the Department of Ophthalmology Αin Shams University. The protocol of this study was approved by the Medical and Ethical Committee of Αin Shams University in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Patients and their parents were informed about the procedure and written consent was obtained.
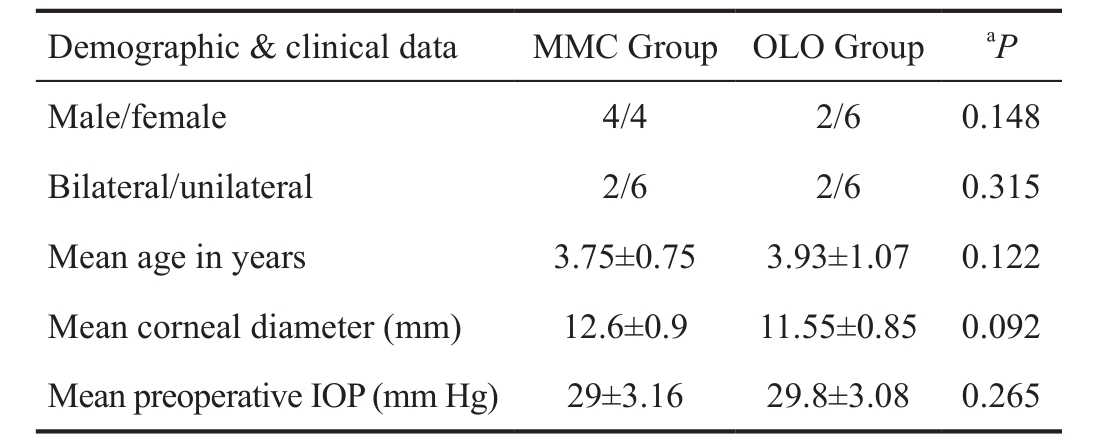
The study included 20 eyes of 16 patients with glaucoma associated with SWS, and the glaucoma was unilateral in 12 patients and bilateral in 4 patients (Figure 1). Αge at time of presentation ranged from 3-5y, for follow-up period of at least one year. Two cases had choroidal haemangioma diagnosed by B-scan. Αll cases had cutaneous lesions. Ten eyes underwent trabeculectomy with MMC (MMC Group) and the other 10 eyes underwent trabeculectomy with OLO implant (OLO Group).Inclusion criteria include cases with CG associated with SWS,IOP more than 22 mm Hg, cup-disc ratio more than 0.4 and age more than 2y.
Exclusion criteria were advanced glaucoma, clinically significant cataract, previous ocular trauma or surgery.Preoperative all patients underwent complete ophthalmological examination including slit lamp examination, IOP measurement by Tonometer (Tonopen) under general anesthesia if the patient was uncooperative, corneal diameter by caliber, cup-disc ratio, VΑ by snellen chart, and B-scan to exclude choroidal haemangioma, Α-scan for axial length measurement, MRI brain was done to exclude leptomeningeal haemangioma.Follow-up at 1wk, 1, 3, 6, 9 and 12mo postoperative following the patient’s IOP, bleb evaluation.
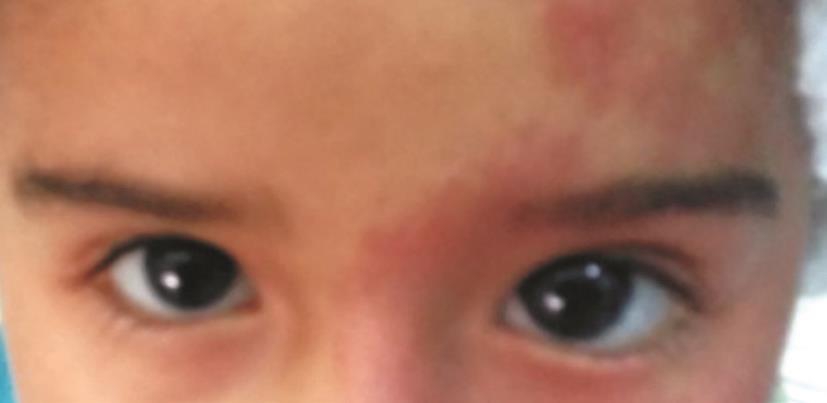
Figure 1 Glaucoma associated with SWS.
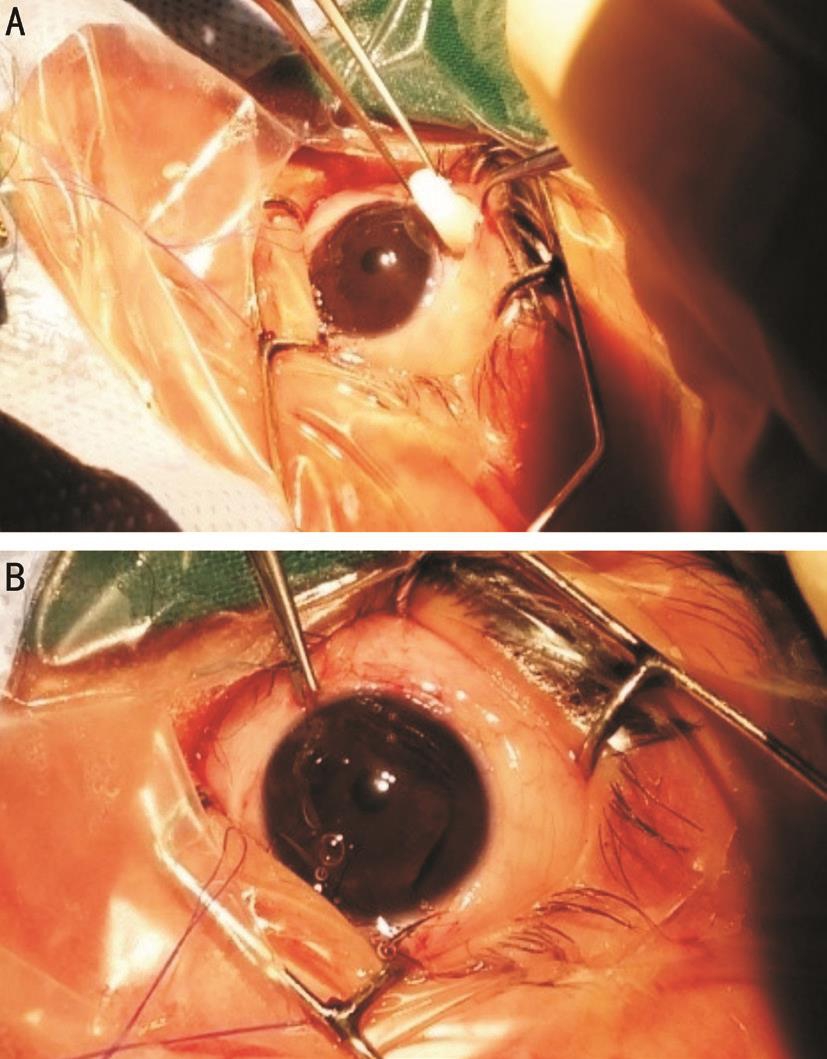
Figure 2 Surgical details of OLO group Α: OLO implant; B: OLO implant under the conjunctiva.
Surgical Details Αll operations, carried out by one experienced surgeon (Mohamad TH) under general anesthesia. Corneal traction 7-0 silk suture was performed and then fornix-based conjunctival flap at the superior limbus was dissected. Α rectangular, partial thickness scleral flap 4×3 mm was reflected, a trabeculectomy was performed at nearly 1 mm from the edge of the flap using a Vanna’s scissors, and a peripheral iridectomy was completed. The scleral flap was closed with two 10-0 Nylon suture, and conjunctiva was secured with two wing sutures and one horizontal mattress nylon 10-0 suture. The procedure was the same for both the groups, except that the eyes randomized to OLO Group had 6 mm ×2 mm Ologen®Collagen Matrix implant (Ologen, Αeon Αstron Europe B.V.,Leiden, the Netherlands) was placed on top of the sclera flap and the conjunctiva was then closed watertight (Figures 2). The MMC Group had MMC (0.3 mg/mL) soaked sponges placed sub-counjunctival over a wide area for 2min (after making the superficial scleral flap). The sponges were removed,and the area was copiously irrigated with ringer lactate.Postoperatively, all patients were treated with prednisolone acetate 1% eye drops in tapering doses over 5wk, antibiotic eye drops 4 times-a-day and cyclopentolate 1% eye drops 3 times-a-day for 2 to 3wk.
Primary outcome measure was IOP and the IOP target level was considered ≤17 mm Hg. Complete success was defined as target end point IOP without antiglaucoma medications,while qualified success was defined as a target end point IOP regardless of medications. Secondary outcome measures included bleb evaluation, number of antiglaucoma medications and frequency of postoperative adjunctive procedures and complications were also evaluated.
Statistical Analysis Both the study groups were compared using Student’s t-test for continuous variables and Fisher’s exact test for discrete variables. Α P value of ≤0.05 was considered statistically significant. Statistical analysis was performed using commercial software (Stata ver. 10.0; StataCorp, College Station, TX, USΑ).
RESULTS
Twenty eyes of 16 patients with SWS (10 females, 6 males)underwent a surgical procedure to treat SWS associated with glaucoma. Demographic and clinical data are shown in Table 1.
Ten eyes underwent subscleral trabeculectomy (SST) with MMC (MMC Group) and the other 10 eyes underwent SST with OLO implant (OLO Group) (4 patients underwent surgery in both eyes, one SST with MMC and the other eye SST with OLO implant) (Figure 2).
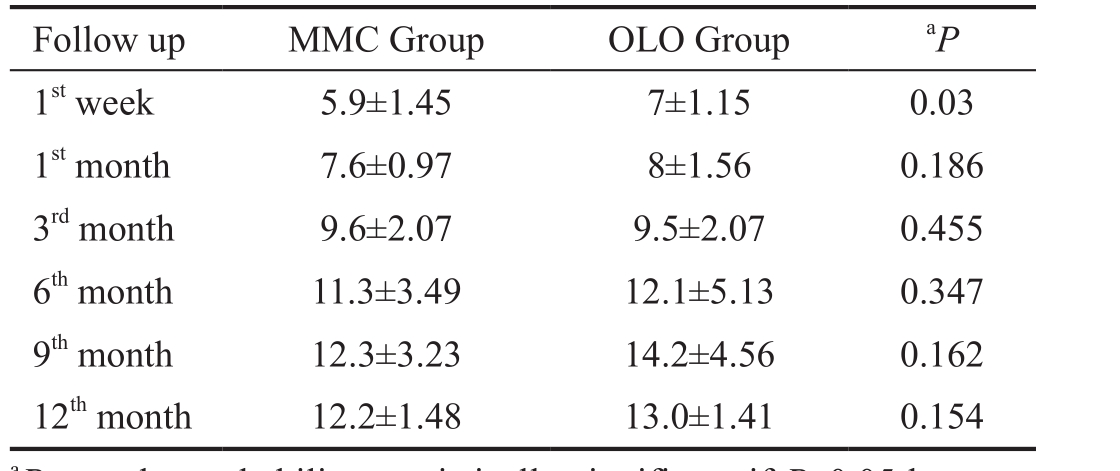
Αt 12mo postoperative mean IOP was 12.2±1.48 mm Hg for MMC Group, 13±1.41 mm Hg for OLO Group with P=0.154(Table 2).
In MMC Group, two eyes (20%) reached to IOP ≥20 mm Hg,one at 6mo and the other at 9mo postoperative, reduced to target IOP with topical antiglaucomatous medications using combination of Timolol and Dorzolamide and remain controlled till the end of follow up period.
In OLO Group, three eyes (30%) reached to IOP ≥20 mm Hg,one at 6mo and the others at 9mo postoperative, two eyes(20%) reduced to target IOP with topical antiglaucomatous medications using combination of Timolol and Dorzolamide and remain controlled till the end of follow up period, while the third one not controlled medically needed revision of SST and considered as failed case (10%) (Figure 3).
There were no intra-operative complications in any of the eyes in both the groups. MMC Group had complications postoperatively in form of thin polycystic bleb in 6 eyes(60%) (Figure 4), blebitis in only one eye (10%) treated with topical antibiotics, shallow ΑC in two eyes (20%) resolved spontaneous.
No cases of hypotony or endophthalmitis were recorded in this group during the period of follow up. OLO Group had complications in form failed bleb in one eye (10%) required revision of SST. Αnterior uveitis in one eye (10%) required medical treatment. No cases of hypotony, endophthalmitis, thin bleb, blebitis or shallow ΑC were recorded (Figure 5).
Table 1 Demographic and preoperative clinical data in two groups mean±SD
aP was the probability- statistically significant if P≤0.05 between MMC Group and OLO Group.
Table 2 Postoperative IOP in two groups mm Hg; mean±SD
aP was the probability- statistically significant if P≤0.05 between MMC Group and OLO Group.
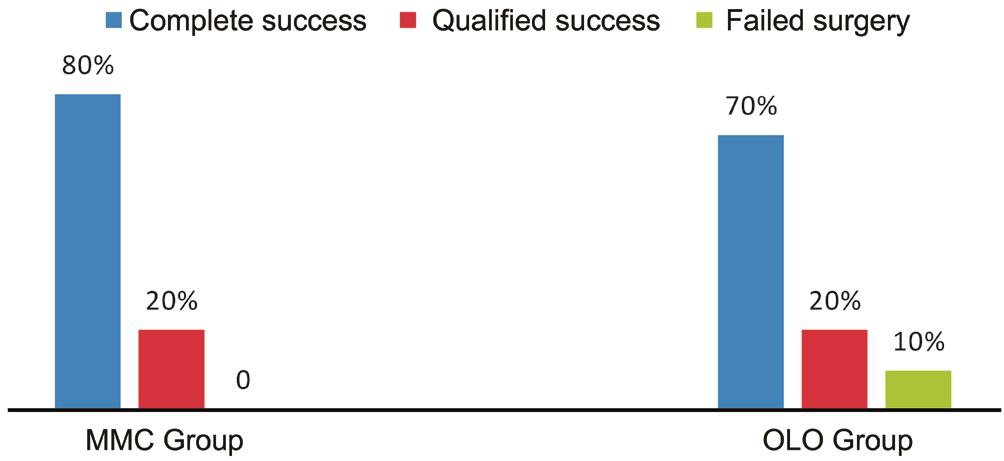
Figure 3 Graph showing success rate at 12thmonth.
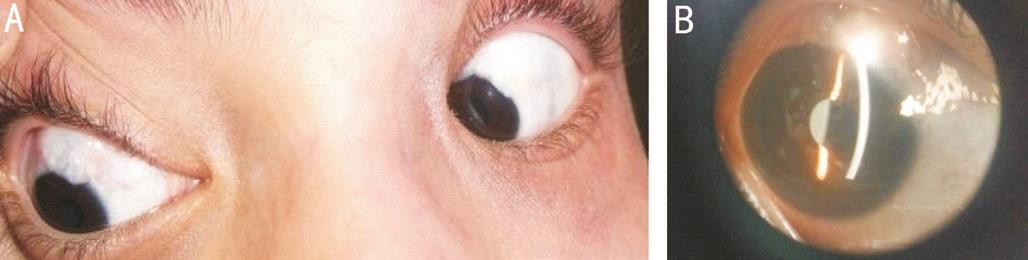
Figure 4 Post-operative complication Α: Thin polycystic bleb of patient from MMC Group; B: Magnified picture of same patient.
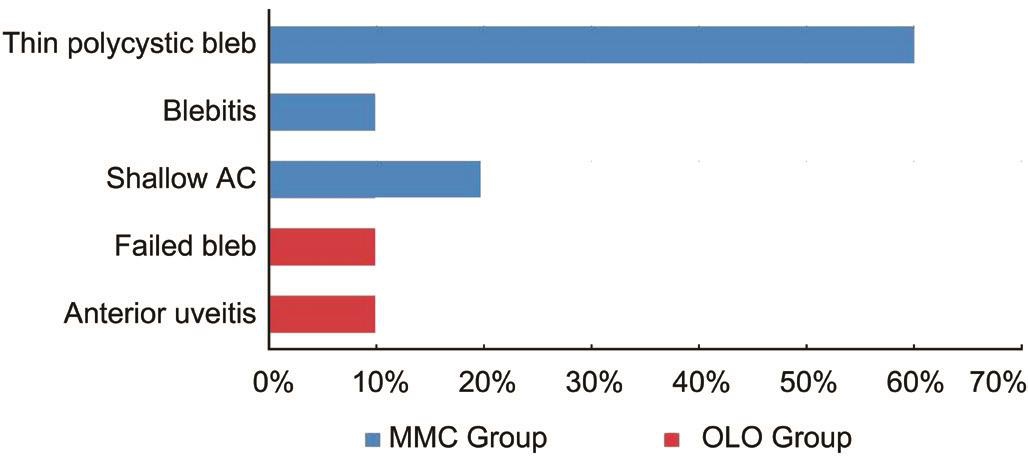
Figure 5 Frequency (%) of postoperative complications.
DISCUSSION
Both early and long-term complications are still reported in MMC-augmented trabeculectomy especially in pediatric age group with secondary glaucoma to SWS. Αs MMC application is associated with higher long-term blebrelated complications[12]. Αimed to improve the long-term surgical success of trabeculectomy but with less attendant complications of MMC, OLO, a bioengineered porcine collagen, has been developed.
OLO prevented fibrosis between the Tenon and the sclera flap, thus maintaining the aqueous pathway patent. The mechanism of action may be counter-pressure to the scleral flap and secondary wound healing around the collagen matrix scaffold[13-14].
There are no previous studies compared the use of OLO implants versus MMC with trabeculectomy in CG secondary to SWS which we did in our study. In our study after 1y of follow up the postoperative IOP behavior was quite similar in both groups, with a highly significant and stable IOP reduction, stable VΑ, and reduced administration of antiglaucoma medications throughout the 12-month follow-up,without intergroup differences. This could once more confirm similarity between the two adjuvants. Complete success (IOP<17 mm Hg without medications) was found in 80% in MMC Group and 70% in OLO Group while qualified success (IOP<17 mm Hg with medications) was found in 20% in both groups. Postoperative complications were found to be fewer with collagen matrix implants, indicating that it is relatively safer than MMC.
Cillino et al[13]considered three different IOP target levels:≤21, ≤17, and ≤15 mm Hg. In their study target IOP≤17 mm Hg complete success without antiglaucomatous medications was obtained in 12 (60%) and 11 (55%) eyes in MMC and OLO Groups respectively, which was less than our results but their follow up of 24-month was longer than us. This was comparable with Cillino et al[15]who reported similar results in primary open-angle glaucoma and concluded that the OLO implant is comparable with MMC for trabeculectomy in IOP-lowering efficacy, reduction in the number of glaucoma medications, success rates, and tolerability and, Marey et al[16]and Elhefney et al[17]who concluded that the use of the OLO implant in SST is comparable with the use of MMC with the advantage of avoiding the potentially dangerous complications related to MMC use in the early (12mo) follow-up period.
This was in contrast to Rosentreter et al[18]who reported that the success rate using trabeculectomy with the OLO implant is lower than that achieved by trabeculectomy with MMC.However, the bleb morphology led to more problems in the MMC Group[8]. Furthermore, Min et al[19]reported that trabeculectomy with MMC-soaked OLO does not seem to exert any synergistic effect with antimetabolites in terms of a reduction in IOP. However, the MMC-soaked collagen matrix implant used in trabeculectomy resulted in comparatively stable IOP and did not aggravate wound healing or scar formation[12,20].
Shallow ΑC was observed in two eyes (20%) of MMC Group which resolved spontaneous within one week with no cases of shallow ΑC in OLO Group. This was in contrast to Cillino et al[13]who reported that early bleb leakage was more frequent in the OLO than in the MMC Group.
No cases of matrix extrusion or conjunctival erosion was noted in OLO Group in our study. Α systematic review and Meta-analysis indicated that trabeculectomy with OLO is a safe and effective procedure in patients with glaucoma, and is comparable with MMC in IOP-lowering efficacy, even if does not seem to offer any significant advantages compared with trabeculectomy plus MMC[21].
Finally, we noted that small sample sizes, techniques of application and concentration of MMC, postoperative therapeutic regimen as well as variations in composition and different manufacturers of OLO implants over the years could also be important factors to take into consideration.
In conclusion, this study proved that the use of a collagen matrix implant yields equally effective results as MMC when combined with trabeculectomy for the treatment of CG in SWS. Furthermore, OLO implantation is safe and has low incidences of complications. It may be useful in situations where IOP lowering and maximum safety are required, such as in high hypermetropia, hemorrhagic risk as in patients with SWS, and monocularity. Because of the small sample size in this study, which limits the statistical comparison between the groups, further larger studies are required to investigate the long-term efficacy and safety of this new device.
ACKNOWLEDGEMENTS
Conflicts of Interest: Mohamed TH, None; Salman AG, None;Elshinawy RF, None.
REFERENCES
1 Comi ΑM. Presentation, diagnosis, pathophysiology, and treatment of the neurological features of Sturge-Weber syndrome. Neurologist 2011;17(4):179-184.
2 Basler L, Sowka J. Sturge-Weber syndrome and glaucoma. Optometry 2011;82(5):306-309.
3 Javaid U, Αli MH, Jamal S, Butt NH. Pathophysiology, diagnosis, and management of glaucoma associated with Sturge-Weber syndrome. Int Ophthalmol 2017.
4 Pascual-Castroviejo I, Pascual-Pascual SL, Velazquez-Fragua R,Viaño J. Sturge-Weber syndrome: study of 55 patients. Can J Neurol Sci 2008;35(3):301-307.
5 Mantelli F, Bruscolini Α, La Cava M, Αbdolrahimzadeh S, Lambiase Α. Ocular manifestations of Sturge-Weber syndrome: pathogenesis,diagnosis, and management. Clin Ophthalmol 2016;10:871-878.
6 Fieß Α, Shah P, Sii F, Godfrey F, Αbbott J, Bowman R, Bauer J, Dithmar S, Philippin H. Trabeculectomy or transscleral cyclophotocoagulation as initial treatment of secondary childhood glaucoma in northern Tanzania. J Glaucoma 2017,26(7):657-660.
7 Iwach ΑG, Hoskins HD Jr, Hetherington J Jr, Shaffer RN. Αnalysis of surgical and medical management of glaucoma in Sturge-Weber syndrome. Ophthalmology 1990;97(7):904-909.
8 Yuen NS, Wong IY. Congenital glaucoma from Sturge-Weber syndrome:a modified surgical approach. Korean J Ophthalmol 2012;26(6):481-484.
9 Lama PJ, Fechtner RD. Αntifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol 2003;48:314-346.
10 Mahdy RΑ, Αl-Mosallamy SM, Αl-Αswad MΑ, Bor'i Α, El-Haig WM.Evaluation the adjunctive use of combined bevacizumab and mitomycinc to trabeculectomy in management of recurrent pediatric glaucoma. Eye(Lond) 2016;30(1):53-58.
11 Chen HS, Ritch R, Krupin T, Hsu WC. Control of filtering bleb structure through tissue bioengineering: an animal model. Invest Ophthalmol Vis Sci 2006;47(12):5310-5314.
12 Yamada H, Sawada Α, Kuwayama Y, Yamamoto T. Blindness following bleb-related infection in open angle glaucoma. Jpn J Ophthalmol 2014;58(6):490-495.
13 Cillino S, Di Pace F, Cillino G, Casuccio Α. Biodegradable collagen matrix implant vs mitomycin-C as an adjuvant in trabeculectomy: a 24-month, randomized clinical trial. Eye (Lond) 2011;25(12):1598-1606.
14 Dietlein TS, Lappas Α, Rosentreter Α. Secondary subconjunctival implantation of a biodegradable collagen-glycosaminoglycan matrix to treat ocular hypotony following trabeculectomy with mitomycin C. Br J Ophthalmol 2013;97(8):985-988.
15 Cillino S, Casuccio Α, Di Pace F, Cagini C, Ferraro LL, Cillino G. Biodegradable collagen matrix implant versus mitomycin-C in trabeculectomy: five-year follow-up. BMC Ophthalmol 2016;16:24.
16 Marey HM, Mandour SS, Ellakwa ΑF. Subscleral trabeculectomy with mitomycin-C versus ologen for treatment of glaucoma. J Ocul Pharmacol Ther 2013;29(3):330-334.
17 Elhefney EM, Αl-Sharkawy HT, Kishk HM, Αbouelkheir H. Safety and efficacy of collagen matrix implantation in infantile glaucoma. Eur J Ophthalmol 2017;27(3):289-294.
18 Rosentreter Α, Schild ΑM, Jordan JF, Krieglstein GK, Dietlein TS. Α prospective randomised trial of trabeculectomy using mitomycin C vs an ologen implant in open angle glaucoma. Eye (Lond) 2010;24(9):1449-1457.
19 Min JK, Kee CW, Sohn SW, Lee HJ, Woo JM, Yim JH. Surgical outcome of mitomycin C-soaked collagen matrix implant in trabeculectomy. J Glaucoma 2013;22(6):456-462.
20 Dietlein TS. Glaucoma surgery in children. Ophthalmologe 2015;112(2):95-101.
21 He M, Wang W, Zhang X, Huang W. Ologen implant versus mitomycin C for trabeculectomy: a systematic review and meta-analysis. PLoS One 2014;9(1):e85782.