INTRODUCTION
Glaucoma is the leading cause of irreversible blindness worldwide, characterized by optic neuropathy and visual field loss. Primary open angle glaucoma (POAG) is a common form of glaucoma, consisting of juvenile open angle glaucoma (JOAG) and adult onset open angle glaucoma as determined by clinical characteristics at different ages.The molecular genetics of POAG or JOAG have not been completely revealed, but evidence that mutations are associated with these heterogeneous diseases has been reported in the literatures. To date, sixteen loci are linked to POAG or JOAG.MYOC, OPTN, CYP1B1 and WDR36 have been reported as the causative genes of POAG or JOAG, amongst which,MYOC is the first identified and the primary gene responsible for JOAG[1-5].
In this study, we enrolled a large Chinese family identified to have five generations of autosomal dominant JOAG.A recurrent mutation c.1099G>A (p.G367R) of MYOC is associated with the phenotypes in this pedigree from southeast China.
SUBJECTS AND METHODS
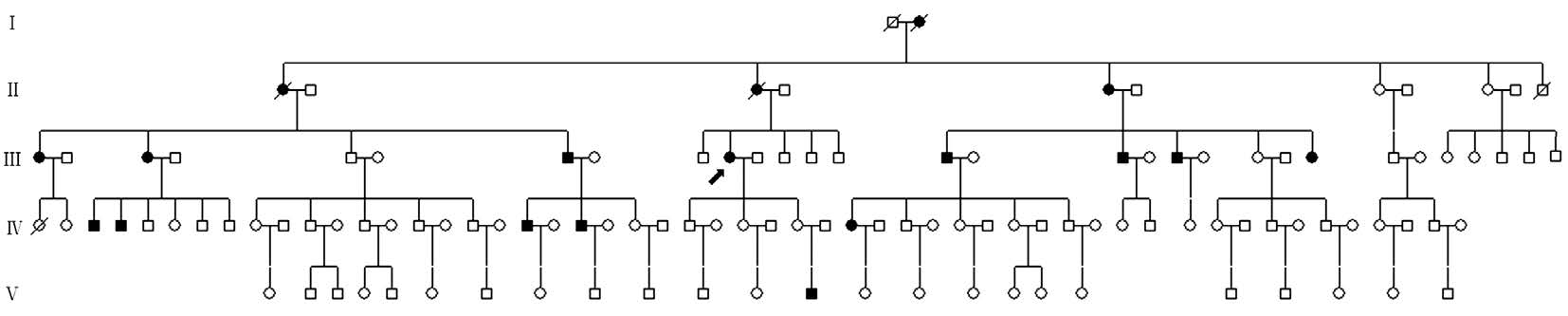
Figure 1 Pedigree with JOAG This five-generational Chinese pedigree was composed of 114 members segregating autosomal dominant JOAG. The proband is marked with an arrow. Squares and circles indicate males and females, respectively. Black and white symbols represent affected and unaffected individuals, respectively.
Patients and DNA SpecimenAccording to the tenets of the Declaration of Helsinki (2008), this study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University, Fuzhou, China. Afive-generational family with 114 members with JOAG was recruited from the Fujian province. Blood samples were collected from 12 members,including three JOAG patients, one suspect, and eight unaffected participants. Totally 100 unrelated individuals without eye diseases were used as controls. All participants gave written informed consent to the publication of their case details prior to enrollment from September 2009 to December 2010. Routine physical and ophthalmological examinations were performed by two experienced glaucoma specialists. A 3 mL peripheral blood sample was collected from each control and the 12 family members. Genomic DNA was extracted from a 300 μL blood sample using a Wizard Genomic DNA Purification Kit(Promega, Beijing, China) according to the manufacturer’s instructions.
Clinical Evaluation and Criteria Medical histories were collected from all family members and ophthalmic evaluations were performed, including visual acuity, anterior segment examination, intraocular pressure (IOP), and gonioscopy.Glaucomatous changes, such as fundus examination, visual field evaluation, the ratio of cup-to-disc, optic nerve head(ONH), and retinal nerve fibre layer (RNFL), were detected using optical coherence tomography. The diagnosis criteria for JOAG have been described previously[6-7]. Brie fly, patients were younger than 35 years old and clinical presentations of JOAG include an initial IOP above 22 mm Hg or higher without any treatments, an open anterior angle, glaucomatous ONH and RNFL damages with typical visual field defects, and the absence of any secondary glaucoma, such as neovascular glaucoma or traumatic glaucoma. The JOAG suspect was diagnosed according to the following conditions: a consistent IOP higher than 22 mm Hg, a suspicious optic neuropathy or abnormity of the visualfield.
Mutation Screen and Analysis MYOC (NM_000261), OPTN(NM_021980), CYP1B1 (NM_000104) and WDR36(NM_139281) were selected as disease-associated genes. The exon and flanking intron sequences of candidate genes were amplified by the polymerase chain reaction (PCR) using a MyCycler thermocycler (BioRad, Hercules, CA, USA). The primers for MYOC were: MYOC1 F: TCTCTGGAGCTCG GGCATGA, R: CTGCTGAACTCAGAGTCCCC;MYOC2 F: AACATAGTCAATCCTTGGGCC, R:TAAAGACCACGTGGGCACAA; MYOC3 F: CCGCA TGATCATTGTCTGTG, R: CTGGCTGGCTCTCCCTTCA;and primers for OPTN, CYP1B1 and WDR36 were not shown in this paper. The reaction mixtures for PCR included 100 ng DNA, 5 μL dNTP Mixture (2.5 mmol/L), 1.0 μmol/L each of the pair primers, 5 μL 10×Ex Taq Buffer (Mg2+plus), 1.5 U TaKaRa Ex Taq, and ddH2O up to 50 μL. The thermal cycling conditions for PCR were incubation at 94℃ for 4min, 30 cycles (30s at 94℃, 30s at 56℃, and 90s at 72℃), followed by 7min at 72℃ for extension. PCR products were sequenced on an ABI3730 Automated Sequencer (ABI, Foster City, CA, USA).The results were compared with the reference sequences in the NCBI gene bank, using Chromas software, and with the reported mutations in the literature. Single nucleotide polymorphisms and intron variants were excluded.Furthermore, different online bioinformatics software (SIFT:http://sift.jcvi.org, PolyPhen-2: http://genetics.bwh.harvard.edu/pph2/, Panther: http://www.pantherdb.org and Mutation Taster: http://www.mutationtaster.org) were used for predicting the pathogenic ity of mutations.
RESULTS
Clinical Findings This five-generational Chinese pedigree was composed of 114 members segregating autosomal dominant JOAG (Figure 1, Table 1). Other ocular or systemic defects were not observed in any of the participants. Eighteen patients (including deceased patients: I:2, II:1 and II:3) were diagnosed with JOAG through current and previous medical histories and examinations. One patient was diagnosed as a JOAG suspect according to a normal IOP and enlarged cupto-disc ratio of 0.6/0.7 (OD/OS). The proband (III:10) was a seventy-three years old woman who was diagnosed with JOAG at the age of 31 and received an operation aged 41.She was diagnosed with advanced glaucoma, presenting with an elevated IOP (52/23 mm Hg, OD/OS) and characteristic glaucomatous visualfield defects when enrolled in this study.
Mutation Screening of MYOC in Juvenile-onset Open Angle Glaucoma A heterozyous mutation (c.1099G>A) of MYOC was identified in the three JOAG and the one suspect individual by sanger sequencing of the coding and flanking regions; mutations in OPTN, CYP1B1 or WDR36 were not detected in this study. The transition mutation was located in the first base pair of codon 367 (GGA>AGA) in exon 3 of MYOC and was predicted to be a missense substitution of glycine to arginine (p.G367R) in myocilin (Figure 2A).This missense mutation was not present in unaffected family members or in 100 ethnically unrelated controls. Other variants in coding regions of candidate genes were not identified for all participants. G367R was not registered in the 1000 Genomes,dbSNP, and the HapMap databases.
Table 1 Clinical data on the patients in this family


Figure 2 Sequencing results of MYOC A: The sequences of the proband and an unaffected member (V: 4) are shown. A heterozygous mutation (c.1099G>A) is detected in exon 3 of MYOC in the affected patient. B: Multiple protein sequence alignments. Multiple-sequence alignment revealed p.G367R located within a highly conserved region. The “mut.” sequence indicates the sequence identified in the present study.
By aligning the amino acid sequence of MYOC across different species, we found that codon 367, where mutant (p.G367R)existed, is a phylogenetically conserved position (Figure 2B). All reported MYOC mutations in Chinese family are summarized in Table 2. The online bioinformatics software predicts that G367R is a causative substitution of MYOC (SIFT: deleterious,PolyPhen-2: probably damage, Panther: probably damaging,Mutation Taster: disease causing). Taken together, these data indicate that the p.G367R substitution is the causative mutation, rather than a simple polymorphism in this pedigree.
DISCUSSION
MYOC was the first causative gene reported for glaucoma[8].Currently, more than 270 variants in MYOC have been identified, with more than half potentially diseasing-causing in JOAG or POAG. These mutations contribute 2%-4% of POAG and 22%-36% of JOAG patients. MYOC comprises three exons and two introns, in which 90% of the reported disease-causing mutations were located in exon 3, nine in exon 1, and only one in exon 2 (http://www. myocilin.com/statistical summary.php). Mutations in MYOC were found in various ethnicities,including American, Australian, Brazilian, Chinese, Japanese,Indian, South African and Spanish subjects. Missense mutations account for the majority of disease-causing variants in MYOC (83.7%), followed by a nonsense mutation (5.8%),small deletion (4.8%), small insertion (4.8%) and small indel(1%). Interestingly, sixteen recorded MYOC mutations were predicted for truncation of myocilin, the protein expressed by MYOC (http://www. myocilin.com/statistical summary.php). In addition, rare mutations were identified as haploinsufficiency and copy number variation[9-10].
In the present study, a recurrent mutation (c.1099G>A) in the MYOC gene of a five-generation Chinese pedigree affected with autosomal dominant JOAG was identified to co-segregate with the glaucoma phenotype. The missense mutation leads to the substitution of glycine for arginine at codon 367 (p.G367R)where the amino acid is highly conserved. Until now, 30 MYOC mutations have been identified in Chinese POAG or JOAG patients. R91X, E300K, S341P, T353I, P370L, D384N and Y471C were previously reported at least twice in the literatures[3,11-13]. As far as we know, this family is the largest with detected, certain MYOC mutants in China, including eighteen JOAG patients and one suspect. The present study confirms the pathogenic effect of G367R mutation in the JOAG pedigree, in the Chinese Han family. Based on previous evidence from different ethnicities, there may be a foundereffect with G367R. In other words, these mutants may result from the same common ancestor[14].
Table 2 All reported MYOC mutations in the Chinese family

Based on published research, the correlation between genotype and phenotype of G367R in affected patients can be summarized as: 1) pedigrees had an autosomal dominant form with increasingly aged-related incomplete penetrance; 2) there was no significant distinction by gender; 3) onset age is associated early on with elevated IOP; 4) a rapid binoculus enlarged cup-disc ratio and characteristic glaucomatous visual field loss can be observed after age 30 or more; 5) carriers were in poor medical condition, but showed good responsiveness to glaucoma filtering operations. The affected carriers in this study were generally in accordance with the features described above, besides which, long-term surgical outcomes were generally poor. There may be some unidentified factors,environmental or genetic, which are responsible for this heterogenous phenotype in this family[15]. A candidate gene,CYP1B1, has been reported to act as the modifying gene of MYOC when myocilin expression occurs in a trabecular meshwork cell[16-17]. A common pathway was speculated to exist between these two glaucoma-causing genes[18]; however,neither mutations in CYP1B1 nor in OPTN and WDR36 were detected in the study and it was suggested that MYOC was the primary genetic cause of JOAG, which was consistent with the literatures[19].
Myocilin is a secreted protein that consists of 504 amino acids, located preferentially in the iris, sclera, trabecular meshwork, ciliary body, retina and optic nerve, as well as other organizations in the human body[20]. The subcellular localizations of myocilin were in the ciliary rootlet and basal body of the connecting cilium of photoreceptor cells, and in the rough endoplasmic reticulum, where myocilin appears to be a modulator of apoptosis by interacting with the apoptotic pathway[21]. Abnormal expression of myocilin results in diseases, only occurring in glaucoma where equal amounts of mutant and normal mRNA result in the same percentage of these two kinds of myocilin at the transcription level[22].That is to say, genetic mutants can lead to the mis-folding and accumulation of abnormal myocilin in the surface of trabecular meshwork, as well as leading to the aggregation of insoluble protein in trabecular meshwork cells. The mechanical occlusion and cell dysfunction of G367R result in an increasing aqueous outflow obstruction and elevated IOP,that can be confirmed by experiments in vivo and in vitro[23-24].A comprehensive general medical history and ophthalmic evaluation should be undertaken every time by ophthalmologists,including anterior segment examination, IOP measurement,ONH and visual field evaluation, following by genetic screening. The progress of glaucoma can be slowed down if the screen for causative genes is adopted in high risk groups.Early onset of glaucoma and notably elevated IOP appear frequently in patients with JOAG for MYOC mutations, with relatively poor response to therapy[19]. For disease-causing mutations, enhancing early diagnosis and treatments with antiglaucoma medicines orfilter surgeries should be recommended to minimize serious visual loss in patients. These physical examinations and treatments can be performed before age 15 or earlier, regardless of any clinical manifestation. The suspect who carried G367R in this study was treated when his binoculus IOP reached 25 mm Hg at age 18. Unfortunately, trabeculotomy was followed by anti-glaucoma agents, because his IOP was out of control and visualfield defects had been progressing.
In conclusion, we identified a MYOC c.1099G>A mutation in an autosomal dominant JOAG family, and summarized the characteristic phenotypes among the patients. Further evidence of a founder effect for the G367R MYOC mutant was provided by our data. Moreover, we found that genetic testing can be utilized for high-risk populations in this study. Screening for disease-causing genes will be helpful for not only genetic counseling, but also for early diagnosis and treatment of patients or carriers affected by JOAG. Although mutations can help us to understand the mechanisms of glaucoma and to enhance early diagnosis and therapeutic interventions for JOAG positive patients, further research is needed to explore the poor drug responsiveness and the well-controlled effects of surgery in patients affected by the MYOC/p.G367R mutation.
ACKNOWLEDGEMENTS
Foundations:Supported by Natural Science Foundation of China (No.81270999; No.81570870); Professor Academic Development Fund of Fujian Medical University (No.JS14019).
Conflicts of Interest:Yao YH, None; Wang YQ, None; Fang WF, None; Zhang L, None; Yang JH, None; Zhu YH, None.
REFERENCES
1 Ying H, Yue BY. Cellular and molecular biology of optineurin. Int Rev Cell Mol Biol 2012;294:223-258.
2 Takamoto M, Araie M. Genetics of primary open angle glaucoma. Jpn J Ophthalmol 2014;58(1):1-15.
3 Huang X, Li M, Guo X, Li S, Xiao X, Jia X, Liu X, Zhang Q. Mutation analysis of seven known glaucoma-associated genes in Chinese patients with glaucoma. Invest Ophthalmol Vis Sci 2014;55(6):3594-3602.
4 Sakurada Y, Mabuchi F. Advances in glaucoma genetics. Prog Brain Res 2015;220:107-126.
5 Yao YH, Zhu YH, Yang JH. Advances in genetics of primary open angle glaucoma. Chinese Journal of Experimental Ophthalmology 2017;35(6):572-576.
6 Prum BE, Jr Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD,Moroi SE, Herndon LW Jr, Lim MC, Williams RD. Primary openangle glaucoma preferred practice pattern(®) guidelines. Ophthalmology 2016;123(1):P41-P111.
7 Su HA, Li SY, Yang JJ, Yen YC. An application of NGS for WDR36 gene in Taiwanese patients with juvenile-onset open-angle glaucoma. Int J Med Sci 2017;14(12):1251-1256.
8 Sheffield VC, Stone EM, Alward WL, Drack AV, Johnson AT, Streb LM, Nichols BE. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat Genet 1993;4(1):47-50.
9 Braghini CA, Neshich IA, Neshich G, Soardi FC, de Mello MP, Costa VP, de Vasconcellos JP, de Melo MB. New mutation in the myocilin gene segregates with juvenile-onset open-angle glaucoma in a Brazilian family.Gene 2013;523(1):50-57.
10 Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS,Tomarev SI, John SW, Johnson RL. Targeted disruption of the myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol Cell Biol 2001;21(22):7707-7713.
11 Zhou XM, Yin Y, Fan N, Cheng HB, Li XH, Wang Y, Yu WH, Cai SP,Liu XY. Single nucleotide polymorphism of MYOC affected the severity of primary open angle glaucoma. Int J Ophthalmol 2013;6(3):264-268.
12 Cheng JW, Cheng SW, Ma XY, Cai JP, Li Y, Lu GC, Wei RL. Myocilin polymorphisms and primary open-angle glaucoma: a systematic review and meta-analysis. PLoS One 2012;7(9):e46632.
13 Janssen SF, Gorgels TG, Ramdas WD, Klaver CC, van Duijn CM,Jansonius NM, Bergen AA. The vast complexity of primary open angle glaucoma: disease genes, risks, molecular mechanisms and pathobiology.Prog Retin Eye Res 2013;37(12):31-67.
14 Iliev ME, Bodmer S, Gallati S, Lanz R, Sturmer J, Katsoulis K, Wolf S, Trittibach P, Sarra GM. Glaucoma phenotype in a large Swiss pedigree with the myocilin Gly367Arg mutation. Eye (Lond) 2007;22(7):880-888.
15 Chen J, Cai SP, Yu WH, Yan NH, Tang L, Chen XM, Liu XY.Sequence analysis of MYOC and CYP1B1 in a Chinese pedigree of primary open-angle glaucoma. Mol Vis 2011;17:1431-1435.
16 Mookherjee S, Acharya M, Banerjee D, Bhattacharjee A, Ray K.Molecular basis for involvement of CYP1B1 in MYOC upregulation and its potential implication in glaucoma pathogenesis. PLoS One 2012;7(9):e45077.
17 Lim SH, Tran-Viet KN, Yanovitch TL, Freedman SF, Klemm T, Call W, Powell C, Ravichandran A, Metlapally R, Nading EB, Rozen S, Young TL. CYP1B1, MYOC, and LTBP2 mutations in primary congenital glaucoma patients in the United States. Am J Ophthalmol 2013;155(3):508-517.
18 Bagiyeva S, Marfany G, Gonzalez-Angulo O, Gonzalez-Duarte R.Mutational screening of CYP1B1 in Turkish PCG families and functional analyses of newly detected mutations. Mol Vis 2007;13(13):1458-1468.19 Miller MA, Fingert JH, Bettis DI. Genetics and genetic testing for glaucoma. Curr Opin Ophthalmol 2017;28(2):133-138.
20 McDowell CM, Luan T, Zhang Z, Putliwala T, Wordinger RJ, Millar JC, John SW, Pang IH, Clark AF. Mutant human myocilin induces strain specific differences in ocular hypertension and optic nerve damage in mice. Exp Eye Res 2012;100(1):65-72.
21 Koch MA, Rosenhammer B, Koschade SE, Braunger BM, Volz C,Jagle H, Tamm ER. Myocilin modulates programmed cell death during retinal development. Exp Eye Res 2014;125:41-52.
22 Menaa F, Braghini CA, Vasconcellos JP, Menaa B, Costa VP,Figueiredo ES, Melo MB. Keeping an eye on myocilin: a complex molecule associated with primary open-angle glaucoma susceptibility.Molecules 2011;16(7):5402-5421.
23 Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K,Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest 2011;121(9):3542-3553.
24 Kanagavalli J, Pandaranayaka PJ, Krishnadas SR, Krishnaswamy S,Sundaresan P. In vitro and in vivo study on the secretion of the Gly367Arg mutant myocilin protein. Mol Vis 2007;13(127):1161-1168.