INTRODUCTION
A neural tract tracing study in Xenopus toad[1]had revealed that central processes of the temporal muscular afferent mesencephalic trigeminal nucleus (Vme) neurons project directly to the oculomotor nucleus (III) and trochlear nucleus (IV). This neuronal circuit may help an amphibian pops eyes to stare a prey meanwhile opens mouth widely to shoot its tongue[1]. Interestingly in human, an oculomotor disorder known as Marcus Gunn syndrome (MGS) or jaw winking is characterized by eyelid retraction and occasionally eye bobbling elicited by jaw movements such as opening the mouth[2-5]. Is there a similar trigeminal proprioceptiveoculomotor system neural pathway exists in human? In relevant clinic studies on the MGS, the authors showed distinct co-firing of masticatory and eyelid muscles when both muscle activities were recorded simultaneously[2,4-5]. Further, Sona[6]found stimulation of pterygoid muscle nerve elicited ipsilateral ptotic eyelid retraction and the elicited retraction was completely relieved by section of this nerve from trigeminal motor root. Hence, Sona[6], Wartenberg and Glaser[5]proposed a“release” hypothesis that jaw-winking is probably a primitively normal re flex but become highly controlled and undetectable during phylogenetic development, since humans have no longer needed to wildly open eyes and mouth simultaneously to prey. However, a congenital miswiring hypothesis has been dominating MGS mechanism until up-to-day because aberrant innervation through misdirected nerve branch has been found in another congenital oculomotor synkinesis such as Duane’s syndrome[7-8]. Well, congenital extraocular muscle fibrosis associated MGS may share the same mutated gene with Duane’s syndrome[8-9]. But, the mechanisms of the MGS without congenital extraocular muscle fibrosis[2,4]and some acquired MGS[10-11]are still enigmatic. In addition, clinical entities of some temporal trigemino-oculomotor synkinesis,and of alternative healing and relapse pattern of the jawwinking also suggest the existence of an instinct trigeminal proprioceptive-oculomotor system pathway[2,4-5].
Therefore, the question is whether a residue of neuronal circuit for primitive jaw-winking re flex still exists in human or mammal?To explore this potential pathway anatomically in present study, we choose the rat, a mammal with a similar brain system to the human to demonstrate this neural pathway by neural tract tracing. A motoneuron marker choline acetyltransferase[12]was assumed to label the III/IV motoneurons. Further, tract tracing of central axons of intracellularly labeled Vme neuron that responding to electric stimulation of the masseter nerve was performed to verify the involvement of jaw muscle afferent Vme neurons. Moreover, anterograde tracing combined with retrograde tracing discovered projection of the Vme neurons onto the pre-oculomotor neurons.
MATERIALS AND METHODS
Fifty-three adult Sprague-Dawley rats (300-350 g, male and female) were used in the experiments. All surgical procedures and animal care were carried out in accordance with the Care of Laboratory Animals in Research issued by the Chinese Academy of Sciences. Experimental protocol was approved by the Shaanxi Provincial Eye Research Institute and Xi’an BRIGHT Eye Hospital.
Anterograde Tract Tracing
Phaseolus vulgaris leucoagglutinin injection The animals were administered with atropine (0.15 mg/kg, i.p.) and anesthetized with sodium pentobarbital (40 mg/kg, i.p.).They were placed on a stereotaxic frame until no limbwithdrawal re flex was elicited by pinching the hind paw. After a craniotomy on parietal skull, a glass micropipettefilled with 2.5% Phaseolus Vulgaris Leucoagglutinin (PHA-L) (Vector Labs, Burlingame, CA, USA) in 0.01 mol/L phosphate buffer saline (PBS, pH 7.4) was advanced into the caudal Vme(P 0.4-1.0 to interaural, L 1.4-1.6 to midline, tilted 16° rostrally,depth 5.2-5.6 mm) and PHA-L was iontophorezed into the caudal Vme unilaterally. The animals were administrated with an analgesic (ibuprophen syrup 20 mg/kg, orally) after recovery and were given food and water ad libitum.
Biotinylated dextran amine injection The whole procedure for Biotinylated Dextran Amine (BDA) injection was the same as that of PHA-L injection, except that 10% BDA (mol/L, Wt.10 000, Molecular Probes, Eugene, OR, USA) in 0.9% saline was delivered to the Vme.
Histological procedure for phaseolus vulgaris leucoagglutinin and biotinylated dextran amine visualization About ten days after PHA-L or BDA injections, the animals were euthanized with sodium pentobarbital (140 mg/kg, i.p.) and perfused transcardially with 0.9% saline, followed by 4%paraformaldehyde in 0.1 mol/L PBS (pH 7.4). The brain was removed and serial coronal sections were cut at 30 mm thickness.Immunohistochemical protocol for PHA-L visualization was the same as described in our previous publication[13]; besides,we used the same procedure for revealing BDA as previously reported[13]. The sections were then counter stained with 1%neutral red and sealed by Permount.
Immuno- fluorescent Double Labeling
Biotinylated dextran amine tract tracing combined with choline acetyltransferase immunocytochemistry Sections were rinsed 3 times after immunoblocking (1% normal goat serum and 1% Triton-X 100 in 0.01 mol/L PBS) and incubated in mouse anti-choline acetyltransferase (ChAT) monoclonal antibody (Chemicon International, Temecula, CA, USA)overnight at 4℃. Then, sections were rinsed 3 times and incubated in a mixture of fluorescein-conjugated streptavidin(Molecular Probes) and Alexa Fluor 568 conjugated rabbit anti-mouse (Molecular Probes) for 6-8h at 4℃. Finally,sections were thoroughly rinsed, mounted and sealed with Vectashield mounting medium (Vector Labs). BDA and ChAT double labeling was observed and photographed with a Nikon E-600 fluorescent LM and Bio-Rad 1024 Laser Scan Confocal Microscope (Olympus).
Biotinylated dextran amine anterograde tracing combined with cholera toxin B retrograde tracing Cholera toxin B(CTB) (Sigma Chemical, St. Louis, MO, USA) was used as the retrograde tracer (2% in PBS) to label pre-oculomotor neurons in the INC and was delivered into the III 5d after BDA injection. Sections (30 mm) were incubated in goat anti-CTB polyclonal antibody (List Biological Labs, Campbell,CA, USA) overnight at 4℃. In next day, sections were rinsed 3 times and incubated in a cocktail of Alexa Fluor 568 conjugated donkey anti-goat and fluorescein conjugated streptavidin (Molecular Probes) 6-8h at 4℃. Then, sections were thoroughly rinsed, mounted and sealed with Vectashield mounting medium (Vector Labs). BDA and CTB double labeling was observed and photographed with the same microscopy facilities aforementioned.
Intracellular labeling of Vme neurons by horseradish peroxidase Glass microelectrodes filled with 4%-8% horseradish peroxidase (HRP) (Sigma VI) dissolved in 0.05 mol/L Tris buffer with 0.25 mol/L KCl (pH 7.6) were used for intracellular recording and labeling. The Vme neurons were approached dorsally. Cells were identified extracellularly by their short-latency (2.9±0.9ms) responses to masseter nerve stimulation, and by their burst discharges evoked by pressing down the jaw. The electrode was then advanced in 10 µm steps at speed of 1.6 m/s. An intracellular penetration was recognized by a negative shift of 30-70 mV in the baseline and/or action potentials of 40-60 mV. After quickly confirminghe type of the cell from the intracellular record, HRP was iontophoretically injected into the neuron with 4-15 nA positive direct current for about 5min.
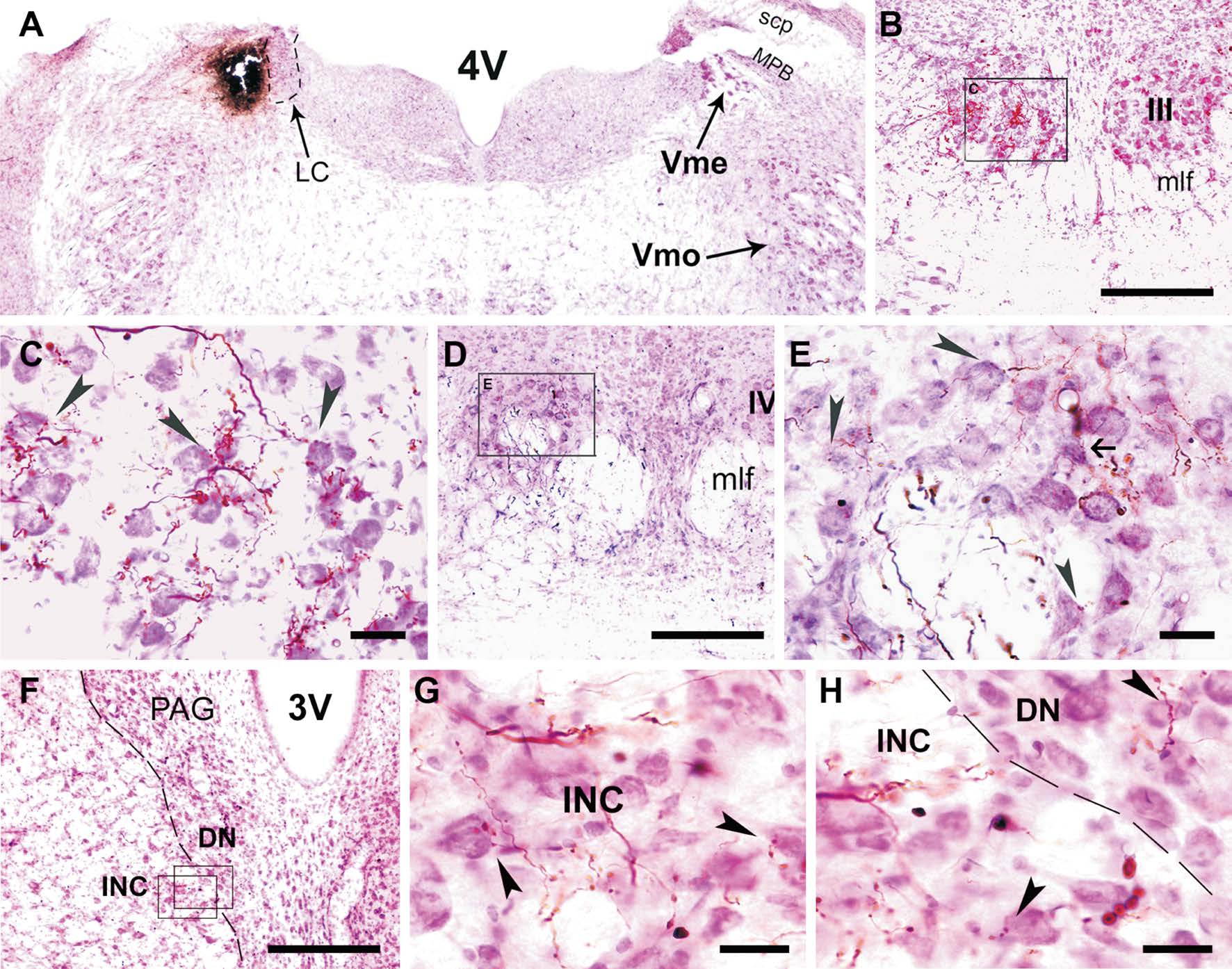
Figure 1 Projections of Vme neurons to III/IV and INC/DN A: Vme injection site; B: PHA-L labeled terminals in ipsilateral III; C: Magnified frame area in B, showing contacts of labeled terminals on Nissl stained motoneurons (arrowheads); D: Labeling in IV; E: Vme afferent terminals ended upon both motoneurons (arrowheads) and interneurons (arrow); F: Distribution of labeled terminals in INC and DN; G: An enlarged frame area in F, showing contacts of labeled terminals on Nissl stained INC (arrowheads) neurons (arrowheads); H: The other magnified frame area in F. 3V: 3rdventricle; 4V: 4thventricle; DN: Darkschewitsch nucleus; INC: Interstitial nucleus of Cajal; LC: Locus ceoruleus; mlf: Medial longitudinal fasciculus; MPB: Medial parabrachial nucleus; PAG: Periaqueductal gray; scp: Superior cerebellar peduncle; Vme: Mesencephalic trigeminal nucleus; Vmo: Trigeminal motor nucleus. Scale bars: 300 μm in B, D; 50 μm in C, E; 400 μm in F and 25 μm in G, H.
RESULTS
Distribution of Vme Neuronal Axons and Terminals in Oculomotor System When PHA-L or BDA was injected into the rat caudal Vme, the anterogradely labeled central axons and terminals were observed in the ipsilateral III/IV and in interstitial nucleus of Cajal and Darks chewitsch nucleus(INC/DN) as well (Figure 1). A typical PHA-L injection site shown in Figure 1A was about 200×250×230 μm3, distributed rostro-caudally in eight coronal sections (30 μm), which is completely restricted in the caudal Vme area. PHA-L labeled terminals were distributed in the III and IV exclusively ipsilateral to the tracer injection (Figure 1B, 1D; Figure 2B,2C). Higher magnification views showed that labeled boutons were closely apposite with neutral red stained motoneurons(arrowheads) in III and IV (Figure 1C, 1E). In addition, The PHA-L positive axons and terminals were found in ipsilateral INC/DN complex (Figure 1F; Figure 3C). Labeled Vme neuronal boutons were also seen in close apposite upon(arrowheads) counterstained neurons in the INC/DN (Figure 1G, 1H).
Is There Any Vme Neuronal Projection to the Abduces Nucleus and Their Premotor Neurons? Interestingly, the Vme neurons project not only to the III/IV but also up to the INC/DN, the later has been well recognized as a premotor control area for vertical and torsional eye movements[14-15].Thus, it raises a question for whether or not the Vme neurons share their projections to horizontal eye moving nucleus and their premotor neurons? Therefore, we carefully examined abduces nucleus (VI) and their premotor neurons region[16]-central mesencephalic and paramedian pontine reticular formation (CMRF/PPRF). There is no any labeling in the VI and CMRF/PPRF.
Confocal Visualization of Contacts Between Vme Neuronal Boutons and Choline Acetyltransferase Positive Oculomotor and Trochlear Nuclei Motoneurons By injection of BDA into the caudal Vme (Figure 2A), labeled Vme neuronal axons and boutons (green) were seen overlapped with ChAT immunostained motoneurons (red) in the III and IV (Figure 2B, 2C) ipsilateral to the tracer injection. Confocal microscopy was applied to explore potential synaptic connections between the Vme neuronal terminals and the III and IV motoneuronsthose are ChAT positive. Contacts between labeled boutons and somata or primary dendrites (arrowheads) were clearly observed in the III (Figure 2D, 2F) and IV (Figure 2E, 2G).
Confocal Visualization of Contacts Between Vme Neuronal Boutons and Cholera Toxin B Retrogradely Labeled Preoculomotor Neurons in Interstitial Nucleus of Cajal and Darkschewitsch Nucleus Following injections of BDA into the caudal Vme (Figure 3A), and of retrograde tracer CTB into the contralateral III (Figure 3B), labeled Vme neuronal axons and boutons (green) were seen overlapping with retrogradely labeled pre-oculomotor neurons (red) in the INC/DN area (Figure 3C) ipsilateral to the Vme injection. Revealed by confocal microscopic observation, the majority of Vme neuronal boutons (green) were located and made contacts onto CTB labeled somata and dendrites (arrowheads) of the pre-oculomotor neurons (red) in the INC (Figure 3C-3F).Appositions of Vme projecting terminals upon retrogradely labeled pre-oculomotor neurons were also found in the DN(Figure 3G).
Intracellularly Labeled Vme Neuronal Axons Traveling into the Oculomotor Nucleus Majority of intracellularly labeled axons of the Vme neurons travel caudally to mastication associated nuclei as had been reported earlier by Luo and colleagues[17]. Notably, a few Vme neurons located in rostral column of the nucleus and responded to masseter nerve stimulation sent their axons to the III (Figure 4). After reconstruction of the intracellular traced trajectories of the axons by camera lucida, it was observed these axons climbed rostral-ventrally into the III area (Figure 4A). Attempting tofind close apposite of labeled boutons upon motoneurons, we performed Nissl stain (Figure 4B) to mark the motoneurons.
DISCUSSION
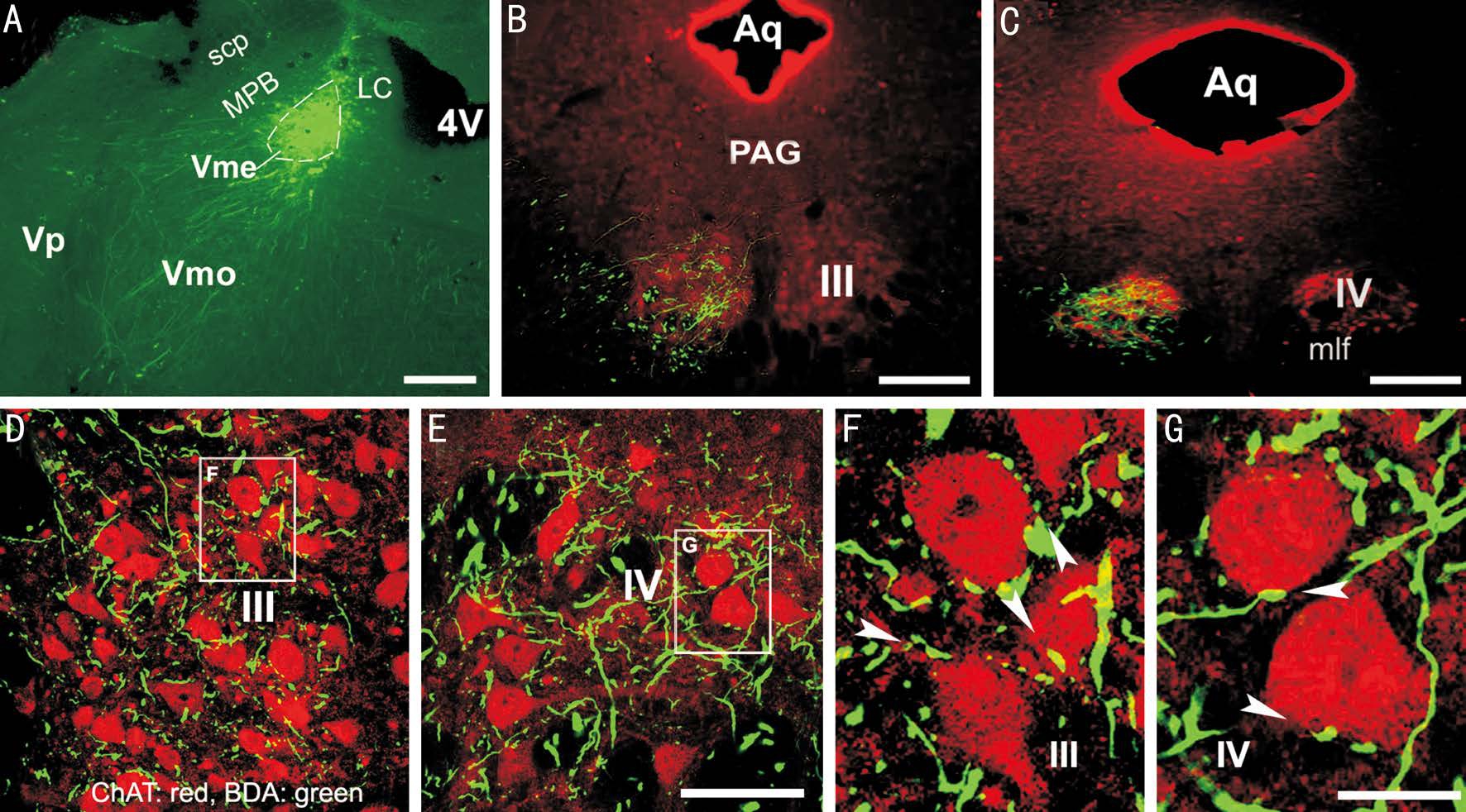
Figure 2 Potential synaptic contacts between Vme neuronal terminals and III/IV motoneurons A: BDA injection site; B: Overlapping of BDA labeling with ChAT positive motoneurons in III; C: Overlapping in IV; D: Confocal microscopic revealing of contacts of BDA terminals on ChAT motoneurons in III; E: Contacts in IV; F: Magnified frame area in D, showing those contacts (arrowheads) in III; G: Enlarged frame area in E, showing contacts in IV (arrowheads). Aq: Aqueduct; Vp: Principal trigeminal sensory nucleus. Scale bars: 400 μm in A-C; 100 μm in D, E; 25 μm in F, G.
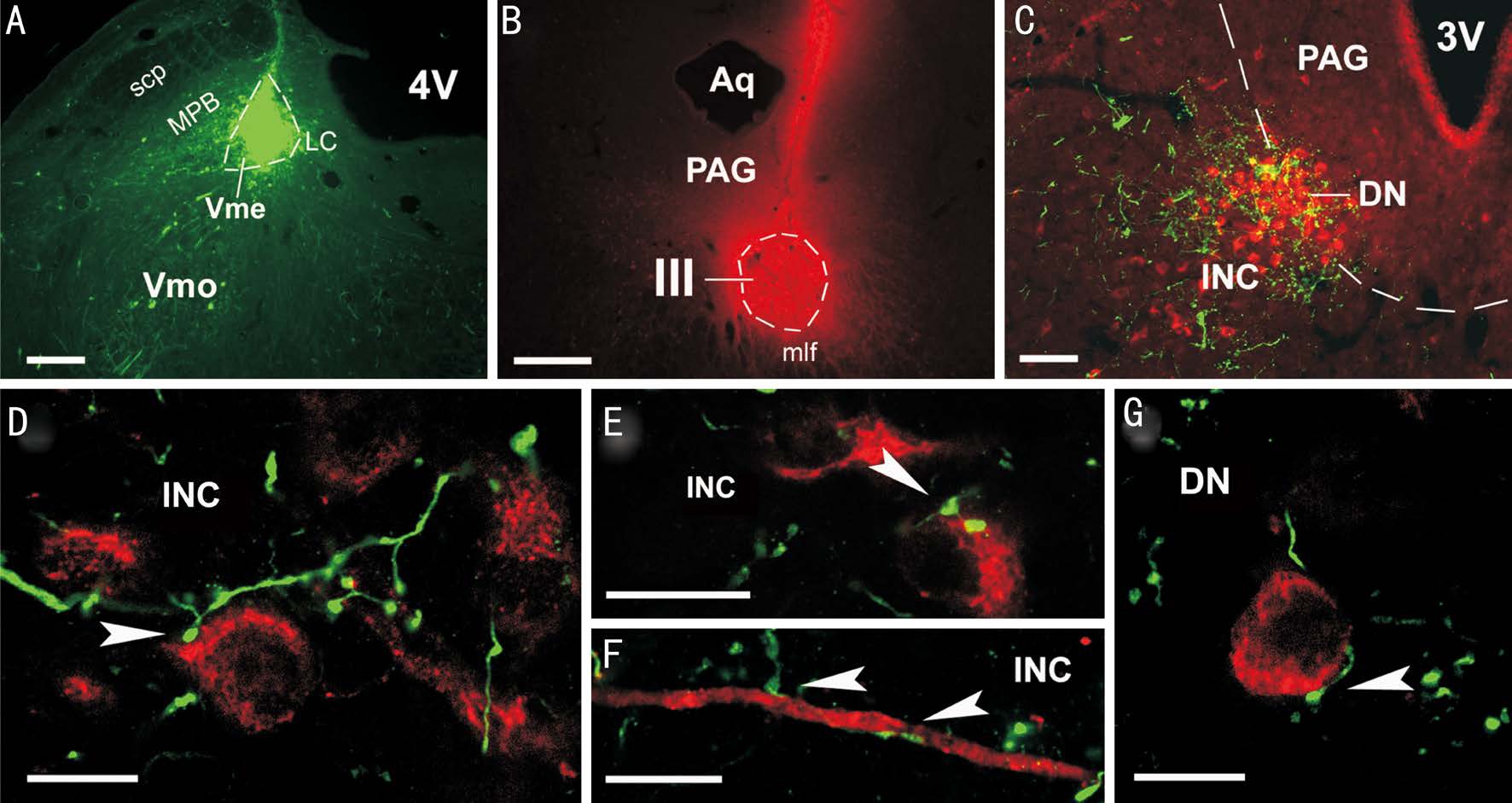
Figure 3 Potential synaptic contacts between Vme neuronal terminals and INC/DN pre-oculomotor neurons A: BDA injection site in caudal Vme; B: CTB injection site in contralateral III; C: Overlapping of BDA labeledfibers and terminals with CTB labeled pre-oculomotor neurons in the INC/DN, ipsilateral to the BDA injection and contralateral to the CTB delivery; D-G: Confocal microscopic viewing of contacts(arrowheads) between BDA labeled boutons and CTB labeled pre-oculomotor somata or dendrite in the INC (D, E and F) and DN (G). Scale bars: 400 μm in A, B; 200 μm in C; 25 μm in D-G.
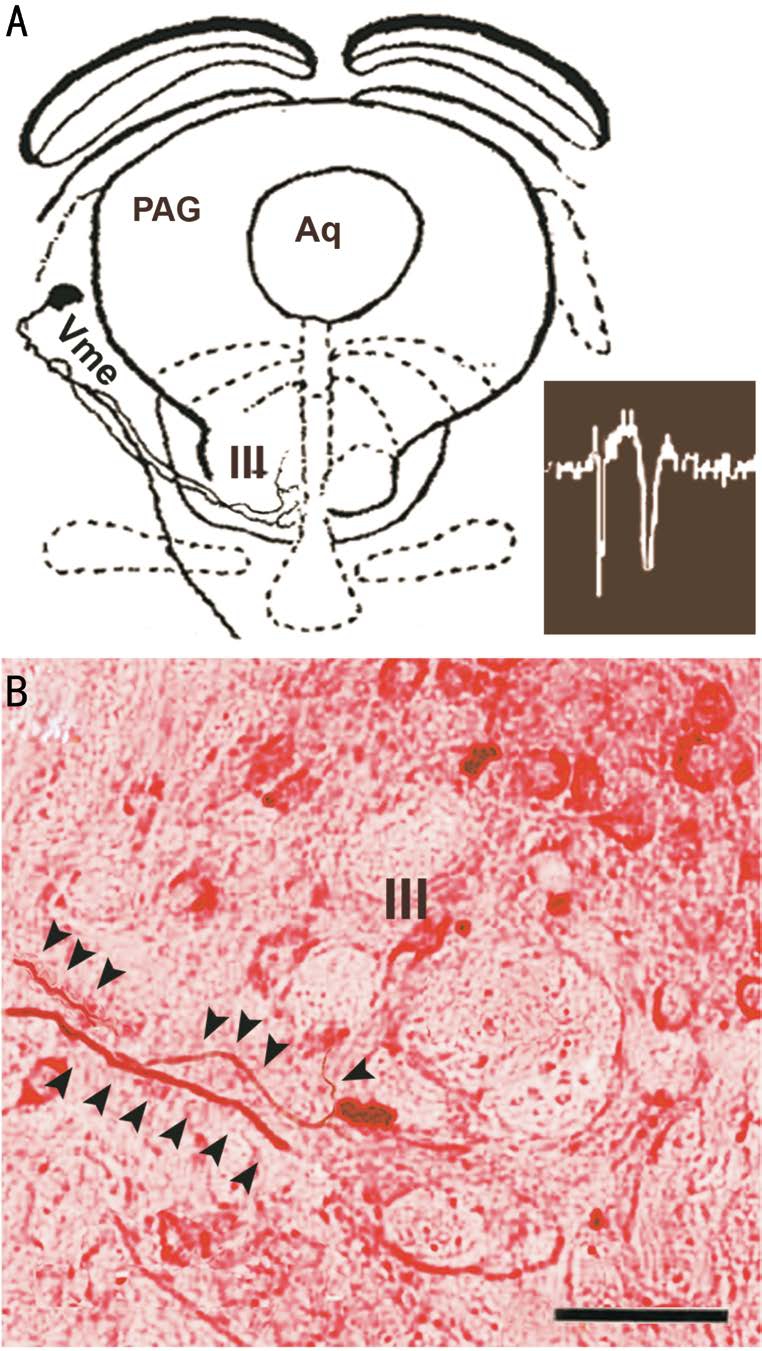
Figure 4 Jaw muscle afferent Vme neurons sending axons to the ipsilateral III A: Camera Lucida reconstructed intracellularly labeled Vme soma and axons in midbrain levels of the brain stem, this Vme neuron responded to electric stimulation of ipsilateral masseter nerve (bottom right); B: Intracellular labeled Vme neuronal axons(arrowheads) traveling through the border area of the III in a neutralred counterstained section. Scale bar =100 μm.
In the present study, we applied two anterograde tracers:PHA-L and BDA because the PHA-L is generally superior to the BDA for anterograde labeling neuronal somata and their axons. The leucoagglutinin receptors are widely distributed in almost all kinds of cells in mammals and humans[18-19].However, they are located primarily on somata in the central nervous system, which makes PHA-L become super anterograde neuronal tracer due to it is selectively taken up by somata when being carefully iontophorized without injuring passing fibers[18-19]. While, high molecular BDA has been used as anterograde tracer and low molecular BDA can be applied to retrograde tract tracing[20]. Highly purified high molecular BDA is also an excellent anterograde tracer. The BDA used in our current study has been verified by many previous experiments to be a reliable anterograde tracer[13,20]. Meanwhile, the case was abandoned if the tracer sprayed out of the Vme nucleus border.Therefore in the rat, we demonstrated that the Vme neurons project to the III/IV as previously reported in toad[1]. We have found and reported the projections from the Vme to the III/IV and INC/DN[13], but it was still not clear what kinds of neurons in the III/IV and INC/DN received these projections. In the present work, we identified III/IV motoneurons by ChAT that has been known to be a reliable marker for all somatic motoneurons[12]. Then, by laser scanned confocal microscopy imagining, we disclosed possible connections between Vme projecting terminals and III/IV motoneurons, without excluding innervation of Vme neurons onto interneurons[15]in the III/IV. We further unveiled Vme neuronal terminals close apposite upon somata and dendrites of retrogradely labeled premotor neuron in the INC/DN following injection of the tracer into the III. This is more important since the INC/DN complex has been well documented as pre-oculomotor area dominates vertical and torsional eye movements[14-15].A pathway to both motor and premotor neurons suggests a functional impact. Reasonably, we would wonder whether the Vme neurons emit any projection to horizontal eye-movement control center. Then, we examined the VI and VI premotor neurons in the CMRF/PPRF[16], we detected labeling in neither PHA-L nor BDA injection cases. Moreover, by means of intracellular injection of HRP after electrophysiological identifying jaw muscle afferent Vme neurons, some HRP labeled Vme neuronalfibers were observed climbing into the ipsilateral III. Both previous and present studies implied an existence of masticatory oculomotor system pathway in the rat,and this pathway might be related to vertical and torsional eye movements.
However, we do not exclude possible participant of periodontal Vme in this pathway, even though we termed it as a masticatory oculomotor system pathway, based on a published study in toad[1]. The properties of the Vme neurons labeled in our current work could be either jaw muscle spindle afferents or periodontal afferents, because the caudal Vme in which we injected tracers in this work contains both kinds of aforementioned Vme neurons[17,21]. Clinically, retraction of ptotic eyelid evoked by tapping tooth was also reported in a congenitalfibrosis patient[22]. Note, neither mutated gene was identified nor anatomy evidence was shown, but the authors summarized the case as aberrant regeneration of superior rectus branch of oculomotor nerve into the trigeminal nerve[22].Evidence from humans that supported the existence of a masticatory oculomotor system pathway was demonstrated by Sona[5-6]. They found distinct masticatory and eyelid muscles co-firing[4-5], retraction of ipsilateral ptotic eyelid by pterygoid nerve stimulation and relieve of the retraction by section of pterygoid nerve[6]. These findings have been misinterpreted until now as congenital misconnection of trigeminal motorfibers and oculomotor branches[2].
However, there was so far as known no direct evidence showing misconnection between trigeminal motor fibers and any branch of oculomotor nerve after searching through either PubMed or Google. Actually, our findings can give rise to a more rational interpretation for these previously reported phenomenon[2,4-5,22]. More interestingly, in a few cases without any diagnosis of congenital neurological disorder,Lehman et al[23]observed retraction of upper eyelid by electric stimulus of ipsilateral pterygoid nerve as Sona had shown in MSG patients[6]. Consistently, Liang et al[24]had witnessed co-firing of upper eyelid and jaw closing muscles during steady occlusion (isometric contraction of masseter without jaw movement) by recording of electromyography from both upper eyelid and jaw muscles simultaneously in physically normal human volunteers. Further, Liang et al[25]had recorded electromyography from rat superior rectum and levator palpebrae by repeatedly down-stretching the lower jaw.These phenomena would be better explained by the trigeminal proprioceptive-oculomotor pathway we have been exploring,rather than by congenital miswiring story.
ACKNOWLEDGEMENTS
Conflicts of Interest:Liang HC, None; Zhang JD, None;Luo PF, None; Qiao Y, None; Su AL, None; Zhang T, None;Zhu HN, None.
REFERENCES
1 Hiscock J, Straznicky C. Peripheral and central terminations of axons of the mesencephalic trigeminal neurons in Xenopus. Neurosci Lett 1982;32(3):235-240.
2 Pearce FC, McNab AA, Hardy TG. Marcus Gunn jaw-winking syndrome: a comprehensive review and report of four novel cases.Ophthal Plast Reconstr Surg 2017;33(5):325-328.
3 Kannaditharayil D, Geyer H, Hasson H, Herskovitz S. Bilateral Marcus Gunn jaw-winking syndrome. Neurology 2015;84(10):1061.
4 Brodsky CM. Pediatric Neuro-Ophthalmology. New York, Dordrecht,Heidelberg, London: Springer; 2010.
5 Saitkowski RM, Glaser JS. Pediatric neuro-ophthalmology: General considerations and congenital motor and sensory anomalies. In Glaser JS, 3rded. Neuro-ophthalmology. Philadelphia, Lippincott: Williams &Wilkins; 1999:461-487.
6 Sona K. Section of the portio minor of the trigeminal nerve for the treatment of the Marcus Gunn phenomenon. Mod Probl Paediatr 1976;18:258-262.
7 Hotchkiss MG, Miller NR, Clark AW, Green WR. Bilateral Duane's retraction syndrome. A clinical-pathologic case report. Arch Ophthalmol 1980;98(5):870-874.
8 Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W,Chan WM, Andrews C, Demer JL, Robertson RL, Mackey DA, Ruddle JB, Bird TD, Gottlob I, Pieh C, Traboulsi EI, Pomeroy SL, Hunter DG,Soul JS, Newlin A, Sabol LJ, Doherty EJ, de Uzcátegui CE, de Uzcátegui N, Collins ML, Sener EC, Wabbels B, Hellebrand H, Meitinger T, de Berardinis T, Magli A, Schiavi C, Pastore-Trossello M, Koc F, Wong AM,Levin AV, Geraghty MT, Descartes M, Flaherty M, Jamieson RV, Møller HU, Meuthen I, Callen DF, Kerwin J, Lindsay S, Meindl A, Gupta ML Jr, Pellman D, Engle EC. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 2010;140(1):74-87.
9 Kaçar Bayram A, Per H, Quon J, Canpolat M, Ülgen E, Doğan H,Gumus H, Kumandas S, Bayram N, Bilguvar K, Çağlayan AO. A rare case of congenitalfibrosis of extraocular muscle type 1A due to KIF21A mutation with Marcus Gunn jaw-winking phenomenon. Eur J Paediatr Neurol 2015;19(6):743-746.
10 García Ron A, Jensen J, Garriga Braun C, Gómez E, Sierra J. Marin-Amat and inverted Marcus-Gunn syndrome. Two case reports. An Pediatr(Barc) 2011;74(5):324-326.
11 Sibony PA, Lessell S, Gittinger JW Jr. Acquired oculomotor synkinesis.Surv Ophthalmol 1984;28(5):382-390.
12 Kimura H, McGeer PL, Peng F, McGeer EG. Choline acetyltransferasecontaining neurons in rodent brain demonstrated by immunohistochemistry.Science 1980;208(4447):1057-1059.
13 Zhang J, Liang H, Luo P, Xiong H. Unraveling a masticatory -oculomotor neural pathway in rat: implications for a pathophysiological neural circuit in human? Int J Physiol Pathophysiol Pharmacol 2011;3(4):280-287.
14 Fukushima K. The interstitial nucleus of Cajal and its role in the control of movements of head and eyes. Prog Neurobiol 1987;29(2):107-192.
15 Sugiuchi Y, Takahashi M, Shinoda Y. Input-output organization of inhibitory neurons in the interstitial nucleus of Cajal projecting to the contralateral trochlear and oculomotor nucleus. J Neurophysiol 2013;110(3):640-657.
16 Büttner-Ennever JA. Neuroanatomy of the oculomotor system. The reticular formation. Rev Oculomot Res 1988;2:119-176.
17 Luo PF, Wang BR, Peng ZZ, Li JS. Morphological characteristics and terminating patterns of masseteric neurons of the mesencephalic trigeminal nucleus in the rat: an intracellular horseradish peroxidase labeling study. J Comp Neurol 1991;303(2):286-299.
18 Groenewegen HJ, Wouterlood FG. Light and electron microscopic tracing of neuronal connections with Phaseolus vulgaris eucoagglutinin(PHA mm), and combinations with other neuroanatomical techniques.Handbook of Chemical Neuroanatomy 1990;8:47-124.
19 Cucchiaro JB, Uhlrich DJ. Phaseolus vulgaris leucoagglutinin(PHA-L): A neuroanatomical tracer for electron microscopic analysis of synaptic circuitry in the cat's dorsal lateral geniculate nucleus. J Electron Microsc Tech 1990;15(4):352-368.
20 Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG.Pathway tracing using biotinylated dextran amines. J Neurosci Methods 2000;103(1):23-37.
21 Nomura S, Mizuno N. Differential distribution of cell bodies and central axons of mesencephalic trigeminal nucleus neurons supplying the jaw-closing muscles and periodontal tissue: a transganglionic tracer study in the cat. Brain Res 1985;359(1-2):311-319.
22 Gottlob I, Jain S, Engle EC. Elevation of one eye during tooth brushing. Am J Ophthalmol 2002;134(3):459-460.
23 Lehman AM, Dong CC, Harries AM, Patel A, Honey CR,Patel MS. Evidence of ancillary trigeminal innervation of levator palpebrae in the general population. J Clin Neurosci 2014;21(2):301-304.
24 Liang H, Song J, Shen D, Qiao Y, Zhang J. Co-firing of levator palpebrae and masseter muscles links the masticatory and oculomotor system in humans. J Biomed Res 2015;29(4):316-320.
25 Liang H, Zhang J, Luo P, Zhu H, Qiao Y, Su A, Zhang T.Electromyography and Fos immunostaining study establish a possible functional link between trigeminal proprioception and the oculomotor system in rats. J Biomed Res 2017;31(3):256-263.