INTRODUCTION
Cataract is a common age-related disease and its incidence continues to rise[1]. Due to the unclear nature of its pathogenesis, non-surgical treatment of cataracts has remained limited. Previous studies of the pathogenesis of cataracts have provided evidence to suggest that oxidative damage may be a key factor in cataract development[2-3]. One study found a significantly elevated level of reactive oxygen species(ROS) in aqueous humor and lens of patients with age-related cataracts[4], while a separate in vitro study has demonstrated that hydrogen peroxide (H2O2) concentration equal to that in the lens of cataract patients may lead to lens opacity and lens epithelial cell apoptosis[5].
Micro RNAs (miRNAs) are the short noncoding RNAs of 21-23 nucleotides in length, which can bind to the 3’-untranslated region (UTR) of target mRNAs, resulting in the translational repression or degradation of mRNA[6]. Prior studies have shown that miRNAs are involved in a variety of physiological and pathological processes[7]. It has also been shown that some miRNAs are associated with the onset of age-related cataracts,suggesting that miRNAs may become a new target for cataract diagnosis and treatment[8].miR-211 is located on intron 6 of the Trpm1 gene at 15q13-q14,a locus that is frequently lost in many neoplasms[9]. miR-211 is one of the most abundant miRNAs in the developing and adult eye[10]. miR-211 belongs to a group of specific miRNAs that are highly expressed in human vitreous[11].
Recent studies have found that miRNAs regulate human lens epithelial cell (hLEC) apoptosis[8,12]and thus may be involved in the development of cataracts. Expression of miR-211 specifically has been found to play a key role in retinal pigment epithelium (RPE) cell differentiation and function[13-14].However, the precise relationship between miR-211 and oxidative damage leading to cataract formation has not been previously reported. Thus, in this study, we measured the expression of miR-211 in age-related cataract lens tissue,and then investigated the role and mechanism of miR-211 in the oxidative stress response and the process of age-related cataract formation.
SUBJECTS AND METHODS
Specimens A total of 21 fresh anterior lens capsules were collected between January 2016 and March 2016 at the Fourth Affiliated Hospital of China Medical University from agerelated cataract patients undergoing phacoemulsification surgery (patients were excluded if they were affected by other eye diseases). Nine of the samples were collected from males and 12 from females, aged 56-72 (61.31±8.23)y. Fifteen transparent (healthy) anterior lens capsules were collected from the Fourth Affiliated Hospital of China Medical University Eye Bank, including 9 from males and 6 from females, aged 51-69 (60.24±7.32)y. All specimens were immediately stored in liquid nitrogen at the time of collection. This study was approved by the Ethics Committee of the Fourth Affiliated Hospital of China Medical University, and signed informed consent was obtained from each patient.
Cell Culture A human lens epithelial cell line (SRA01/04)was generously donated for experimental use by Dr. Yi-sin Liu of the Doheny Eye Institute. This cell line was cultured in a medium containing 10% fetal bovine serum (Gibco, USA),100 U/mL penicillin and 100 mg/mL streptomycin (Thermo,USA) in DMEM medium (Invitrogen, USA), and was placed in a 37℃, 5% CO2constant temperature incubator.
Real-time Quantitative Polymerase Chain Reaction Trizol reagent (Invitrogen, USA) was used to extract total cell RNA. Then, a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, USA) was used to obtain miRNA cDNAs. TaqMan MicroRNA Assays (Applied Biosystems,expression USA) were used to detect miR-211 expression, and RNU6B was employed as an internal control. RNA was reverse transcribed using the PrimerScript RT reagent kit (Takara,China) and SIRT1 mRNA expression was detected using a TaqMan Universal Master Mix II (Applied Biosystems, USA),with β-actin designated as an internal control. The upstream and downstream primers of miR-211 and RNU6B were purchased from ThermoFisher (USA) and their sequences can be found on their website. The p53 primer sequences are as follows:forward: 5’-CAGCAGTCAAGCACTGCCAAG-3’, reverse:5’-AGACAGGCATGGCACGGATAA-3’. The Bax primer sequences are as follows: forward: 5’-AGATGAACTGG ACAGCAATATG-3’, reverse: 5’-CCTACCCAGCCTCCG TTAT-3’. The β-actin primer sequences were: forward:5’-CATCCGTAAAGACCTCTATGCCAAC-3’, reverse:5’-ATGGAGCCACCGATCCACA-3’. PCR was performed using the ABI 7500 (Applied Biosystems, USA). Three independent experiments were performed and 2-ΔΔCtquantitative analysis was performed to analyze relative expression levels.
Detection of Endogenous Intracellular Reactive Oxygen Species A 2’,7’-dichloro-fluorescein diacetate (DCFHDA) probe was used to detect fluorescence derived from endogenous ROS in hLECs. Totally 1×104cells were seeded into each well of a 96-well plate and were cultured for 16h,until cells were observed adhering to the sides of the well.The cells were then exposed to 400 μmol/L H2O2for 1h whereupon the culture medium was aspirated and 10 μmol/L fluorescent probe DCFH-DA was added to each well. This mixture was then incubated in a 37℃ incubator for 20min.The cells were then washed three times with phosphate buffer solution and their DCF fluorescence intensity value (i.e. the mean fluorescence intensity of DCF, representing the level of intracellular ROS) was read using a multifunctional microplate reader. The excitation wavelength used was 485 nm and the emission wavelength was set at 530 nm.
Cell Transfection SRA01/04 cells were seeded in 24-well cell culture plate for 24h. Then Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, USA) was used according to the manufacturer instructions to transfect the cells with miR-211 mimics, mimic controls, miR-211 inhibitors, or inhibitor controls. After 72h, cells were exposed to 400 μmol/L H2O2for 1h whereupon mRNA and protein levels of p53 and Bax were measured using RT-qPCR and Western blotting. An MTS assay was used to assess cell viability.
Cell Viability Assay Cell viability was detected using the MTS assay kit (Promega, China). Seventy-two hours after transfection, SRA01/04 cells were exposed to 400 μmol/L H2O2for 1h. According to the manufacturer’s instructions,20 μL MTS solution was added to each well and the cells were then incubated for 4h at 37℃, 5% CO2. The absorbance of each group was read using an absorbance plate reader set to 490 nm wavelength.
Western Blotting Protein RIPA lysis buffer (Pierce, USA)was used to extract total protein and a BCA kit (Thermo,USA) was employed to quantify protein concentration. Forty microgramme of protein solution was added to each well of 10% NuPAGE Bis-Tris precast gels (Invitrogen, USA) for electrophoretic separation of proteins. Proteins were then transferred to PVDF membranes and blocked with 5% nonfat dry milk for 1h at room temperature. The protein was then incubated with primary antibody: rabbit anti-p53 (1:1000,abcam, USA), rabbit anti-Bax (1:1000, abcam, USA) or rabbit anti-GAPDH (1:2000, abcam, USA) overnight at 4℃.HRP-labeled goat anti-rabbit IgG (H+L) secondary antibody(1:2500, Promega, USA) was then added and samples were incubated at room temperature for 2h. Proteins were then detected using an ECL kit after which analysis of protein bands was conducted using Image J software (USA).
Statistical Analysis Each experiment was performed independently at least 3 times with similar results. Measurement data were presented as mean±standard deviation (SD).Differences between the groups were calculated using unpaired Student’s t-tests. Statistical significant difference was considered at P<0.05. Statistical analysis was done using SPSS 16.0 (USA).
RESULTS
miR-211 Expression Increased in the Anterior Lens Capsules of Patients with Age-related Cataracts RT-qPCR detection of the expression level of miR-211 indicated that,as compared to the healthy transparent lens capsule group(normal group), the level of miR-211 expression in anterior capsules of patients with age-related cataracts (cataract group)was significantly higher (4.23±0.27, P<0.001).
miR-211 Expression Increased in hLECs Subjected to Oxidative Stress Based on the results of the DCFHDA fluorescent probe assay, endogenous ROS levels were significantly increased in SRA01/04 lens epithelial cells exposed to 400 μmol/L H2O2(H2O2group, 264.64±32),relative to the control group (53.35±9.12, P<0.001). miR-211 expression in the H2O2group was also significantly higher than the control group as measured by RT-qPCR (5.24±0.38,P<0.001).
miR-211 Regulated the Oxidative Stress Response in hLECs Seventy-two hours after transfection, SRA01/04 cells were exposed to 400 μmol/L H2O2for 1h. Cell viability was detected using the MTS assay. It was found that, compared with the mimic control group, the cell viability of the miR-211 mimic group lens epithelial cells was significantly reduced(P<0.001; Figure 1). Compared to the control group, the cell viability of the lens epithelial cells in the miR-211 inhibitor group was significantly increased (P<0.001; Figure 1)suggesting that miR-211 suppresses proliferation in hLECs and diminishes their ability to cope with oxidative stress.
miR-211 Increased the Relative Expression of p53 and Bax mRNA in hLECs Using RT-qPCR, it was found that,compared with the mimic control group, the miR-211 mimic group hLECs had significantly increased levels of p53 and Bax mRNA expression (P<0.001; Figure 2). Conversely, p53 and Bax mRNA expression was significantly reduced in the miR-211 inhibitor group as compared to the inhibitor control group(P<0.001; Figure 2).
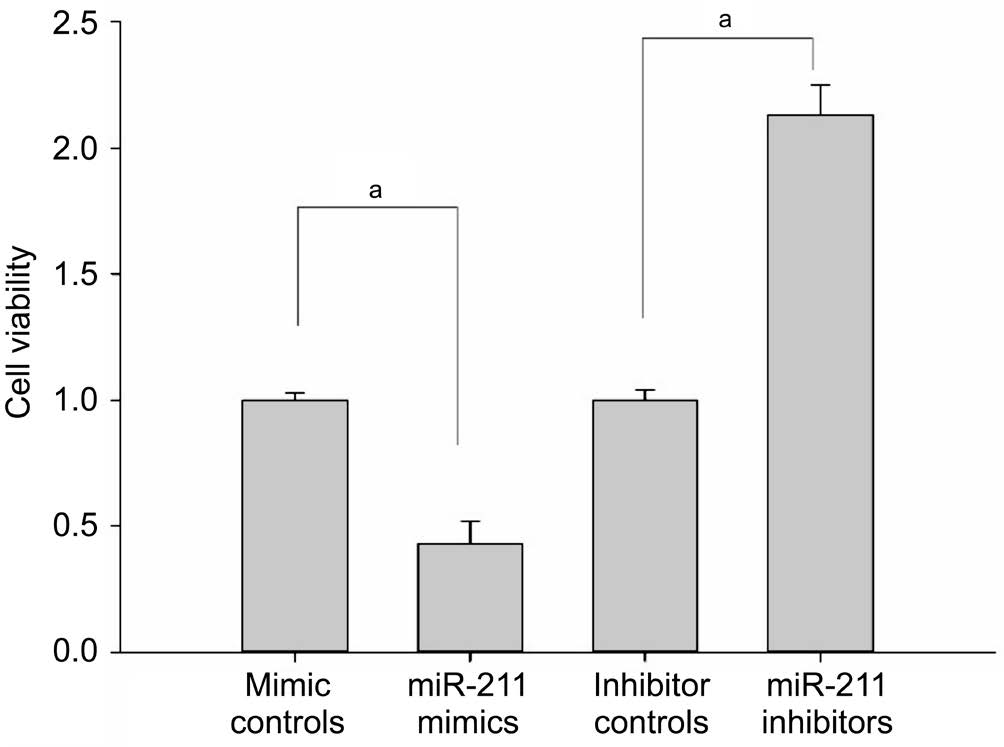
Figure 1 Cell viability of hLECs measured by MTS assay Seventytwo hours after transfection, SRA01/04 cells were exposed to 400 μmol/L H2O2for 1h. Cell viability was detected using the MTS assay (aP<0.001).
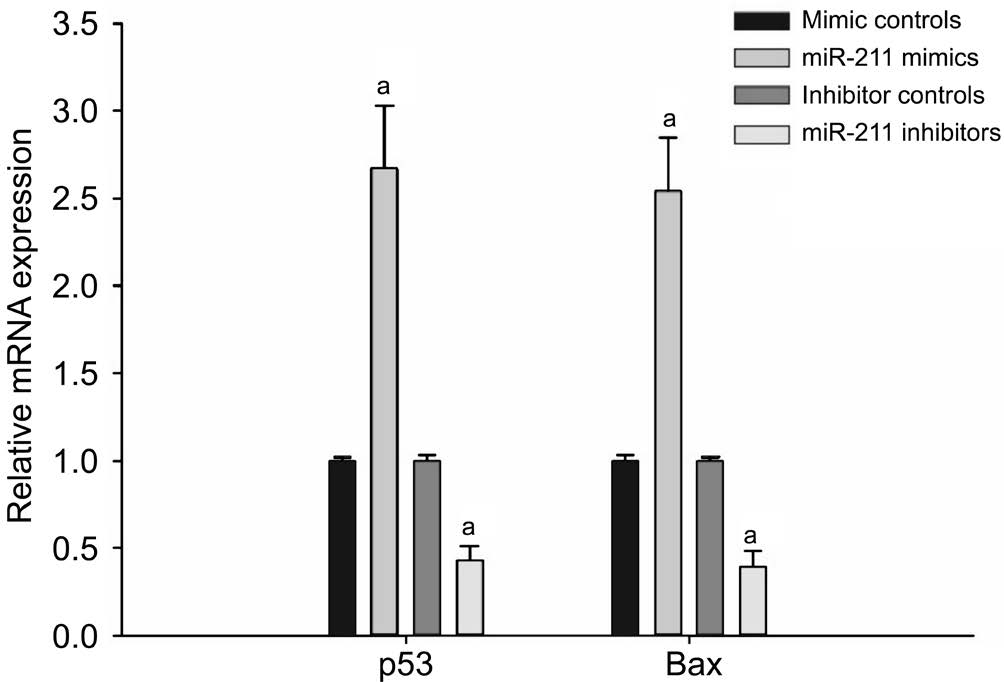
Figure 2 RT-qPCR detection of the expression of p53 and Bax mRNA in hLECs Compared with the mimic control group, the miR-211 mimic group hLECs had significantly increased levels of p53 and Bax mRNA expression. Conversely, p53 and Bax mRNA expression were significantly reduced in the miR-211 inhibitor group as compared to the inhibitor control group (aP<0.001).
miR-211 Increased the Relative Expression of p53 and Bax Protein in hLECs By Western blotting, it was found that the miR-211 mimic group hLECs had significantly increased levels of p53 and Bax protein expression when compared with the mimic control group (P<0.001; Figure 3). Conversely, p53 and Bax protein expression were significantly reduced in the miR-211 inhibitor group as compared to the inhibitor control group (P<0.001; Figure 3).
DISCUSSION
As stated in the introduction, cataracts are one of the most common ocular diseases, and a leading cause of blindness worldwide[15]. Current treatments of this condition are almost exclusively surgical, and cataract surgery is the most common surgical procedure performed on individuals over 65y[16-17]. Surgical treatment, while effective, often incurs a large economic cost and generates a great deal of fear in patients[18-19]. Therefore, investigation into effective nonsurgical interventions for cataracts is an important endeavor with the potential for widespread application and great social benefit.
Previous research has reported that a variety of stimuli have the potential for generating oxygen free radicals in the eye and that the resultant oxidative stress plays an important role in the pathogenesis of cataracts[20-22]. Oxidative stress refers when to the exogenous or endogenous ROS exceeds the antioxidant capacity of a cell and exerts a deleterious impact on the cellular signal transduction system, and further damages nucleic acids, proteins, lipids and other macromolecules[4,23-24].Excess of ROS are often associated with pathology and have been implicated in causing cellular aging, apoptosis and necrosis[20,25-26]. It has also been previously reported that hydrogen peroxide (H2O2) levels are significantly increased in the aqueous humor of cataract[4]. A study by Spector and Garner[27]showed that early cataract development is associated with an increase of oxidative stress. This study further demonstrated that H2O2levels in cataract patients’ aqueous humor increased as much as 30 times higher than normal.In vitro studies have demonstrated that equivalent levels of hydrogen peroxide can induce apoptosis in lens epithelial cells and generate lens opacity in a similar manner as that occurs in the eyes of cataract patients[5]. However, the mechanism by which increased H2O2causes such pathological changes in the lens remains unclear.
miRNAs are a class of endogenous noncoding RNA that often regulate gene expression by either complementary pairing with the 3'-UTR of a target gene or mediating the degradation of target gene mRNA[28]. One miRNA may target more than one mRNA, and they are involved in a variety of physiological and pathological processes including cell growth and apoptosis, hormone secretion, aging, organ development,immune response and other pathologies[7,29]. Although many miRNAs have been studied in relation to age-related cataract development, the relationship between miR-211 and oxidative damage in the development of cataracts has not been previously investigated. Previous research has reported that miR-211 expression is low in breast cancer, malignant melanoma, liver cancer, and a variety of other diseases such as vitiligo[30-33]. Unrelated studies have also shown that miR-211 expression plays a key role in the differentiation and normal function of retinal pigment epithelium cells[13-14].
This study found that miR-211 is highly expressed in agerelated cataract tissue, suggesting that miR-211 may be involved in the incidence of age-related cataracts. miR-211 expression was also found to be increased in lens epithelial cells with H2O2-induced oxidative stress, further suggesting that miR-211 is involved in the oxidative stress pathway of hLECs. The results of the subsequent experiments employing an H2O2-induced oxidative stress model with miR-211 mimic transfection further established this relationship. The group of lens epithelial cells transfected with miR-211 mimics had a greatly decreased cell viability when compared to the control group while the lens epithelial cells transfected with miR-211 inhibitors had increased cell viability. In addition, p53 and Bax(key regulators of apoptosis) mRNA and protein expression levels displayed a direct relationship with miR-211 expression,suggesting that miR-211 may decrease lens epithelial cell viability and its ability to cope with oxidative stress by modulating p53 and Bax expression.
In summary, miR-211 expression is increased in age-related cataract tissues and miR-211 appears to participate in the development of this disease as it diminishes the ability of hLECs to defend against oxidative stress, and inhibits hLEC proliferation and repair by up-regulating p53 and Bax. The findings of this study provide strong evidence that miR-211 could be a potential new target for the diagnosis and treatment of cataracts.
ACKNOWLEDGEMENTS
conflicts of Interest: Lu B, None; Christensen IT, None; Ma LW, None; Yu T, None; Jiang LF, None; Wang CX, None;Feng L, None; Zhang JS, None; Yan QC, None; Wang XL,None.
REFERENCES
1 Goutham G, Manikandan R, Beulaja M, Thiagarajan R, Arulvasu C,Arumugam M, Setzer WN, Daglia M, Nabavi SF, Nabavi SM. A focus on resveratrol and ocular problems, especially cataract: from chemistry to medical uses and clinical relevance. Biomed Pharmacother 2017;86:232-241.
2 Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res 2010;44(3):155-165.
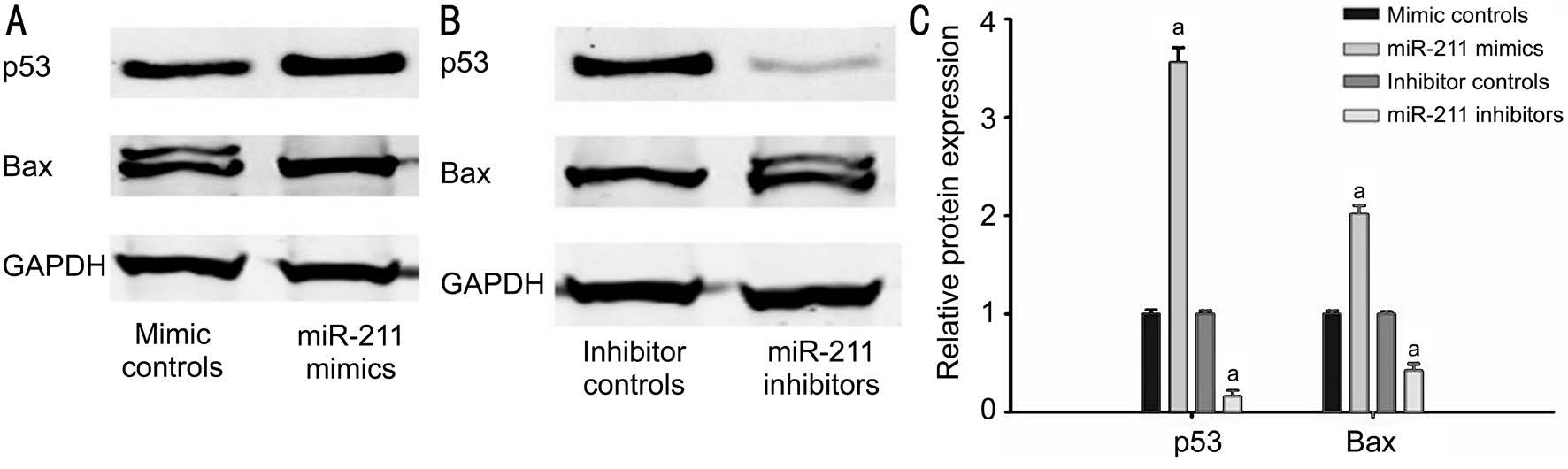
Figure 3 The expression of p53 and Bax protein in hLECs A, C: The miR-211 mimic group hLECs had significantly increased levels of p53 and Bax protein expression when compared with the mimic control group; B and C: p53 and Bax protein expression were significantly reduced in the miR-211 inhibitor group as compared to the inhibitor control group (aP<0.001).
3 Petrou AL, Terzidaki A. A meta-analysis and review examining a possible role for oxidative stress and singlet oxygen in diverse diseases Biochem J 2017;474(16):2713-2731.
4 Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol 2003;15(2):247-254.
5 Steven Zatechka D, Lou MF. Studies of the mitogen-activated protein kinases and phosphatidylinositol-3 kinase in the lens. 2. The intercommunications. Exp Eye Res 2002;75(2):177-192.
6 Chen X, Xiao W, Chen W, Liu X, Wu M, Bo Q, Luo Y, Ye S, Cao Y, Liu Y. MicroRNA-26a and -26b inhibit lens fibrosis and cataract by negatively regulating Jagged-1/Notch signaling pathway. Cell Death Differ 2017;24(8):1431-1442.
7 Zhou X, Jiao Z, Ji J, Li S, Huang X, Lu X, Zhao H, Peng J, Chen X, Ji Q, Ji Y. Characterization of mouse serum exosomal small RNA content: the origins and their roles in modulating in flammatory response.Oncotarget 2017;8(26):42712-42727.
8 Zhang F, Meng W, Tong B. Down-regulation of microRNA-133b suppresses apoptosis of lens epithelial cell by up-regulating BCL2L2 in age-related cataracts. Med Sci Monit 2016;22:4139-4145.
9 Poetsch M, Kleist B. Loss of heterozygosity at 15q21.3 correlates with occurrence of metastases in head and neck cancer. Mod Pathol 2006;19(11):1462-1469.
10 Barbato S, Marrocco E, Intartaglia D, Pizzo M, Asteriti S, Naso F,Falanga D, Bhat RS, Meola N, Carissimo A, Karali M, Prosser HM,Cangiano L, Surace EM, BanfiS, Conte I. MiR-211 is essential for adult cone photoreceptor maintenance and visual function. Sci Rep 2017;7(1):17004.
11 Ragusa M, Caltabiano R, Russo A, Puzzo L, Avitabile T, Longo A,Toro MD, Di Pietro C, Purrello M, ReibaldiM. MicroRNAs in vitreus humor from patients with ocular diseases. Mol Vis 2013;19:430-440.
12 Qin Y, Zhao J, Min X, Wang M, Luo W, Wu D, Yan Q, Li J, Wu X, Zhang J. MicroRNA-125b inhibits lens epithelial cell apoptosis by targeting p53 in age-related cataract. Biochim Biophys Acta 2014;1842(12 Pt A):2439-2447.
13 Wang FE, Zhang C, Maminishkis A, Dong L, Zhi C, Li R, Zhao J,Majerciak V, Gaur AB, Chen S, Miller SS. MicroRNA-204/211 alters epithelial physiology. FASEB J 2010;24(5):1552-1571.
14 Kutty RK, Samuel W, Boyce K, Cherukuri A, Duncan T, Jaworski C,Nagineni CN, Redmond TM. Proinflammatory cytokines decrease the expression of genes critical for RPE function. Mol Vis 2016;22:1156-1168.
15 Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol 2017;28(1):98-103.
16 Fukuoka H, Afshari NA. The impact of age-related cataract on measures of frailty in an aging global population. Curr Opin Ophthalmol 2017;28(1):93-97.
17 Hahn U, Krummenauer F. Results and methodology of cost-utility evaluation of cataract surgery in developed countries: quality-adjusted life years and cataract. J Cataract Refract Surg 2017;43(6):839-847.
18 Wang W, Yan W, Fotis K, Prasad NM, Lansingh VC, Taylor HR,Finger RP, Facciolo D, He M. Cataract surgical rate and socioeconomics:a global study. Invest Ophthalmol Vis Sci 2016;57(14):5872-5881.
19 Babizhayev MA, Yegorov YE. Biomarkers of oxidative stress and cataract. Novel drug delivery therapeutic strategies targeting telomere reduction and the expression of telomerase activity in the lens epithelial cells with N-acetylcarnosine lubricant eye drops: anti-cataract which helps to prevent and treat cataracts in the eyes of dogs and other animals. Curr Drug Deliv 2014;11(1):24-61.
20 Brennan L, Khoury J, Kantorow M. Parkin elimination of mitochondria is important for maintenance of lens epithelial cell ROS levels and survival upon oxidative stress exposure. Biochim Biophys Acta 2017;1863(1):21-32.
21 Liu XF, Hao JL, Xie T, Malik TH, Lu CB, Liu C, Shu C, Lu CW, Zhou DD. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell 2017;16(5):934-942.
22 Pescosolido N, Barbato A, Giannotti R, Komaiha C, Lenarduzzi F.Age-related changes in the kinetics of human lenses: prevention of the cataract. Int J Ophthalmol 2016;9(10):1506-1517.
23 Kruk J, Kubasik-Kladna K, Aboul-Enein HY. The role oxidative stress in the pathogenesis of eye diseases: current status and a dual role of physical activity. Mini Rev Med Chem 2015;16(3):241-257.
24 Babizhayev M, Yegorov Y. Telomere attrition in human lens epithelial cells associated with oxidative stress provide a new therapeutic target for the treatment, dissolving and prevention of cataract with n-acetylcarnosine lubricant eye drops. kinetic, pharmacological and activity-dependent sceparation of therapeutic targeting: transcorneal penetration and delivery of l-carnosine in the aqueous humor and hormone-like hypothalamic antiaging effects of the instilled ophthalmic drug through a safe eye medication technique. Recent Pat Drug Deliv Formul 2016;10(2):82-129.
25 Zarrouk A, Vejux A, Mackrill J, O'Callaghan Y, Hammami M, O'Brien N, Lizard G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res Rev 2014;18:148-162.
26 Tarwadi KV, Chiplonkar SA, Agte V. Dietary and nutritional biomarkers of lens degeneration, oxidative stress and micronutrient inadequacies in Indian cataract patients. Clin Nutr 2008;27(3):464-472.
27 Spector A, Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res 1981;33(6):673-681.
28 Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of posttranscriptional regulation by micro RNAs: are the answers in sight? Nat Rev Genet 2008;9(2):102-114.
29 Zhuang L, Wang X, Wang Z, Ma X, Han B, Zou H, Wu Z, Dong S, Qu Z, Zang Y, Wu L. Micro RNA-23b functions as an oncogene and activates AKT/GSK3β/β-catenin signaling by targeting ST7L in hepatocellular carcinoma. Cell Death Dis 2017;8(5):e2804.
30 Chen LL, Zhang ZJ, Yi ZB, Li JJ. MicroRNA-211-5p suppresses tumour cell proliferation, invasion, migration and metastasis in triplenegative breast cancer by directly targeting SETBP1. Br J Cancer 2017;117(1):78-88.
31 Jiang G, Wen L, Deng W, Jian Z, Zheng H. Regulatory role of miR-211-5p in hepatocellular carcinoma metastasis by targeting ZEB2. Biomed Pharmacother 2017;90:806-812.
32 Babapoor S, Horwich M, Wu R, Levinson S, Gandhi M, Makkar H,Kristjansson A, Chang M, Dadras SS. microRNA in situ hybridization for miR-211 detection as an ancillary test in melanoma diagnosis. Mod Pathol 2016;29(5):461-475.
33 Sahoo A, Lee B, Boniface K, Seneschal J, Sahoo SK, Seki T, Wang C, Das S, Han X, Steppie M, Seal S, Taieb A, Perera RJ. MicroRNA-211 regulates oxidative phosphorylation and energy metabolism in human vitiligo. J Invest Dermatol 2017;137(9):1965-1974.