INTRODUCTION
Glaucoma is an ocular neurodegenerative disease that usually accompanied by raised intraocular pressure(IOP), which characterized by optic atrophy and visual field defect[1]. The primary pathological basic is selective apoptosis of retinal ganglion cells (RGCs), which results in irreversible damage of vision and even permanent blindness[2]. At present,glaucoma usually resorts to surgery or control of IOP with drugs, but they do not work for everyone. Lowering IOP alone will not completely inhibit RGCs apoptosis and axon neuropathy. Therefore, investigating the molecular mechanisms underlying RGCs apoptosis to prevent RGCs apoptosis is the nowadays treatment direction.
Oxidative stress injury is considered as a major mechanism of RGCs apoptosis in the development of glaucoma[3],which reflects the imbalance of the oxidative system and antioxidative system in certain pathological changes of human body[4]. In vivo, studies showed that glaucoma and other retinopathy diseases were accompanyed by ischemic and hypoxic and made direct damage to the retina, causing oxidative stress that involved in RGCs[5]. Ischemia-reperfusion injury in retina could aggravate oxidative stress in retina,which led to excessive producing of free radicals and cellular energy metabolism disturbance, furthermore this induced DNA damage to promote RGCs apoptosis[6]. Additionally, it also found that the process of oxidative stress-induced RGCs apoptosis in vitro relied on casepase to some extent[3,7]. Inhibition of RGCs apoptosis induced by oxidative stress injury is of a very vital significance to glaucoma therapy and causes concerns from more and more researchers. Currently, the studies of inhibitory effect of anti-oxidative stress drugs on RGCs apoptosis and its mechanisms have been carried out to look for an effective therapeutic targets, which has important value in the treatment of glaucoma.
It is accepted that endoplasmic reticulum stress (ERS) is a critical aspect in the response mechanism of cells and it may trigger ERS mediated apoptosis signals in certain conditions[8-9].Recent studies found that ERS played an important role in retinopathy of experimental animal models, such as diabetic retinopathy, retinitis pigmentosa, glaucoma[10-11].ERS inducer tunicamycin was injected into the vitreous and then significantly promoted RGCs death and thinning of the retina[12], suggesting that ERS could cause degenerative nerve of the retina. Cauterization of episcleral vein is a new method to create an animal model of chronic glaucoma[13]. In this animal model, the protein expression of Chop, ERS related protein, in the retina was gradually increased with prolonged time, accompanied with the lose of RGCs[14]. Intravitreal injection of NMDA could induce Chop over-expression in NMDA-induced neurodegeneration in mice, and the mice with Chop gene knocked out were able to resist neurological damage induced by NMDA[15]. These findings indicate that ERS exerts very important role in neuronal damage and thus involves in the occurrence and development of glaucoma.
Gurigumu-13 (GRGM), a mongolian medicine, has been used in ophthalmology clinical treatment for many years and achieve significant efficacy. It is a complex compound which is constituted with 13 kinds of Chinese herbs, Carthamus tinctorius L. (Xinjiang, China), SyZygium aromaticum(Yunnan, China), Semen Nelumbinis (Hunan, China),Ophiopogon japonicas (Thunb.) Ker-Gawl (Sichuan, China),Radix Aucklandiae (Yunnan, China), Fructus Chebulae(Guangxi, China), Fructus Toosendan (Sichuan, China),Gardenia jasminoides Ellis (Jiangxi, China), Pterocarpus santalinus (Hainan, China), Moschus (Beijing, China), Pulvis Cornus Bubali Concentratus (Hebei, China), Calculus Bovis(Hunan, China), Cinnabar (Hunan, China). Mongolian medicine believes that the liver tends to be over-active, resulting in glaucoma. Although GRGM is used in the clinical treatment of glaucoma to have the full pharmacology theory basis, the underlying mechanism remains to be fully elucidated. In the present study, we evaluated the protective effects of GRGM on RGCs in DBA/2J mice and further investigated its mechanism.The study provides experimental bases for the further application of GRGM in the treatment of glaucoma.
MATERIALS AND METHODS
Animal Experiments The animal experiments were approved by the Ethics Committee of Affiliated Hospital of Inner Mongolia University for the Nationalities. All procedures for animals were conducted in accordance with the ARVO Guidelines for the Use of Animals in Research. DBA/2J and C57BL/6J mice were obtained from the Laboratorial Animal Center at Affiliated Hospital of Inner Mongolia University for the Nationalities. DBA/2J mice (3mo) were randomly divided into four groups, and 10 mice per group, and mice were given different doses of GRGM (0, 0.4, 0.8, 1.6 g/kg·d) once a day lasting 7mo. The control group has 10 C57BL/6J mice. All mice were in a standarded light-dark cycle with food and water freely.
Intraocular Pressure Measurements in Mice The IOP of all mice was measured by a rebound tonometer (TonoLab; Colonial Medical Supply, Franconia, NH, USA) with intraperitoneal injection of 10% chloral hydrate. The probe tip of tonometer was placed at the site of 2-3 mm away from the surface of mouse cornea, then started the tonometer to make the probe tip hit the center of cornea surface. Three consecutive sets of six measurements of IOP were averaged as the IOP of mice. All IOP measurements were performed by the same researcher.
Tissue Preparation After 7mo perfusion, retinas and optic nerves were quickly isolated from the eyes of mice. Retinas and optic nerves were rinsed andfixed with 4% paraformaldehyde and 0.5% glutaraldehyde respectively for RGCs, neurons and axon counting. For Western blot and PCR, retinal tissues were quickly dissected, stored in liquid nitrogen.
Axon Counting The glutaraldehyde-fixed optic nerves were cut into small pieces, and then stained by p-phenylenediamine according to the instruction. Number of axon was calculated on 18 visual fields chosen from each image of optic nerve under a inverted microscope, including center, mid-periphery and peripheral margin of optic nerve. The axon number of 18 visualfields were counted manually and then normalized to the total area of the optic nerve.
Retinal Ganglion Cells and Neurons Counting RGCs and neurons were counted as the previous study with small modification. Brie fly, DAPI was used to label RGCs, and the whole retina was divided into central, middle and peripheral regions. RGCs number per retina was calculated with 10 visualfields through DAPI-labeled RGCs counting under a inverted microscope.
Western Blot Total protein was extracted from retinas or RGCs using RIPA buffer (1% NP-40, 0.25% Na-deoxycholate,50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L EDTA,1 mmol/L PMSF, 1 μg/mL Aprotinin, 1 μg/mL leupeptin,1 μg/mL pepstatin, 1 mmol/L Na3VO4, 1 mmol/L NaF). The concentration of total protein was measured with BCA Protein Assay Kit (Thermo Fisher Scientific). Total protein (25 μg per sample) was diluted in Tris-HCl (pH 7.4) and separated by SDS-PAGE. The gel-separated proteins were transferred to the PVDF membrane, and then the PVDF membrane was immersed in 5% skim milk for 30min. The primary antibodies(Abcam) were added and incubated with the target proteins on PVDF membrane at 4℃ overnight. Proteins were then incubated with corresponding HRP-conjugated secondary antibodies (Abcam). The target proteins were observed by the ECL development solution (Thermo Fisher Scientific). Relative expression level was measured with an ImageMaster®VDS and normalized to β-actin. Data were mean±standard deviation(SD).

Figure 1 GRGM alleviated the damage of RGCs in DBA/2J mice A, B: Numbers of total neurons, RGCs and RGC axons in retinas of DBA/2J mice treated with GRGM at different concentrations; C: Changes in IOP levels of C57BL/6J and DBA/2J mice.aP<0.01 vs C57BL/6J;bP<0.05,cP<0.01 vs DBA/2J without GRGM treatment.
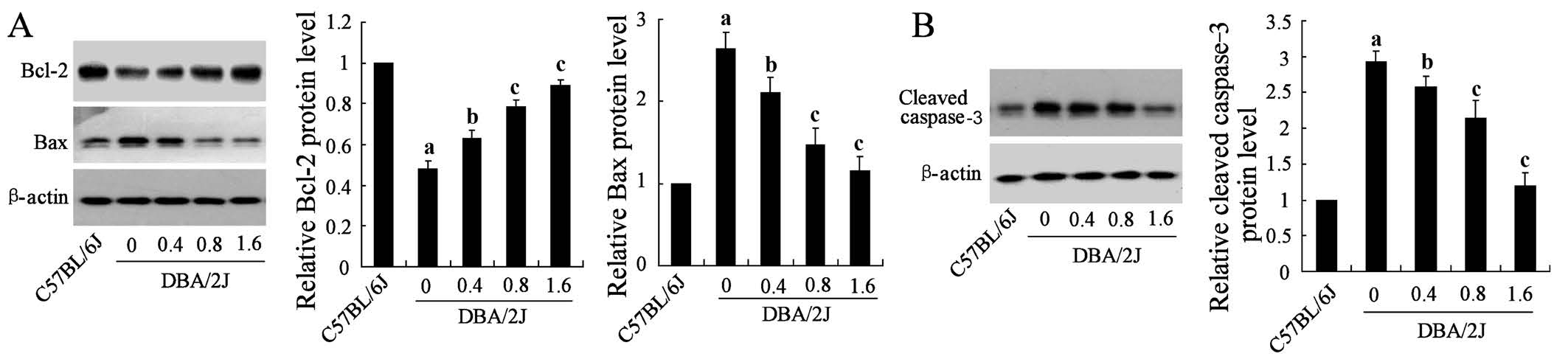
Figure 2 Effect of GRGM on the expression of apoptosis pathway related molecules in RGCs A, B: Western blot analysed the expression of Bcl-2, Bax and cleaved caspase-3. β-actin acted as the internal reference.aP<0.01 vs C57BL/6J;bP<0.05,cP<0.01 vs DBA/2J without GRGM treatment.
Detection of Malondialdehyde Malondialdehyde (MDA) in retinas of mice was measured with commercial kit (Beyotime Biotechnology) according to the kit instructions. The relative contents of MDA were expressed as nmol/mg protein.
Statistical Analysis All statistical analysis were performed with SPSS 17.0 software (IBM Corporation). The numbers of cells, protein expression, IOP and relative content of MDA were expressed as the mean±SD. A P value of <0.05 was considered as statistically significant. The Student’s t-test was used to compare paired groups.
RESULTS
Effect of GRGM on the Damage of Retinal Ganglion Cells in DBA/2J Mice After continuously treated with 0, 0.4, 0.8 and 1.6 g/kg·d GRGM for 7mo, the numbers of total neurons,RGCs and RGC axons in retinas were counted and IOP levels of experimental mice were detected. We found that total neurons, RGCs and RGC axons in retinas of DBA/2J mice were obviously lower than those in C57BL/6J mice; GRGM significantly increased the number of total neurons, RGCs and RGC axons in retinas of DBA/2J mice with the dependence of concentration (Figure 1A, 1B). However, IOP levels of DBA/2J mice were much higher than those of C57BL/6J mice,and GRGM had no effect on the IOP levels of DBA/2J mice(Figure 1C).
Effect of GRGM on the Expression of Apoptosis Pathway Related Molecules in Retinal Ganglion Cells To investigate the protective mechanisms of GRGM in retinas of DBA/2J mice, the expressions of apoptosis pathway related proteins in RGCs were detected in this part. Western blot assays showed that GRGM could dramatically promote the expression of Bcl-2 protein, while the expression of Bax and cleaved caspase-3 in the levels of protein were markedly decreased with the increase of GRGM concentration (Figure 2).
Inhibition of GRGM on the Retinal Lipid Peroxidation in DBA/2J Mice MDA content reflects the degree of lipid peroxidation, so we further examined the relative content of MDA of mouse retinas. The relative content of MDA in retinas of DBA/2J mice was significantly higher than that of C57BL/6J mice. Furthermore, the relative content of MDA was the highest in retinas of DBA/2J mice at 7 months old and then decreased. Importantly, GRGM could obviously decrease MDA content in retinas of DBA/2J mice at 7, 10 months old,while the content of MDA in retinas of DBA/2J mice did not significant change with different concentrations of GRGM at 3 months old (Figure 3).
Inhibition of GRGM on the Oxidative Stress in Retinas of DBA/2J Mice Oxidative stress involved in multiple pathophysiological processes through induced oxidative damage. Therefore, we evaluated the effect of GRGM on the expression of oxidative stress related proteins including Gfap,Cp, HO-1, Nos-2, and found that the expressions of Gfap, Cp,HO-1, Nos-2 were significantly inhibited in retinas of DBA/2J mice treated with GRGM in a concentration-dependent manner(Figure 4).
Effect of GRGM on Antioxidative Ability of Retina in DBA/2J Mice The antioxidative ability of retina is an important parameter of maintaining the function of the retina.Ourfindings showed that the expression of antioxidant protein Nrf2, GST, GPx4, Prdx2 in retinas of DBA/2J mice were markedly lower than that of C57BL/6J mice. However, GRGM could significantly promote antioxidant proteins expression in retinas of DBA/2J mice (Figure 5).
Inhibition of GRGM on Endoplasmic Reticulum Stress in Retinal Ganglion Cells of DBA/2J Mice ERS-mediated apoptosis was closely related to retinal damage, so we further detected the expression of ERS related protein Bip and Chop in RGCs. The results showed that both Bip and Chop highly expressed in RGCs of DBA/2J mice, but their protein expression levels were significantly decreased in RGCs after DBA/2J mice treated with GRGM (Figure 6).
DISCUSSION
Herein, the study demonstrated that mongolian medicine GRGM could possess significantly protective effect on RGCs loss of DBA/2J mice. In recent studies, DBA/2J mice were used extensively for antiglaucoma research because of their pigment dispersion, iris atrophy and RGCs loss, which were similar to human glaucoma. In our study, GRGM were administrated to DBA/2J mice from 3 to 10 months old. First,we examined the effects of GRGM on the number of neurons,RGCs and RGC axons and IOP in the retinas. We found that GRGM distinctly improved retina damage via increasing the number of neurons, RGCs and axons in a concentration dependent manner. However, GRGM had no significant effect on IOP of DBA/2J mice at the concentration range.Numerous studies show that apoptosis is the main way of the loss of RGCs, and RGCs apoptosis is the final common pathway in many diseases with optic nerve damage including glaucoma[16-17]. Hence, we believe that GRGM is able to inhibit RGCs apoptosis that prevents RGCs loss in retinas. To further verify this conclusion, we evaluated the effects of GRGM on the expression of apoptosis-related proteins. Western blot results supported that GRGM could promote Bcl-2 expression while Bax and cleaved caspase-3 expression were reduced by GRGM.
More significantly, the study also found that GRGM could obviously down-regulate the high level of MDA in retinas of DBA/2J mice since they were 7 months old with the dependence of concentration. MDA is one of the most important products of the lipid peroxidation and also the very important detection target for oxidative cell damage. Oxidative stress injury is considered as a major mechanism during RGCs apoptosis of glaucoma, and the lipid peroxidation is a part of oxidative stress. So we speculated that GRGM protected RGCs through regulating oxidative stress. Further study showed that GRGM significantly inhibited the expression of oxidative stress-related proteins including Gfap, Cp, HO-1,Nos-2, but promoted the expression of antioxidant proteins.The imbalance of the oxidative system and a ntioxidative system in certain pathological changes of human body causes oxidative stress[18-19]. Corporately, these results demonstrated that GRGM could suppress oxidative stress and subsequent RGCs apoptosis.
In recent years, many researches have confirmed that ERS could contribute to the occurrence and development of glaucoma via inducing RGCs apoptosis[20-21]. A small number of primary angle-closure glaucoma could been caused by musclefiber protein mutation in trabecular meshwork cells[22].When the mutant protein highly expressed in trabecular meshwork cells, ERS activation and a series of glaucoma phenotypes were observed in mice[23]. In our study, GRGM treatment evidently reduced the protein expression of Bip and Chop, ERS related proteins. Bip, a chaperone protein,can bind the transmembrane proteins located in endoplasmic reticulum to regulate ERS-induced apoptosis[11,24]. Increase in Bip expression is considered to be one of the important indicators for the initiation of ERS-induced apoptosis. Chop plays a crucial role in the process of ERS-induced apoptosis,and serves as a transcription factor for down-regulation of Bcl-2 expression[25]. It have been reported that ERS inducer tunicamycin could cause the death of embryonic fibroblasts in wild-type mice, and Chop deletion mice were able to resist tunicamycin-induced cell death[26-27]. Our results indicated that GRGM had significant inhibitory effect on ERS in retinas, and then demonstrated the antiapoptosis effects.
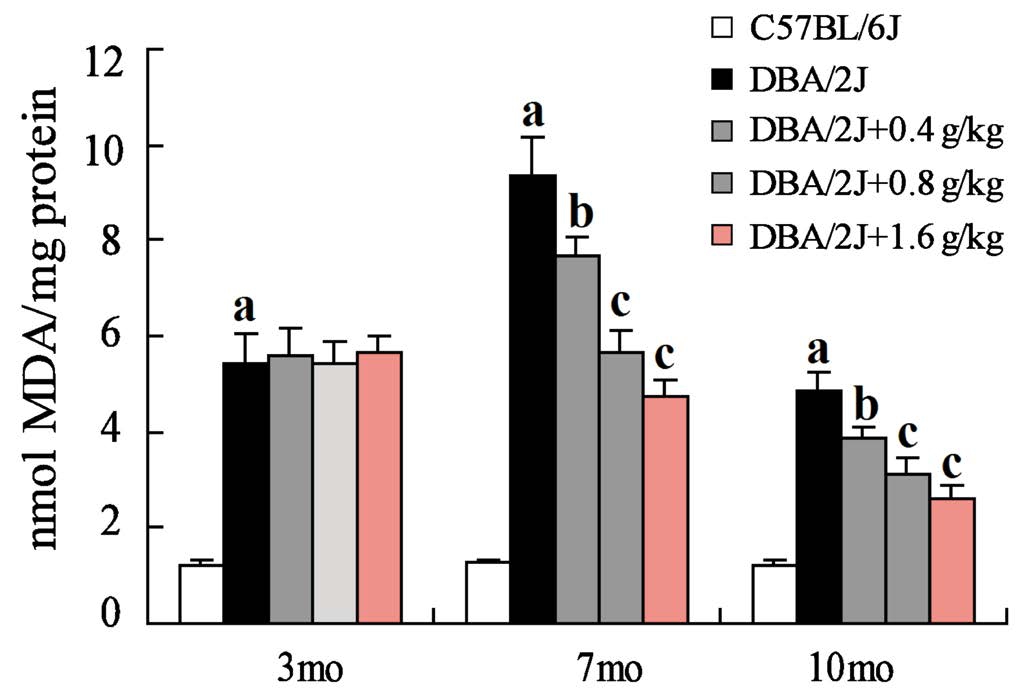
Figure 3 Effect of GRGM on lipid peroxidation of DBA/2J mouse retinas. The relative content of MDA of mouse retinas was evaluated at 3, 7, 10mo respectively β-actin acted as the internal reference.aP<0.01 vs C57BL/6J;bP<0.05,cP<0.01 vs DBA/2J without GRGM treatment.
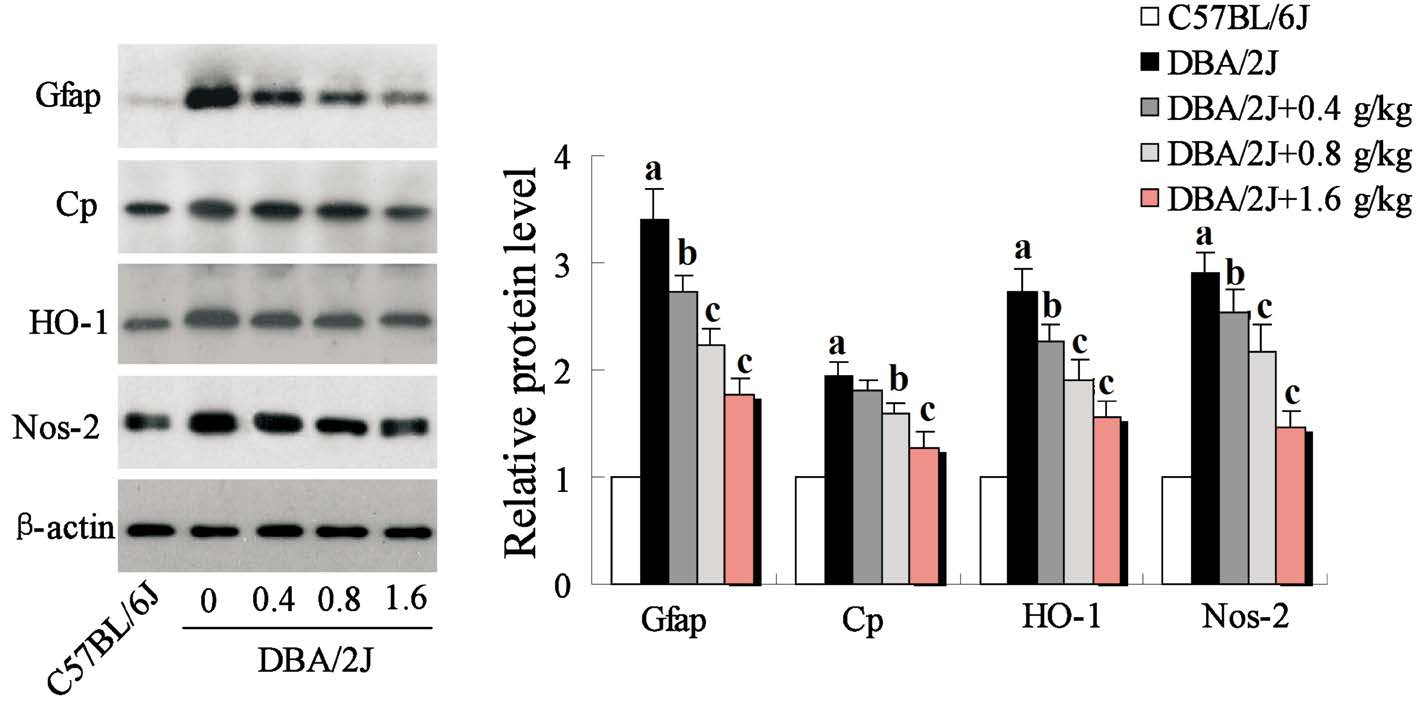
Figure 4 Effect of GRGM on the expression of oxidative stress related proteins of DBA/2J mouse retinas Western blot was employed to determine the expression of Gfap, Cp, HO-1, Nos-2, and β-actin acted as the internal reference.aP<0.01 vs C57BL/6J;bP<0.05,cP<0.01 vs DBA/2J without GRGM treatment.

Figure 5 GRGM increased antioxidative ability of retina in DBA/2J mice The expressions of Nrf2, GST, GPx4, Prdx2 were quantified and relative protein levels were shown. β-actin acted as the internal reference.aP<0.01 vs C57BL/6J;bP<0.05,cP<0.01 vs DBA/2J without GRGM treatment.
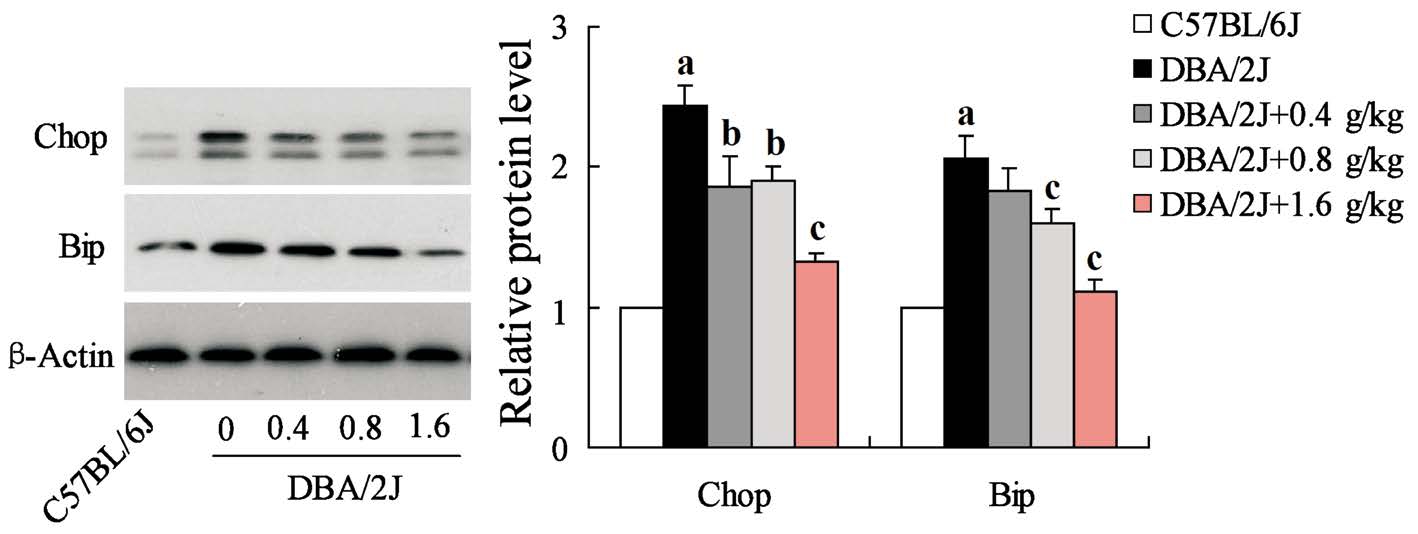
Figure 6 GRGM inhibited the ERS in RGCs of DBA/2J mice The expressions of ERS protein Bip and Chop were detected by Western blot analysis, and β-actin acted as the internal reference.aP<0.01 vs C57BL/6J;bP<0.05,cP<0.01 vs DBA/2J without GRGM treatment.
In summary, our results provided strong evidence that GRGM had obvious protective effects on RGCs in DBA/2J mice via inhibiting oxidative stress and ERS as shown by increased the number of RGCs and axons. The study provides experimental basis for the further application of GRGM in the treatment of glaucoma.
ACKNOWLEDGEMENTS
Foundation:Supported by the National Natural Science Foundation of China (No.81660734).
Conflicts of Interest:Zhang QL, None; Wang W, None;Jiang Y, None; Zhang TZ, None; Lu ZJ, None; Jiang A, None.
REFERENCES
1 Song BJ, Caprioli J. New directions in the treatment of normal tension glaucoma. Indian J Ophthalmol 2014;62(5):529-537.
2 Nuschke AC, Farrell SR, Levesque JM, Chauhan BC. Assessment of retinal ganglion cell damage in glaucomatous optic neuropathy: axon transport, injury and soma loss. Exp Eye Res 2015;141:111-124.
3 Lee KY, Nakayama M, Aihara M, Chen YN, Araie M. Brimonidine is neuroprotective against glutamate-induced neurotoxicity, oxidative stress,and hypoxia in purified rat retinal ganglion cells. Mol Vis 2010;16(31-32):246-251.
4 Anderson G, Maes M. Oxidative/nitrosative stress and immunoin flammatory pathways in depression: treatment implications. Cur Pharm Des 2014;20(23):3812-3847.
5 Kaur C, Foulds WS, Ling EA. Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol 2008;2(4):879-889.
6 Dvoriantchikova G, Degterev A, Ivanov D. Retinal ganglion cell (RGC)programmed necrosis contributes to ischemia-reperfusion-induced retinal damage. Exp Eye Res 2014;123:1-7.
7 Ohia SE, Awe OS, Opere CA, Leday AM, Harris LC, Sharif NA.Hypoxia-induced [(3)H]D-aspartate release from isolated bovine retina:modulation by calcium-channel blockers and glutamatergic agonists and antagonists. Curr Eye Res 2001;23(5):386-392.
8 Ge HW, Hu WW, Ma LL, Kong FJ. Endoplasmic reticulum stress pathway mediates isoflurane-induced neuroapoptosis and cognitive impairments in aged rats. Physiol Behav 2015;151:16-23.
9 Mandl J, Mészáros T, Bánhegyi G, Csala M. Minireview: endoplasmic reticulum stress: control in protein, lipid, and signal homeostasis. Mol Endocrinol 2013;27(3):384-393.
10 Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal in flammation and diabetic retinopathy. FEBS Lett 2009;583(9):1521-1527.
11 Kroeger H, Messah C, Ahern K, Gee J, Joseph V, Matthes MT,Yasumura D, Gorbatyuk MS, Chiang WC, Lavail MM. Induction of endoplasmic reticulum stress genes, BiP and chop, in genetic and environmental models of retinal degeneration. Invest Ophthalmol Vis Sci 2012;53(12):7590-7599.
12 Boriushkin E, Wang JJ, Li J, Jing G, Seigel GM, Zhang SX.Identification of p58IPK as a novel neuroprotective factor for retinal neurons. Invest Ophthalmol Vis Sci 2015;56(2):1374-1386.
13 Danias J, Shen F, Kavalarakis M, Chen B, Goldblum D, Lee K,Zamora MF, Su YL, Brodie SE, Podos SM. Characterization of retinal damage in the episcleral vein cauterization rat glaucoma model. Exp Eye Res 2006;82(2):219-228.
14 Sun X, Cheng F, Meng B, Yang B, Song W, Yuan H. Pregnenolone sulfate decreases intraocular pressure and changes expression of sigma receptor in a model of chronic ocular hypertension. Mol Biol Rep 2012;39(6):6607-6614.
15 Awai M, Koga T, Inomata Y, Oyadomari S, Gotoh T, Mori M, Tanihara H. NMDA-induced retinal injury is mediated by an endoplasmic reticulum stress-related protein, CHOP/GADD153. J Neurochem 2006;96(1):43-52.
16 Tatham AJ, Meira-Freitas D, Weinreb RN, Marvasti AH, Zangwill LM,Medeiros FA. Estimation of retinal ganglion cell loss in glaucomatous eyes with a relative afferent pupillary defect. Invest Ophthalmol Vis Sci 2014;55(1):513-522.
17 Wu JH, Zhang SH, Nickerson JM, Gao FJ, Sun Z, Chen XY, Zhang SJ,Gao F, Chen JY, Luo Y, Wang Y, Sun XH. Cumulative mtDNA damage and mutations contribute to the progressive loss of RGCs in a rat model of glaucoma. Neurobiol Dis 2015;74:167-179.
18 Circu ML, Aw TY. Intestinal redox biology and oxidative stress. Semin Cell Dev Biol 2012;23(7):729-737.
19 Izuta H, Matsunaga N, Shimazawa M, Sugiyama T, Ikeda T, Hara H. Proliferative diabetic retinopathy and relations among antioxidant activity, oxidative stress, and VEGF in the vitreous body. Mol Vis 2010;16(16):130-136.
20 Ito Y, Shimazawa M, Inokuchi Y, Yamanaka H, Tsuruma K, Imamura K, Onoe H, Watanabe Y, Aihara M, Araie M, Hara H. Involvement of endoplasmic reticulum stress on neuronal cell death in the lateral geniculate nucleus in the monkey glaucoma model. Eur J Neurosci 2011;33(5):843-855.
21 Ha Y, Saul A, Tawfik A, Zorrilla EP, Ganapathy V, Smith SB. Diabetes accelerates retinal ganglion cell dysfunction in mice lacking sigma receptor 1. Mol Vis 2012;18:2860-2870.
22 Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet 2001;10(2):117-125.
23 Peters JC, Bhattacharya S, Clark AF, Zode GS. Increased endoplasmic reticulum stress in human glaucomatous trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci 2015;56(6):3860-3868.
24 Corazzari M, Lovat PE, Armstrong JL, Fimia GM, Hill DS, Birch-Machin M, Redfern CP, Piacentini M. Targeting homeostatic mechanisms of endoplasmic reticulum stress to increase susceptibility of cancer cells to fenretinide-induced apoptosis: the role of stress proteins ERdj5 and ERp57. Br J Cancer 2007;96(7):1062-1071.
25 Liu M, Wang XR, Wang C, Song DD, Liu XH, Shi DZ. Panax quinquefolium saponin attenuates ventricular remodeling after acute myocardial infarction by inhibiting chop-mediated apoptosis. Shock 2013;40(4):339-344.
26 Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R,Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 2004;18(24):3066-3077.
27 Shimazawa M, Inokuchi Y, Ito Y, Murata H, Aihara M, Miura M,Araie M, Hara H. Involvement of ER stress in retinal cell death. Mol Vis 2007;13(62-63):578-587.