INTRODUCTION
Retinal vein occlusion (RVO), including the central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO), is a common retinal vascular disorder and a frequent cause of visual loss. With the increased prevalence of RVO[1], timely and effective treatment for this sightthreatening condition has become more important. Macular edema (ME) is a common cause of visual loss in patients with RVOs. ME secondary to RVO is generally treated locally with photocoagulation and/or intravitreal anti-vascular endothelial growth factor (VEGF) agents. If further treatment is needed,medical therapies include anticoagulants, acetazolamide, and corticosteroids; and surgical treatments include pars plana vitrectomy, laminar puncture, and radial optic neurotomy[2].However, no consensus has been reached on what should be the “standard of care” treatment protocol for patients with ME secondary to RVO.
All current treatment options have their benefits and flaws.Photocoagulation used to be the treatment of choice for treating ME, particularly as lasers became more precise[3-4].However, in cases of severe ME, retinal swelling reduces the laser energy penetration, leading to poor therapeutic response. Additionally, laser energy is absorbed by blood and photocoagulation cannot be performed in eyes with retinal hemorrhage. Therefore, treatments must be delayed until hemorrhage has largely resolved, potentially leading to poorer visual outcomes. However, the best time to treat ME remains controversial and photocoagulation therapy often results in limited visual outcome[4-5]. Therefore, laser photocoagulation is generally used as a rescue treatment and to maintain, not improve, visual function[6-7].
Intravitreal anti-VEGF therapy has been shown to be most beneficial in treating ME secondary to RVO, in both the research and clinical settings[6-8]. These agents both improve visual acuity and reduce ME[9], but generally require multiple repeat intravitreal injections to maintain vision gain. This can be problematic because multiple injections increase the risk of retinal ischemia, vasoconstriction, and vitreous traction; and impose great economic burden on health care systems[10-12]and patients.
Studies have shown that combination of anti-VEGF and laser photocoagulation therapies is effective in reducing RVO-associated ME and reduces the need for repeat anti-VEGF agent injections[13-14]. However, further investigations are needed because the timing between anti-VEGF injection and laser treatment was not well described. Additionally, laboratory studies have shown that VEGF is expressed in both intraocular and retrobulbar tissues[15-16]. Therefore, we speculate that administering intraocular therapies without systemic treatment may explain recurrent and/or persistent ME.
Here, we examine the safety and efficacy of intravitreal anti-VEGF, oral glucocorticoids, and laser photocoagulation combination therapy in treating ME secondary to RVO.This therapy should theoretically provide the rapid action of intravitreal anti-VEGF agents and the stability of standard laser photocoagulation.
SUBJECTS AND METHODS
This prospective, non-randomized, non-controlled, interventional,clinical study protocol was approved by the Medical Ethics Committee of the Zhongshan Ophthalmic Center at Sun Yatsen University (Guangdong, China; No.2013MEKY028). All study conduct strictly adhered to the tenets of the Declaration of Helsinki and all patients provided written informed consent prior to participation.
Study Patients Patients who developed an RVO within 6mo of the enrollment date with ME secondary to either a BRVO or non-ischemic CRVO [as confirmed on angiography or optical coherence tomography (OCT)] were consecutively recruited into the study. All patients were identified at Zhongshan Ophthalmic Center (Guangzhou, China) Outpatient Clinic.The inclusion criteria included the following: age ≥18y,intraocular pressure (IOP) <21 mm Hg, adequate pupillary dilation, and central retinal thickness (CRT) >250 µm. Patients were excluded if any of the following were present: visually significant cataract, media opacities, retinal disease (other than RVO and related sequelae), and history of intraocular surgery.Patients were also excluded if they had abnormal blood biochemical test results, pregnancy and systemic diseases such as poorly controlled diabetes or hypertension which are contraindications for steroid therapy, prior systemic anti-VEGF therapy, or undergone intraocular steroid therapy or retinal laser treatment in the studied eye within the last 3mo.
Study Examinations All patients underwent a comprehensive ophthalmologic examination, which included measurement of Snellen best-corrected visual acuity (BCVA), IOP (Canon TX-20, Canon Corporation, Tokyo, Japan), CRT [spectraldomain OCT (Spectralis, Heidelberg Engineering, Heidelberg,Germany)], fluorescein angiography (FFA) and retinal vessel oxygenation [vessel diameter and oxygen saturation;retinal oximetry (Oxymap, Reykjavik, Iceland)]. Slit-lamp biomicroscopy and indirect ophthalmoscopy were also performed. Visual acuity measurements were converted to the logarithm of the minimum angle of resolution (logMAR) for data analyses. Systemic and ophthalmic medical history, as reported by patients, was also carefully reviewed to assess the safety of administering oral glucocorticoid treatment. Only one eye from each patient was included in the study.
Study Treatments All patients were initially treated with oral prednisone (0.5 mg/kg body weight for 6wk followed by a 5 mg/wk taper). Intravitreal anti-VEGF therapy (0.5 mg conbercept or ranibizumab) was also administered within 3d of beginning systemic corticosteroid therapy. Two weeks after the intravitreal anti-VEGF injection, patients underwent standard laser photocoagulation. The size of the spot was 300-500 μm.The exposure time was 0.08-0.15s. The power was adjusted and started at 300 mW and increased in steps of 10 mW to produce mild intensity burns covering areas of capillary leakage as seen on FFA, 1 burn width apart. All lesion reaction grades were Tso II[17-18].
Patients were administered rescue treatments if any of the following were true: 1) presence of new or persistent cystic retinal changes, subretinal fluid, or neuroepithelial detachment;2) increase in CRT of >50 µm; 3) presence of new macular hemorrhage, occlusion, or retinal neovascularization. Patients were also administered rescue treatments at the treating physician’s discretion. Rescue treatments included additional intravitreal anti-VEGF injections, changing oral glucocorticoid dose, and laser photocoagulation (Figure 1).
Outcome Measures Study follow-up evaluations were conducted 2wk and 1, 3, 6 and 12mo after laser therapy. At all study visits, BCVA, CRT, retinal vessel oxygen saturation, and retinal vessel diameter were measured. The primary outcome measure of therapy efficacy was the change from baseline in BCVA at month 6 and 12. Secondary outcome measures of efficacy were changes from baseline in CRT, retinal vessel oxygen saturation, retinal vessel diameter, proportion of patients with logMAR BCVA≥1.0 (20/200), and proportion of patients with CRT>250 µm to month 12.
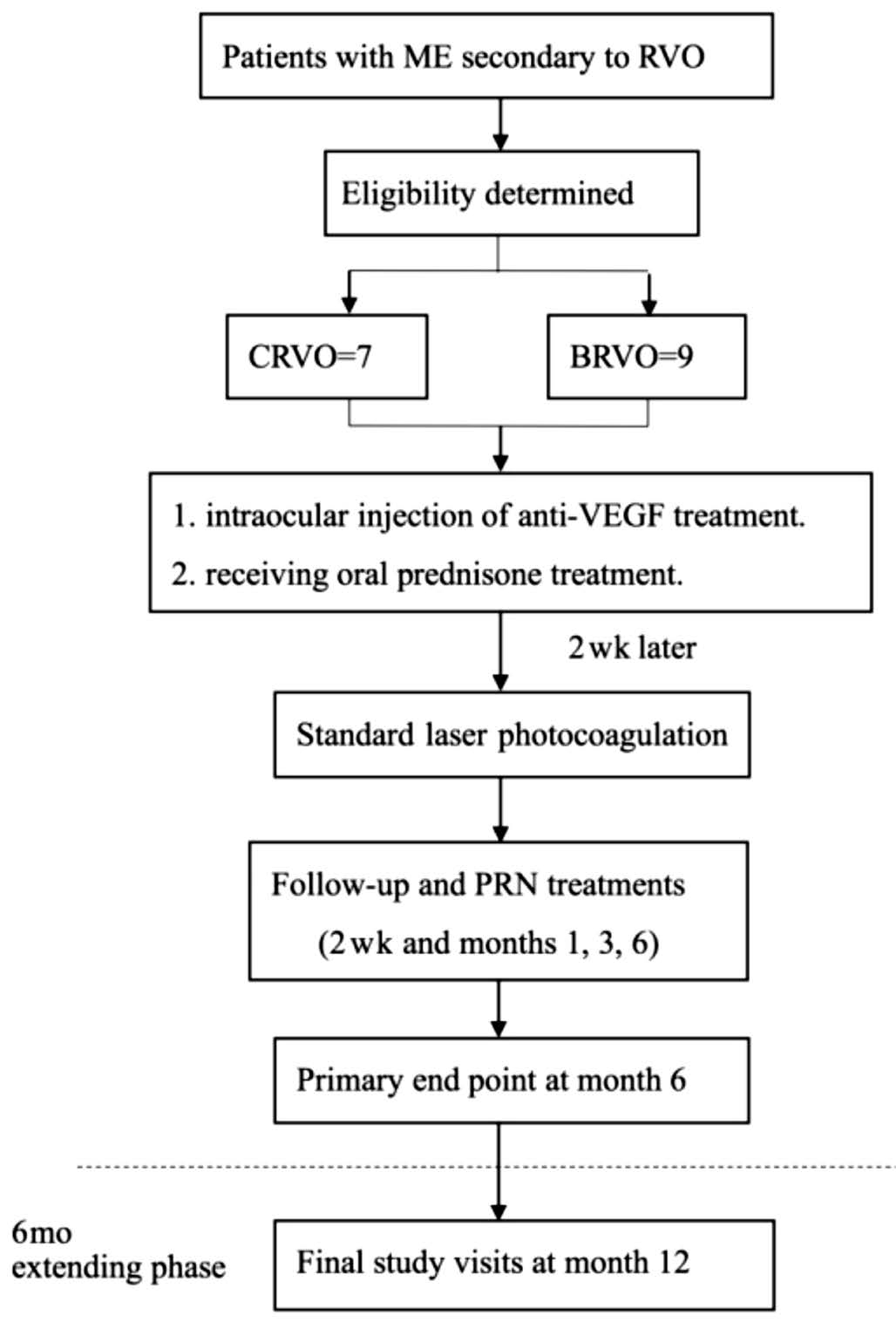
Figure 1 Study flow chart Sixteen patients included 7 patients with CRVO and 9 patients with BRVO, who received oral prednisone treatment and 0.5 mg of intraocular injection of anti-VEGF treatment(Conbercept or ranibizumab). Then at two weeks’ follow-up after the injection, subjects were performed with the standard laser photocoagulation. The primary end point at month 6 and the final study visit was at month 12. The rescue treatments included: 1)received more injection of anti-VEGF treatment; 2) adjusted the oral glucocorticoid dose; 3) laser photocoagulation.
Modified “SAVE” score used to evaluate therapy for ME with FFA and OCT by comparing pre-treatment, before laser therapy and final follow-up based on the former described[19-20].The “SAVE” scoring were as follows: 1) “S”=subretinal fluid(score: present=1, absent=0); 2) “A”=area of retinal thickening(score: greater than one-disc diameter=1, less than one-disc diameter=0; 3) “V”=vitreomacular abnormalities, as ischemia,hemorrhage, neovascularization, atrophic or vitreo-retinal traction (score: present=1, absent=0); 4) “E”=the etiology(score: focal leakage=0, non-focal leakage=1). The mean number of injections administered and the difference in BCVA changes between eyes with BRVO and CRVO were also examined. The incidences of ocular and non-ocular adverse events (AEs) and serious AEs were evaluated to determine treatment safety.
Statistical Analyses Changes from baseline in BCVA,CRT, and retinal oxygenation parameters were examined for statistical significance using paired Student’s t-tests.Differences between eyes with BCVO and CRVO were evaluated using the repeated measures analysis of variance.Differences in proportions of patients with logMAR BCVA≥1.0 and with CRT>250 µm were examined using Chi-square tests. Differences in proportions between eyes with BRVO and CRVO were analyzed using Fisher’s exact tests. The influence of various parameters on the change in BCVA from baseline was assessed using the multi-variable linear regression model. Oxygen saturation and retinal vessel caliber were automatically analyzed using Oxymap specialized software (version 2.5). Missing data were added using the lastobservation-carried-forward method.
All statistical analyses were performed using SPSS statistical software (SPSS, Inc., Chicago, IL, USA). All statistical tests were two-sided and statistical significance was defined as P<0.05.
RESULTS
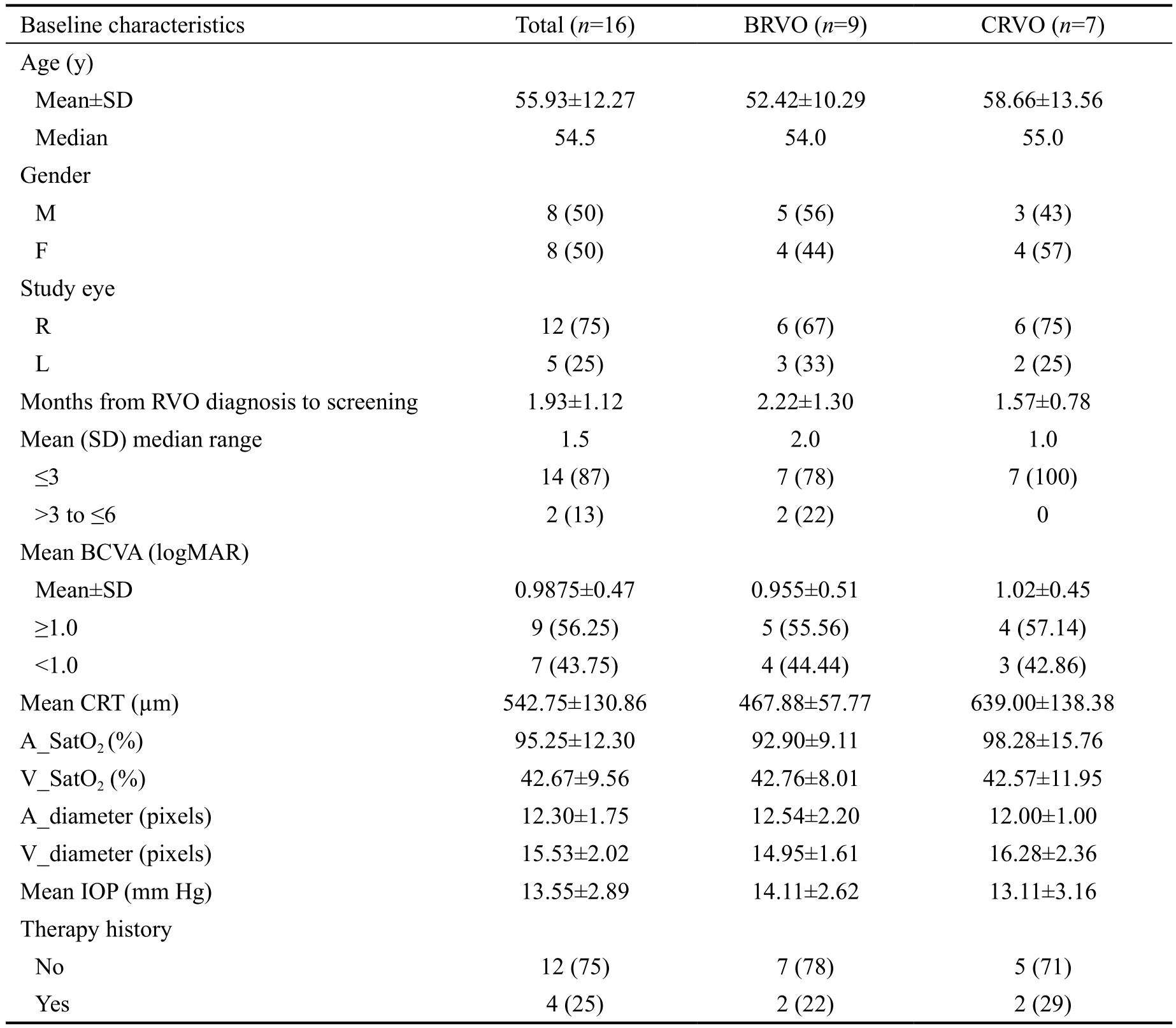
Patient Characteristics A total of 16 eyes of 16 patients (8 men, 8 women) with ME secondary to RVO were included in this study. All patients received treatment between September 2016 and September 2017. Study patient characteristics were summarized in Table 1. The average patient age was 55.93±12.27y. Seven patients had a CRVO and nine patients had a BRVO. Baseline BCVA was 0.99±0.47 (20/200) and baseline CRT was 542.75±130.86 µm in the eyes under study. The mean time from RVO diagnosis to screening was 1.93±1.12mo.
All patients completed the treatment protocol and the 12-month follow-up. Four of 7 patients with CRVO (57.10%)chose intravitreal conbercept therapy while the other 3 patients(42.9%) chose intravitreal ranibizumab therapy. Similarly, 5 of 9 (55.60%) patients with BRVO chose intravitreal conbercept therapy while the other 4 patients (44.40%) chose intravitreal ranibizumab. Until month 12, the mean number of injections administered across all patients was 1.43±0.81 injections(interquartile range=1-4 injections). Four patients (2 CRVO,2 BRVO) required 2 anti-VEGF agent injections and 1 patient with BRVO required 4 injections. At the final study visit,routine examination with slit-lamp biomicroscopy and FFA showed that retinal hemorrhages had largely absorbed and that venous dilation and tortuosity had markedly decreased in all eyes. None of the 16 patients examined developed ischemic disease.
Combination Treatment Efficacy The change in BCVA at 6mo was significantly better than at baseline in both CRVO[baseline: 0.96±0.51 (20/178), month 6: 0.33±0.95 (20/43);P<0.00] and BRVO [baseline: 1.02±0.45 (20/209), month 6: 0.78±0.60 (20/121); P=0.011] patients (Figure 2). Thechange in BCVA at 12mo also was significantly better than at baseline in both CRVO [baseline: 0.96±0.51 (20/178),month 12: 0.31±0.88 (20/40), P<0.00] and BRVO [baseline:1.02±0.45 (20/209), month 12: 0.60±0.49 (20/80); P<0.00]patients. The change in BCVA at month 6 and 12 both were not significantly different between the BRVO and CRVO groups(P=0.51, 0.38). Unfortunately, 1 of 9 patients with BRVO(11.10%) and 2 of 7 patients with CRVO (28.60%) had afinal logMAR BCVA≥1.0 (worse than 20/200), which was worse than at baseline. This slight difference between groups was not significant (P>0.99). Additionally, the change in BCVA at 12mo was significantly correlated with baseline BCVA in both BRVO and CRVO patients (r=0.77, P=0.02; r=0.73, P<0.00).No other baseline characteristics correlated significantly with the change in BCVA (Table 2).
Table 1 Baseline characteristics n (%), mean±SD
The reduction in CRT at 12mo was significant in patients with BRVO (238.37±18.31 µm, P<0.00) and CRVO (243.12±14.40 µm,P<0.00; Figure 2). Eyes with CRVO had a significantly smaller CRT reduction than eyes with BRVO (P<0.00). At 12mo,2 of 9 BRVO patients (22.20%) and 3 of 7 CRVO patients(42.80%) had CRT>250 µm. Though large, this difference between groups was not significant (P=0.36).Arterial oxygen saturation did not change during the study period in eyes with CRVO (baseline: 98.28%±15.76%,12mo 95.14%±2.67%; P=0.58) or BRVO (baseline:92.90%±9.11%, 12mo: 93.11%±3.25%; P=0.94). In contrast,venous oxygen saturation significantly increased during the study period in both the CRVO (baseline: 42.57%±11.95%,12mo: 60.00%±4.39%; P<0.00) and BRVO (baseline:42.76%±8.01%, 12mo: 57.22%±4.71%; P<0.00) groups.Arterial and venous diameter did not significantly change during the study period in either the CRVO (P=0.20 and 0.67,respectively) or BRVO (P=0.29 and 0.10, respectively) group.Additionally, after 12mo of treatment, there was no significant difference between eyes with BRVO and CRVO in any oxygenation parameter examined (arterial oxygen saturation:P=0.20, venous oxygen saturation: P=0.58, arterial diameter:P=0.13, and venous diameter: P=0.35; Table 3).
Table 2 Correlation about change of BCVA from baseline to month 12 with baseline variables
A: Arterial; V: Venous.
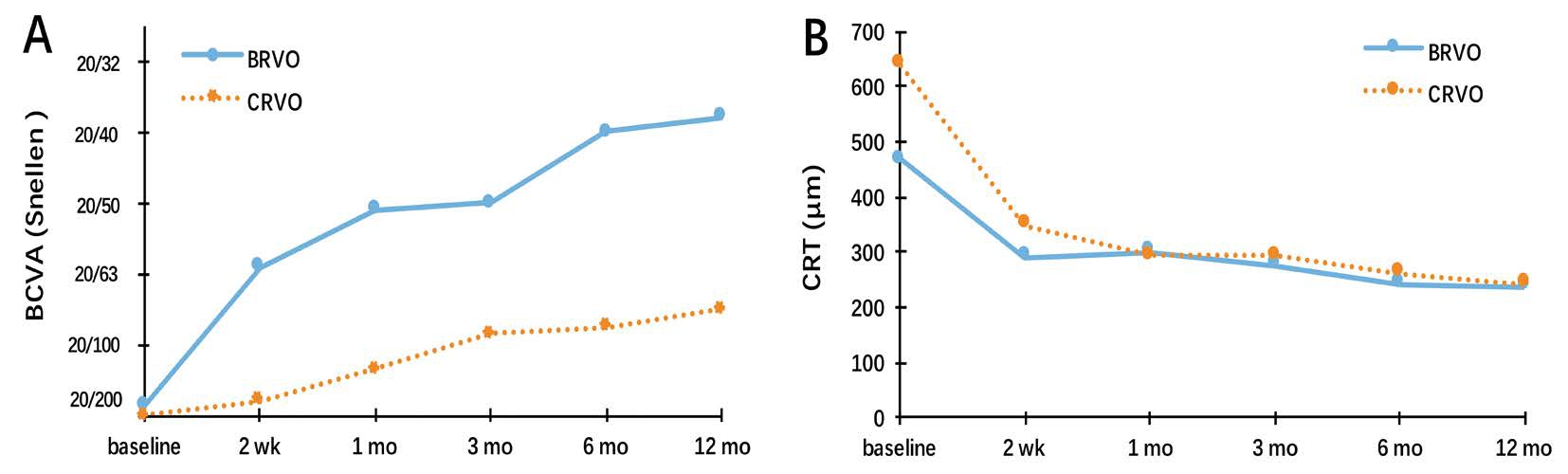
Figure 2 Visual outcomes and OCT outcomes A: The mean change of BCVA from baseline to month 12; B: The mean change of CRT from baseline to month 12.
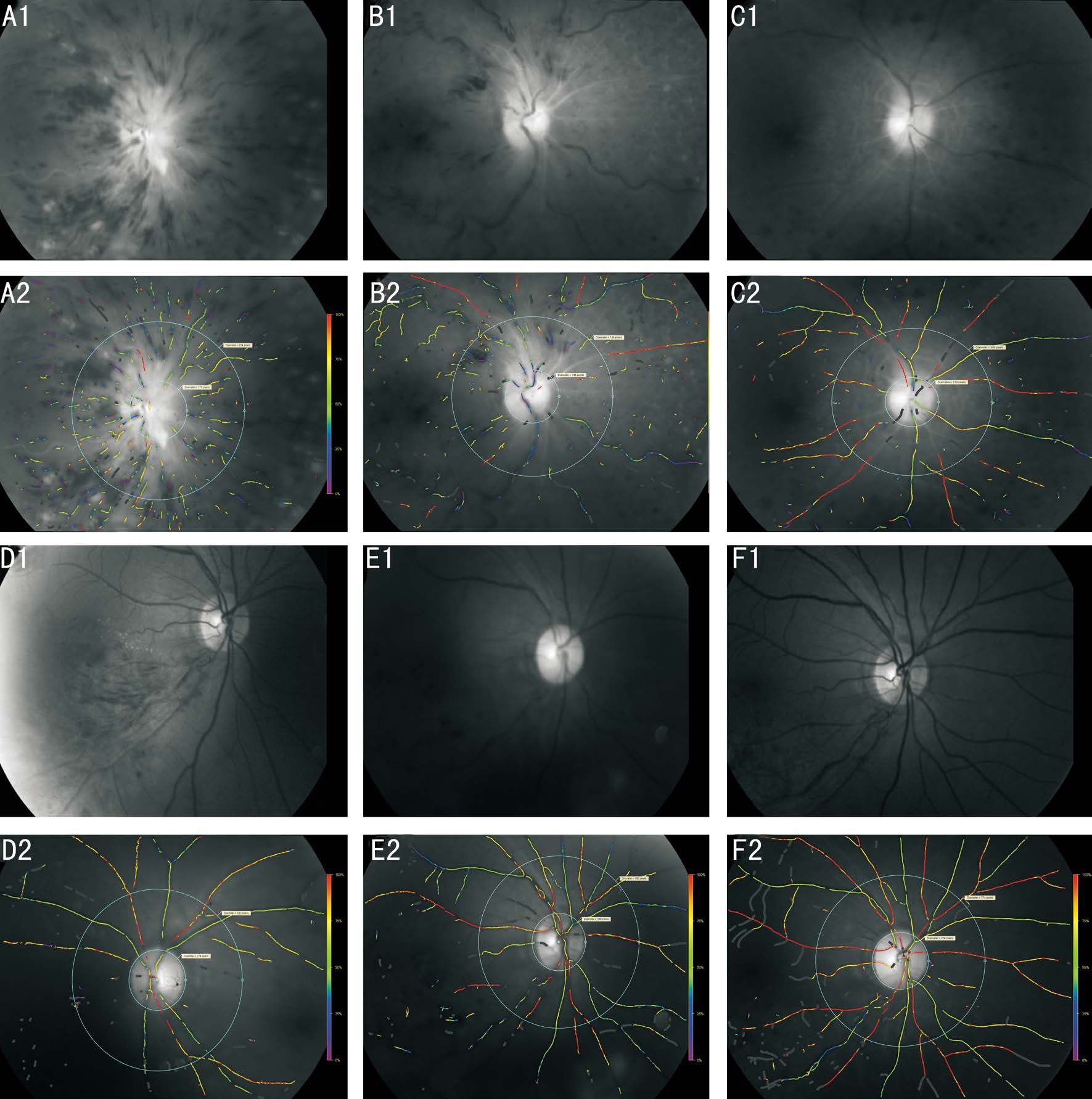
Figure 3 Oxygen saturation outcomes at baseline (A1-A2, D1-D2), after 6mo of treatment (B1-B2, E1-E2) and after 12mo of treatment(C1-C2, F1-F2) A-C: The images showed the change of the oxygen saturation outcomes of a patient with CRVO; D-F: The images showed the change of the oxygen saturation outcomes of a patient with BRVO.
Figure 3 summarizes changes that occurred in retinal vessel oxygenation during the study period. The results of “SAVE”scores (Table 4) showed that the mean scores improvedsignificantly in the subretinal fluid (P<0.00) and vitre-retinal abnormalities (P<0.00) grading post drug treatment, and this trend was sustained to thefinal follow-up. The area of retinal thickening and etiology showed no significant improvement after drug treatment (P=0.27, 0.75). However, post laser therapy, the scores of “A” and “E” decreased significantly(P<0.00, 0.02).
Table 3 Comparison of oxygen saturation and vessel diameter in CRVO and BRVO

BCVA: Best-corrected visual acuity; OCT: Optical coherence tomography.aValues indicate the difference between CRVO and BRVO, and the results from repeated measures analysis of variance;bValues indicate the difference between baseline and month 12, and the results from paired t-test.
Table 4 Results of “SAVE” scores mean±SD

S: Subretinal fluid; A: Area; V: Vitreo-retinal abnormalities; E: Etiology. P values indicate results from paired t-test.aValues indicate the difference between pre-treatment and before laser therapy;bValues indicate the difference between before laser therapy and final followed-up.
Combination Therapy Safety One of the 9 patients with BRVO (11.10%) had a temporary elevation in IOP and 2 of the 7 patients with CRVO (25.60%) developed conjunctival hyperemia. No patient experienced any serious injectionrelated (e.g. endophthalmitis, retinal detachment, retinal tears,and cataract) or drug-related (e.g. thromboembolic event,glaucoma, gastric ulcer, osteoporosis, or myopathy) AEs during the study period.
DISCUSSION
This study examined the safety and efficacy of treating ME secondary to RVO with intravitreal anti-VEGF, oral glucocorticoids, and laser photocoagulation combination therapy. Rapid improvements in functional and anatomical parameters were observed and these improvements were sustained for at least 12mo. Importantly, most patients did not require repeat intravitreal anti-VEGF agent injections.
Treatment efficacy observed here is in agreement with that observed in prior studies, including the studies of Sun et al[21]and Campochiaro et al[22-23]. On an average, both BCVA and CRT showed improvement over baseline values at the primary end point and final study visit. However, compared to patients with BRVO and CRVO in the study of Sun et al[21], who received a mean of 7.14±1.90 and 7.59±1.39 injections, respectively,over 9mo, patients in this study received a mean of 1.37±0.61 injections by month 6. Even until month 12, the mean number of injections was 1.43±0.81. As a matter of fact, earlier studies have reported that repeated intraocular injections increase vitreous traction and the risk of retinal tear, retinal detachment,and endophthalmitis[6]. Intravitreal anti-VEGF injections have also been associated with retinal artery constriction and ischemia[10,24]. Additionally, intravitreal anti-VEGF injections are expensive and minimizing the number of treatments needed eases the large economic burden associated with this therapy.This combination therapy used here in place of repeated anti-VEGF injection successfully reduced the number of required injections from 7-8 per patient over 9mo[21]to 1-2 per patient over 12mo. As indicated by the fewer number of injections, the functional and anatomical improvements observed here were largely sustained and progressively increased until month 12.This good prognosis obtained with fewer number of injections is superior to that with earlier treatment methods.
The use of intravitreal anti-VEGF therapies is somewhat controversial. Recent studies have shown that intravitreal anti-VEGF agents can severely disturb the retinal blood flow, and that these changes may be harmful to the retinal structure and function[24-26]. The combination therapy used here did not induce changes in either the retinal arterial or venous diameter at any point of examination. In contrast, Sacu et al[27]observed a 14%-15% decrease in retinal vessel diameter (veins and arteries), 3mo after initiating intravitreal anti-VEGF therapy in eyes with BRVO. It should be noted that Sacu et al[27]used the retinal vessel analyzer to measure retinal vessel caliber, and not the Oxymap that was used in the current study. However,in agreement with a previous study that also measured retinal vessel oximetry[28], we found an improvement in the retinal blood supply after treatment (indicated by a rise in the retinal vein oxygen saturation with no significant change in arterial oxygen saturation). It should be noted that, though not significant, a modest arterial oxygen saturation decrease was observed following treatment. Therefore, we cannot ignore the possible effects of intravitreal anti-VEGF agents on the retinal blood flow. Additionally, none of our study patients experienced any drug- or procedure-related serious AEs,including neovascular complications. Some views suggest that the upregulation of VEGF has been implicated as a major cause of ME, but anti-VEGF therapy may block the neuroprotective actions of VEGF (e.g. promoting proliferation, differentiation,and survival) on the endothelial, retinal ganglion, Müller,and photoreceptor cells[29-31]. These risks likely increase when injections are repeatedly administered. However, appropriate administration of intravitreal anti-VEGF agents has been shown to be safe. The high cost of each intravitreal anti-VEGF injection also contributes to this controversy and some patients discontinue treatment for economic reasons,particularly in developing countries and when insurance does not cover treatment costs. Hence, reducing overall therapy cost by lowering the number of injections may make anti-VEGF therapy more accessible.
Our patients were also treated with oral glucocorticoids.Glucocorticoids reduce edema,fibrin deposition, and in flammatory cells in RVO by effectively downregulating the expression of metalloproteases, inflammatory cytokines, chemokines, and subsequently decreasing VEGF-A expression and increasing tight junction-associated protein production[32-34]. Due to these mechanisms, glucocorticoids are used in variety of ocular diseases, such as keratitis, allergic conjunctivitis, uveitis,choroiditis, ME, and for reducing inflammation following surgeries[35]. Hence, we added glucocorticoids to improve the rapidity of reducing ME. The results of OCT and “SAVE”scores had shown that oral glucocorticoids reduce the ME and achieve rapid improvements in subretinal fluid and vitreoretinal abnormalities before the laser therapy. Furthermore,the strategies of effective treatment in clinical settings and research studies usually focus on achieving and maintaining the therapeutic concentrations, which can be controlled by the appropriate administration route. Oral administration,intravenous injection, andintravitreal implants help the drugs reach the ocular posterior segment. Intravitreal injections were widely used for delivering the glucocorticoids into the vitreous humor. The complications are caused by the cytotoxic effects and intraocular injections. For the former, studies reported that glucocorticoids had a concentration- and timedependent cytotoxic effect on retinal cells, lens, and trabecular meshwork[36-38]. The latter complications include vitreous hemorrhage, endophthalmitis, and retinal detachment, etc[39].The intravitreal implant Ozurdex was approved by the FDA for treatment of ME secondary to RVO. Clinical research has proven the efficacy and safety of treatments; however, the complications (increased IOP, cataract, etc.), particularly with repeated treatment, still need to be addressed[40-41]. We chose to administer oral glucocorticoidsas part of the treatment regimen,in order to avoid invasive therapy (injection or surgery).Doubtless, it was essential to prevent potential side-effects associated with nonspecific accumulation in other organs[42]. In this study, each patient was administered a personalized dose of glucocorticoids, for safety's sake. Throughout the clinical period, we also followed the patients for glucocorticoidsrelated AEs, such as changes in blood pressure, blood sugar,blood biochemistry, or organ injury (thromboembolic event,glaucoma, gastric ulcer, osteoporosis or myopathy) etc. The final results showed that no patient experienced any drugrelated AEs during the study period. Thus, at least in our trial,oral glucocorticoids were safe and effective. Unfortunately, 1 patient in the current study did not take the oral glucocorticoids as prescribed between months 1 and 2. Three subsequent intravitreal anti-VEGF injections were required to stabilize this patient’s vision, and one more injection was needed at month 7 for persistent ME. Therefore, clinical patients and study patients who begin oral glucocorticoid therapy should be educated on the importance of compliance.
Laser photocoagulation was also administered to patients with ME secondary to RVO, 2wk following intravitreal anti-VEGF injection. The spot size was 50-75 μm and the exposure time was 0.08-0.15s. The power was adjusted and initiated at 300 mW and increased in steps of 10 mW to produce mild intensity burns covering areas of capillary leakage as seen on FFA, 1 burn width apart. “SAVE” scores displayed that the area of retinal thickening and etiology improved significantly after laser therapy. It might be mentioned that laser therapy is perhapsbetter than drugs in reducing the area of edema and leakage. Laser treatment was delayed because intravitreal anti-VEGF therapy reduced retinal thickness (via relieving retinal swelling) and promoted retinal hemorrhage absorption, both of which likely improved laser energy penetration. Additionally,photocoagulation led to a reduction in vascular leakage and stabilized the retina after the short-term effects of anti-VEGF agents wore off, reducing the number of repeat anti-VEGF injections. The 2-week timing was chosen because the vitreous half-life of ranibizumab in monkeys (48 kDa) and conbercept in rabbits (143 kDa) is 2.6-4.0d[43]and 4.2d[44], respectively.Given that species with bigger eyes and longer diffusion paths have a slower vitreous clearance, we theorized that the halflife of ranibizumab and conbercept in the human eye would be 9-14d (vitreous volume is 4.5 mL in humans and 1.5 mL in rabbits and monkeys[45]).
This pilot study examined the safety and efficacy of a new combination therapy for ME secondary to RVO. It had several limitations. First, the small sample size of this pilot study limited the statistical significance of observed changes and differences between patients with BRVO and CRVO. Second,though it was prospective, our study was not randomized,controlled, or masked. Third, oral glucocorticoid dose was tailored to each patient for safety reasons. This may have confounded our results because of varying steroid effects among patients. Therefore, our results should be validated with future multi-center, randomized, controlled studies on a larger number of patients. These studies should also standardize the timing and dose of all therapies administered.
This study demonstrates important benefits of intravitreal anti-VEGF, oral glucocorticoid, and grid laser photocoagulation combination therapy in treating ME secondary to RVO. In most patients, this combination treatment resulted in rapid improvement of retinal function and anatomy that was sustained for at least 12mo. Furthermore, the need for repeat intravitreal anti-VEGF injections was markedly reduced compared to other studies that examined anti-VEGF therapy alone. Therefore, physicians should consider combination therapy for treating ME secondary to RVO.
ACKNOWLEDGEMENTS
Authors’ contributions: All aspects of the study were carried out under the auspices of writers. Xiao-Xiao Feng, Cheng Li,Wan-Wen Shao, Yong-Guang Yuan collated data, Qi-Shan Zheng, Xiao-Bing Qian, Yu-Jie Li contributed to the discussions of the results, analyzing data and commented on drafts of the report. The project was organized by Xiao-Xiao Feng, who was responsible for formulating the question, developing the protocol, receiving, and checking data. The project was managed by Qian-Ying Gao. The report was drafted by Xiao-Xiao Feng.
Foundation:Supported by Technology Planning Project of Guangdong Province, China (No.2015B020211004).
Conflicts of Interest:Feng XX, None; Li C, None; Shao WW, None; Yuan YG, None; Qian XB, None; Zheng QS,None; Li YJ, None; Gao QY, None.
REFERENCES
1 Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY; International Eye Disease Consortium. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia.Ophthalmology 2010;117(2):313-319. e1.
2 Ho M, Liu DT, Lam DS, Jonas JB. Retinal vein occlusions, from basics to the latest treatment. Retina 2016;36(3):432-448.
3 Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol 1984;98(3):271-282.
4 Clarkson JG, Chuang E, Gass D, Pedroso M, Cubillas T, Duria ES,Muniz N. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion: the central vein occlusion study group M report.Ophthalmology 1995;102(10):1425-1433.
5 Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC,Rubio RG. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study.Ophthalmology 2010;117(6):1102-1112.
6 Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P,Schlingemann RO, Weichselberger A. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118(4):615-625.
7 Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P,Massin P, Mitchell P. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology 2014;121(5):1045-1053.
8 Varma R, Bressler NM, Suñer I, Lee P, Dolan CM, Ward J, Rubio RG.Improved vision-related function after ranibizumab for macular edema after retinal vein occlusion: results from the BRAVO and CRUISE trials.Ophthalmology 2012;119(10):2108-2118.
9 Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, Sone T,Minamoto A. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol 2005;140(2):256-e1.
10 Mansour AM, Bynoe LA, Welch JC, Pesavento R, Mahendradas P, Ziemssen F, Pai SA. Retinal vascular events after intravitreal bevacizumab. Acta Ophthalmologica 2010;88(7):730-735.
11 Yilmaz T, Cordero-Coma M. Use of bevacizumab for macular edema secondary to branch retinal vein occlusion: a systematic review. Graefe's Arch Clin Exp Ophthalmol 2012; 250(6):787-793.
12 Azad R, Chandra P, Gupta R. The economic implications of the use of anti-vascular endothelial growth factor drugs in age-related macular degeneration. Indian J Ophthalmol 2007;55(6):441.
13 Kartasasmita AS, Takarai S, Switania A, Enus S. Efficacy of single bevacizumab injection as adjuvant therapy to laser photocoagulation in macular edema secondary to branch retinal vein occlusion. Clin Ophthalmol 2016;10:2135.
14 Shah NJ, Shah UN. Long-term effect of early intervention with single intravitreal injection of bevacizumab followed by panretinal and macular grid photocoagulation in central retinal vein occlusion (CRVO) with macular edema: a pilot study. Eye (Lond) 2011;25(2):239.
15 Amoaku WM, Chakravarthy U, Gale R, Gavin M, Ghanchi F, Gibson J, Mahmood S. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond) 2015;29(6):721.
16 Paulus YM, Sodhi A. Anti-angiogenic therapy for retinal disease.Handb Exp Pharmacol 2017;242:271-307.
17 Tso MOM. Retinal photocoagulation therapy: clinical application and biological basis of therapeutic effects. Retinal Diseases. Philadelphia: JB Lippincott Company;1988:246-262.
18 Nork TM. Laser surgery in ophthalmology: practical applications. Arch Ophthalmol 1994;112(9):1153-1154.
19 Bolz M, Lammer J, Deak G, Pollreisz A, Mitsch C, Scholda C,Schmidt-Erfurth U. SAVE: a grading protocol for clinically significant diabetic macular oedema based on optical coherence tomography and fluorescein angiography. Br J Ophthalmol 2014;98(12):1612-1617.
20 Reznicek L, Bolz M, Garip A, Kampik A, Kernt M, Mayer WJ.Evaluation of the new “SAVE” protocol in diabetic macular edema over the course of anti-VEGF treatment. Curr Eye Res 2016;41(8):1082-1086.
21 Sun Z, Zhou H, Lin B, Jiao X, Luo Y, Zhang F, Liu X. Efficacy and safety of intravitreal conbercept injections in macular edema secondary to retinal vein occlusion. Retina 2017;37(9):1723.
22 Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N,Rubio RG. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology 2011;118(10):2041-2049.
23 Campochiaro PA, Clark WL, Boyer DS, Heier JS, Brown DM, Vitti R, Soo Y. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study.Ophthalmology 2015;122(3):538-544.
24 Fontaine O, Olivier S, Descovich D, Cordahi G, Vaucher E, Lesk MR.The effect of intravitreal injection of bevacizumab on retinal circulation in patients with neovascular macular degeneration. Invest Ophthalmol Vis Sci 2011;52(10):7400-7405.
25 Kim JE, Mantravadi AV, Hur EY, Covert DJ. Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular endothelial growth factor agents. Am J Ophthalmol 2008;146(6):930-934.
26 Yokoyama K, Choshi T, Kimoto K, Shinoda K, Nakatsuka K. Retinal circulatory disturbances following intracameral injection of bevacizumab for neovascular glaucoma. Acta Ophthalmol 2008;86(8):927-928.
27 Sacu S, Pemp B, Weigert G, Matt G, Garhofer G, Pruente C, Schmidt-Erfurth U. Response of retinal vessels and retrobulbar hemodynamics to intravitreal anti-VEGF treatment in eyes with branch retinal vein occlusion. Invest Ophthalmol Vis Sci 2011;52(6):3046-3050.
28 Traustason S, la Cour M, Larsen M. Retinal vascular oximetry during ranibizumab treatment of central retinal vein occlusion. Br J Ophthalmol 2014;98(9):1208-1211.
29 D'Amore PA. Vascular endothelial cell growth factor-a: not just for endothelial cells anymore. Am J Pathol 2007;171(1):14-18.
30 Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, D'Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors.PLoS One 2008;3(11):e3554.
31 Kilic U, Kilic E, Jarve A, Guo Z, Spudich A, Bieber K, Hermann DM. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. J Neurosci 2006;26(48):12439-12446.
32 Coursey TG, Henriksson JT, Marcano DC, Shin CS, Isenhart LC,Ahmed F, Acharya G. Dexamethasone nanowafer as an effective therapy for dry eye disease. J Control Release 2015;213:168-174.
33 Bian F, Shin CS, Wang C, P flugfelder SC, Acharya G, De Paiva CS.Dexamethasone drug eluting nanowafers control in flammation in alkaliburned corneas associated with dry eye. Invest Ophthalmol Vis Sci 2016;57(7):3222-3230.
34 Cholkar K, Hariharan S, Gunda S, Mitra AK. Optimization of dexamethasone mixed nanomicellar formulation. AAPS PharmSciTech 2014;15(6):1454-1467.
35 Blizzard C, Desai A, Driscoll A. Pharmacokinetic studies of sustainedrelease depot of dexamethasone in beagle dogs. J Ocul Pharmacol Ther 2016;32(9):595-600.
36 El-Zaoui I, Behar-Cohen FF, Torriglia A. Glucocorticoids exert direct toxicity on microvasculature by triggering cell death of endothelial cells.Invest Ophthalmol Vis Sci 2014;55(13):4895.
37 Kwak HW, D’Amico DJ. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol 1992;110(2):259-266.
38 Overby DR, Bertrand J, Tektas OY, Boussommier-Calleja A, Schicht M, Ethier CR, Lutjen-Drecoll E. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Invest Ophthalmol Vis Sci 2014;55(8):4922-4933.
39 Michalska-Malecka K, Gaborek A, Nowak M, Halat T, Pawlowska M,Spiewak D. Evaluation of the effectiveness and safety of glucocorticoids intravitreal implant therapy in macular edema due to retinal vein occlusion. Clin Interv Aging 2016;11:699-705.
40 Shah AR, Xi M, Abbey AM, Yonekawa Y, Faia LJ, Hassan TS,Wolfe JD. Short-term efficacy of intravitreal dexamethasone implant in vitrectomized eyes with recalcitrant diabetic macular edema and prior anti-VEGF therapy. J Ophthalmic & Vis Res 2016;11(2):183.
41 Jusufbegovic D, Schaal S. Quiescent herpes simplex keratitis reactivation after intravitreal injection of dexamethasone implant. Retin Cases Brief Rep 2017;11(4):296-297.
42 Katzung BG, Masters SB, Trevor AJ. Basic & Clinical Pharmacology 2004.
43 Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet 2012; 379(9827):1728-1738.
44 Li H, Lei N, Zhang M, Li Y, Xiao H, Hao X. Pharmacokinetics of a long-lasting anti-VEGF fusion protein in rabbit. Exp Eye Res 2012;97(1):154-159.
45 Del Amo EM, Vellonen KS, Kidron H, Urtti A. Intravitreal clearance and volume of distribution of compounds in rabbits: In silico prediction and pharmacokinetic simulations for drug development. Eur J Pharm Biopharm 2015;95:215-226.